Clinical Outcomes of the Adapted AAP 2019 Guidelines on Early Onset Sepsis in Thailand
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Methods Study Design and Data Collection
- Inclusion and Exclusion Criteria
- Pre–adapted AAP 2019 guidelines period (1 February 2017–31 January 2018): This period was specifically chosen to ensure that no neonates received treatment according to the AAP 2019 guidelines. Neonates with suspected EOS were managed according to the CDC 2010 guidelines [36] and the AAP 2012 guidelines [37], supplemented by the clinical judgment of attending physicians. Suspected EOS was not defined by standardized criteria; inclusion was based on the treating physician’s assessment of clinical signs. EOS cases were classified as culture-proven EOS.
- Post–adapted AAP 2019 guidelines period (1 February 2023–31 January 2024): This period was selected because the adapted AAP 2019 guidelines were formally implemented as the standard protocol at Panyananthaphikkhu Chonprathan Medical Center in January 2023. Prior to implementation, the neonatology team provided detailed guidance to all clinicians involved in neonatal care, including pediatricians and interns. During this period, periodic reviews of guideline implementation were conducted, and medical records consistently documented neonatal signs, diagnostic steps, and management. This system ensured that neonates were treated in accordance with the adapted AAP 2019 guidelines. Suspected EOS during this period was defined according to the EOSC guideline, and EOS cases were classified as culture-proven EOS.
4.2. EOS Guidelines Before the Adapted AAP 2019 Implementation
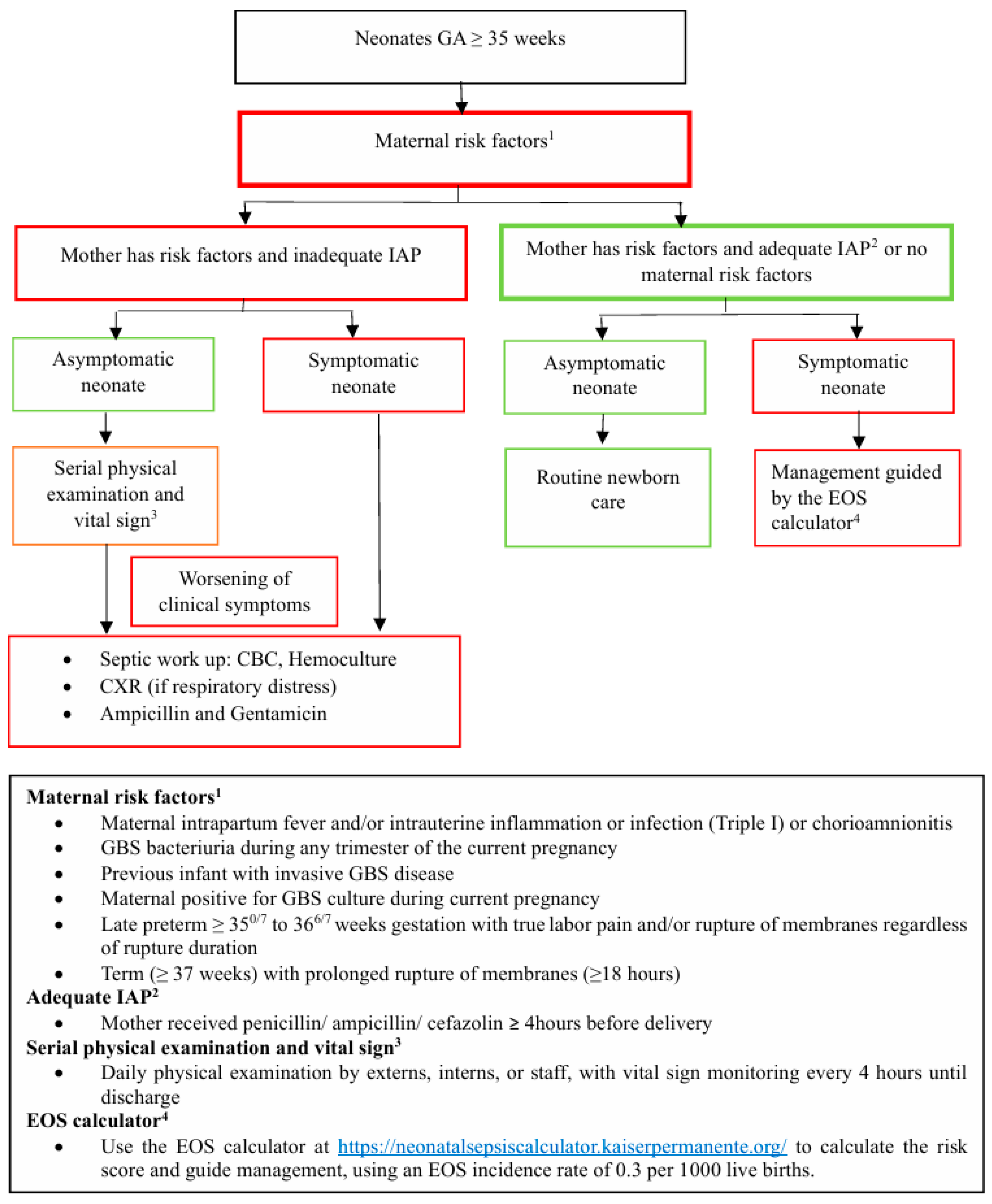
4.3. EOS Guidelines After the Adapted AAP 2019 Implementation
4.4. Definition
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 2018, 142, e20182882. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J. Early onset group B streptococcal disease. J. Pediatr. 1978, 93, 124–125. [Google Scholar] [CrossRef]
- Coggins, S.A.; Puopolo, K.M. Neonatal group B streptococcus disease. Pediatr. Rev. 2024, 45, 63–73. [Google Scholar] [CrossRef]
- Escobar, G.J.; Puopolo, K.M.; Wi, S.; Turk, B.J.; Kuzniewicz, M.W.; Walsh, E.M.; Newman, T.B.; Zupancic, J.; Lieberman, E.; Draper, D. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics 2014, 133, 30–36. [Google Scholar] [CrossRef]
- Nanduri, S.A.; Petit, S.; Smelser, C.; Apostol, M.; Alden, N.B.; Harrison, L.H.; Lynfield, R.; Vagnone, P.S.; Burzlaff, K.; Spina, N.L.; et al. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006 to 2015: Multistate laboratory and population-based surveillance. JAMA Pediatr. 2019, 173, 224–233. [Google Scholar] [CrossRef]
- Michael, W.; Eileen, M.; Sherian, L.; Allen, F.; Gabriel, J. Development and implementation of an early-onset sepsis calculator to guide antibiotic management in late preterm and term neonates. Jt. Comm. J. Qual. Patient Saf. 2016, 42, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Deleon, C.; Shattuck, K.; Jain, S.K. Biomarkers of neonatal sepsis. NeoReviews 2015, 16, e297–e308. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Pamer, E.G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 2016, 352, 535–538. [Google Scholar] [CrossRef]
- Baumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Esaiassen, E.; Fjalstad, J.W.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic exposure in neonates and early adverse outcomes: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 1858–1870. [Google Scholar] [CrossRef]
- Fjalstad, J.W.; Esaiassen, E.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: A systematic review. J. Antimicrob. Chemother. 2018, 73, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Ryan, C.A.; Dempsey, E.; Ross, P.R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiol. Open 2019, 8, e1260. [Google Scholar] [CrossRef]
- Sourour, W.; Sanchez, V.; Sourour, M.; Burdine, J.; Lien, E.R.; Nguyen, D.; Jain, S.K. The association between prolonged antibiotic use in culture-negative infants and length of hospital stay and total hospital costs. Am. J. Perinatol. 2023, 40, 525–531. [Google Scholar] [CrossRef]
- Aeimcharnbanchong, K. Incidence rate and associated factors of early-onset sepsis among neonates born at ≥35 weeks’ gestation in a Thai tertiary hospital. Infect. Drug Resist. 2023, 16, 4093–4100. [Google Scholar] [CrossRef]
- Dhudasia, M.B.; Flannery, D.D.; Pfeifer, M.R.; Puopolo, K.M. Updated guidance: Prevention and management of perinatal group B Streptococcus infection. NeoReviews 2021, 22, e167–e179. [Google Scholar] [CrossRef]
- Strunk, T.; Buchiboyina, A.; Sharp, M.; Nathan, E.; Doherty, D.; Patole, S. Implementation of the neonatal sepsis calculator in an Australian tertiary perinatal centre. Neonatology 2018, 113, 379–382. [Google Scholar] [CrossRef]
- Stipelman, C.H.; Smith, E.R.; Diaz-Ochu, M.; Spackman, J.; Stoddard, G.; Kawamoto, K.; Shakib, J.H. Early-onset sepsis risk calculator integration into an electronic health record in the nursery. Pediatrics 2019, 144, e20183186. [Google Scholar] [CrossRef] [PubMed]
- Klingaman, C.; King, L.; Neff-Bulger, M. Improved newborn care: Evidence-based protocol for the evaluation and management of early-onset sepsis. Am. J. Med. Qual. 2018, 33, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Akangire, G.; Simpson, E.; Weiner, J.; Noel-MacDonnell, J.; Petrikin, J.; Sheehan, M. Implementation of the neonatal sepsis calculator in early-onset sepsis and maternal chorioamnionitis. Adv. Neonatal Care 2020, 20, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Strunk, D.; Furqan, S.H.; Schweig, L.; Lefaiver, C.; George, J.; Prazad, P. Optimizing antibiotic use for early-onset sepsis: A tertiary NICU experience. J. Neonatal Perinat. Med. 2019, 12, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Achten, N.B.; Dorigo-Zetsma, J.W.; van der Linden, P.D.; van Brakel, M.; Plötz, F.B. Sepsis calculator implementation reduces empiric antibiotics for suspected early-onset sepsis. Eur. J. Pediatr. 2018, 177, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Eason, J.; Ward, H.; Danko, O.; Richardson, K.; Vaitkute, R.; McKeon-Carter, R. Early-onset sepsis: Can we screen fewer babies safely? Arch. Dis. Child. 2021, 106, 86–88. [Google Scholar] [CrossRef]
- Gong, C.L.; Dasgupta-Tsinikas, S.; Zangwill, K.M.; Bolaris, M.; Hay, J.W. Early-onset sepsis calculator-based management of newborns exposed to maternal intrapartum fever: A cost-benefit analysis. J. Perinatol. 2019, 39, 571–580. [Google Scholar] [CrossRef]
- Pontello, E.; Favero, V.; Mainini, N.; Tormena, F.; Giovannini, M.; Galeazzo, B.; Frigo, A.C.; Lago, P. Neonatal early-onset sepsis: Impact of the Kaiser calculator in an Italian tertiary perinatal center. Pediatr. Infect. Dis. J. 2022, 41, 161–165. [Google Scholar] [CrossRef]
- Carola, D.; Vasconcellos, M.; Sloane, A.; McElwee, D.; Edwards, C.; Greenspan, J.; Aghai, Z.H. Utility of early-onset sepsis risk calculator for neonates born to mothers with chorioamnionitis. J. Pediatr. 2018, 195, 48–52.e1. [Google Scholar] [CrossRef]
- Money, N.; Newman, J.; Demissie, S.; Roth, P.; Blau, J. Antimicrobial stewardship: Antibiotic use in well-appearing term neonates born to mothers with chorioamnionitis. J. Perinatol. 2017, 37, 1304–1309. [Google Scholar] [CrossRef]
- Pettinger, K.J.; Mayers, K.; McKechnie, L.; Phillips, B. Sensitivity of the Kaiser Permanente early-onset sepsis calculator: A systematic review and meta-analysis. EClinicalMedicine 2019, 19, 100227. [Google Scholar] [CrossRef]
- Berardi, A.; Bedetti, L.; Spada, C.; Lucaccioni, L.; Frymoyer, A. Serial clinical observation for the management of newborns at risk of early-onset sepsis. Curr. Opin. Pediatr. 2020, 32, 245–251. [Google Scholar] [CrossRef]
- Vaccina, E.; Luglio, A.; Ceccoli, M.; Lecis, M.; Leone, F.; Zini, T.; Toni, G.; Lugli, L.; Lucaccioni, L.; Iughetti, L.; et al. Brief comments on three existing approaches for managing neonates at risk of early-onset sepsis. Ital. J. Pediatr. 2021, 47, 159. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.S.; Gupta, A.; Allan, J.M.; Cohen, R.S.; Aby, J.L.; Weldon, B.; Kim, J.L.; Benitz, W.E.; Frymoyer, A. Clinical monitoring of well-appearing infants born to mothers with chorioamnionitis. Pediatrics 2018, 141, e20174032. [Google Scholar] [CrossRef]
- Joshi, N.S.; Gupta, A.; Allan, J.M.; Cohen, R.S.; Aby, J.L.; Kim, J.L.; Benitz, W.E.; Frymoyer, A. Management of chorioamnionitis-exposed infants in the newborn nursery using a clinical examination-based approach. Hosp. Pediatr. 2019, 9, 227–233. [Google Scholar] [CrossRef]
- Cavigioli, F.; Viaroli, F.; Daniele, I.; Paroli, M.; Guglielmetti, L.; Esposito, E.; Cerritelli, F.; Zuccotti, G.; Lista, G. Neonatal early-onset sepsis calculator plus universal serial physical examination: A prospective two-step implementation. Antibiotics 2022, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Dedeke, I.; Arowosegbe, A.; Shittu, O.; Ojo, D.; Akingbade, O. Neonatal sepsis in a Nigerian tertiary hospital: Clinical features, outcome, aetiology, and antibiotic susceptibility. S. Afr. J. Infect. Dis. 2017, 32, 127–131. [Google Scholar] [CrossRef]
- You, T.; Zhou, Y.R.; Liu, X.C.; Li, L.Q. Risk factors and clinical characteristics of neonatal acute respiratory distress syndrome caused by early-onset sepsis. Front. Pediatr. 2022, 10, 847827. [Google Scholar] [CrossRef] [PubMed]
- Verani, J.R.; McGee, L.; Schrag, S.J.; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC. Prevention of perinatal group B streptococcal disease—Revised guidelines from CDC, 2010. MMWR Recomm. Rep. 2010, 59, 1–36. [Google Scholar] [PubMed]
- Polin, R.A.; Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012, 129, 1006–1015. [Google Scholar] [CrossRef]
- Hershkovich-Shporen, C.; Hofi, L.; Khodajev, A.; Flidel Rimon, O. Feeding intolerance in newborns. Am. J. Biomed. Sci. Res. 2022, 16, 210–215. [Google Scholar] [CrossRef]
- Sahni, M.; Jain, S. Hypotension in neonates. NeoReviews 2016, 17, e579–e589. [Google Scholar] [CrossRef]
- Mayfield, S.R.; Bhatia, J.; Nakamura, K.T.; Rios, G.R.; Bell, E.F. Temperature measurement in term and preterm neonates. J. Pediatr. 1984, 104, 271–275. [Google Scholar] [CrossRef]
| Characteristics | Before AAP 2019 (n = 1639) | After AAP 2019 (n = 1401) | p † |
|---|---|---|---|
| GA, weeks, mean ± SD | 38.3 ± 1.2 | 38.2 ± 1.2 | 0.109 |
| Mode of delivery, n (%) | 0.904 | ||
| Vaginal delivery | 812 (49.5) | 691 (49.3) | |
| Cesarean section | 827 (50.5) | 710 (50.7) | |
| Male, n (%) | 817 (49.8) | 740 (52.8) | 0.102 |
| Birth body weight, grams, mean ± SD | 3072.1 ± 413.9 | 3080.5 ± 418.9 | 0.577 |
| Maternal risk factors, n (%) | |||
| Preterm labor pain/ROM | 68 (4.1) | 72 (5.1) | 0.194 |
| Prolong ROM | 21 (1.3) | 10 (0.7) | 0.121 |
| Suspected Triple I/Chorioamnionitis | 2 (0.1) | 2 (0.1) | 0.875 |
| Previous infant with invasive GBS | 0 (0) | 1 (0.1) | 0.279 |
| Maternal UTI | 0 (0) | 1 (0.1) | 0.279 |
| GBS in vaginal swab culture | 0.210 | ||
| Negative | 18 (1) | 23 (1.6) | |
| Unknown | 1621 (99) | 1378 (98.4) | |
| Culture-proven EOS, n (%) | 1 (0.1) | 1 (0.1) | 0.912 |
| Clinical Manifestations | Before AAP 2019 (n = 291) | After AAP 2019 (n = 210) | p † |
|---|---|---|---|
| Respiratory distress, n (%) | 259 (15.8) | 191 (13.6) | 0.093 |
| Feeding intolerance, n (%) | 13 (0.8) | 9 (0.6) | 0.625 |
| Hypotension, n (%) | 11 (0.7) | 10 (0.7) | 0.887 |
| Temperature instability, n (%) | 7 (0.4) | 2 (0.1) | 0.150 |
| Birth asphyxia, n (%) | 2 (0.1) | 0 (0) | 0.191 |
| Outcomes | Before AAP 2019 (n = 1639) | After AAP 2019 (n = 1401) | p † | Odds Ratios * (95% CI) |
|---|---|---|---|---|
| Investigation, n (%) | 41 (2.5) | 1 (0.1) | <0.001 | 35.92 (4.93 to 261.47) |
| Investigation and Antibiotic use, n (%) | 181 (11) | 110 (7.9) | <0.001 | 1.46 (1.14 to 1.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aeimcharnbanchong, K.; Jangmeonwai, P. Clinical Outcomes of the Adapted AAP 2019 Guidelines on Early Onset Sepsis in Thailand. Antibiotics 2025, 14, 1048. https://doi.org/10.3390/antibiotics14101048
Aeimcharnbanchong K, Jangmeonwai P. Clinical Outcomes of the Adapted AAP 2019 Guidelines on Early Onset Sepsis in Thailand. Antibiotics. 2025; 14(10):1048. https://doi.org/10.3390/antibiotics14101048
Chicago/Turabian StyleAeimcharnbanchong, Kanokwan, and Patraporn Jangmeonwai. 2025. "Clinical Outcomes of the Adapted AAP 2019 Guidelines on Early Onset Sepsis in Thailand" Antibiotics 14, no. 10: 1048. https://doi.org/10.3390/antibiotics14101048
APA StyleAeimcharnbanchong, K., & Jangmeonwai, P. (2025). Clinical Outcomes of the Adapted AAP 2019 Guidelines on Early Onset Sepsis in Thailand. Antibiotics, 14(10), 1048. https://doi.org/10.3390/antibiotics14101048






