Antimicrobial Resistance in the Era of Climate Change: Why We Should All Embrace and Integrate the One Health Approach in Clinical Practice?
Abstract
1. Introduction
2. Antimicrobial Resistance
2.1. How Does AMR Develop?
2.2. The Role of the Resistome and Biofilm Formation
2.3. Drivers of AMR
2.3.1. Healthcare-Associated Factors and AMR
2.3.2. Animal Farming, Food Production, and AMR
2.3.3. Economical Components as Drivers of Antimicrobial Resistance: Is Geographical Distribution Implicated in Antimicrobial Resistance?
3. Climate Change as a Driver of Antimicrobial Resistance: Data and Trends
4. Challenges and Future Perspectives in Clinical Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- WHO. Ten Health Issues WHO Will Tackle This Year. 2023. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 31 August 2025).
- O’Neill, J. Review on Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. London: Review on Antimicrobial Resistance. 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 1 June 2025).
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 40, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Sandu, A.M.; Chifiriuc, M.C.; Vrancianu, C.O.; Cristian, R.E.; Alistar, C.F.; Constantin, M.; Paun, M.; Alistar, A.; Popa, L.G.; Popa, M.I. Healthcare-Associated Infections: The Role of Microbial and Environmental Factors in Infection Control-A Narrative Review. Infect. Dis. Ther. 2025, 14, 933–971. [Google Scholar] [CrossRef]
- Kutlu, L.; Wang, R. Greenhouse Gas Emission Inefficiency Spillover Effects in European Countries. Int. J. Environ. Res. Public Health 2021, 18, 4479. [Google Scholar] [CrossRef]
- Singaravadivelan, A.; Sachin, P.B.; Harikumar, S.; Vijayakumar, P.; Vindhya, M.V.; Farhana, F.M.B.; Rameesa, K.K.; Mathew, J. Life cycle assessment of greenhouse gas emission from the dairy production system -review. Trop. Anim. Health Prod. 2023, 55, 320. [Google Scholar] [CrossRef]
- Khorram-Manesh, A.; Gray, L. Global health and human well-being—A systematic review. AIMS Public Health 2025, 12, 310–328. [Google Scholar] [CrossRef]
- Kirwan, M.; Egan, E.; Matthews, A. Missed Nursing Care Infection Prevention and Control Practices in Acute Hospitals in Ireland During the COVID-19 Pandemic. Int. J. Nurs. Pract. 2025, 31, e70062. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Vitale, M. Antibiotic Resistance: Do We Need Only Cutting-Edge Methods, or Can New Visions Such as One Health Be More Useful for Learning from Nature? Antibiotics 2023, 12, 1694. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-Tincu, A.L.; Gligor, F.G.; Mureșan, M.L.; Dobrea, C.M. Microbial Resistance to Antibiotics and Effective Antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Sakalauskienė, G.V.; Radzevičienė, A. Antimicrobial Resistance: What Lies Beneath This Complex Phenomenon? Diagnostics 2024, 14, 2319. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial Resistance and Its Drivers—A Review. Antibiotics 2022, 11, 1362. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Wang, P.; Asare, E.; Pitzer, V.E.; Dubrow, R.; Chen, K. Associations between long-term drought and diarrhea among children under five in low- and middle-income countries. Nat. Commun. 2022, 13, 3661. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Ye, Z.; Li, M.; Jing, Y.; Liu, K.; Wu, Y.; Peng, Z. What Are the Drivers Triggering Antimicrobial Resistance Emergence and Spread? Outlook from a One Health Perspective. Antibiotics 2025, 14, 543. [Google Scholar] [CrossRef]
- Na, S.; Intanon, M.; Srithanasuwan, A.; Chaisri, W.; Suriyasathaporn, W. Evidence of vancomycin-resistant Staphylococcus aureus, multidrug-resistant S. aureus, and Enterococcus faecium-causing mastitis in Thailand and Cambodia. Vet. World. 2025, 18, 202–209. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Pascoe, M. Disinfectants and antiseptics: Mechanisms of action and resistance. Nat. Rev. Microbiol. 2024, 22, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Machado, I.; Simões, L.C.; Simões, M. Biocides as drivers of antibiotic resistance: A critical review of environmental implications and public health risks. Environ. Sci. Ecotechnol. 2025, 25, 100557. [Google Scholar] [CrossRef]
- van Dijk, H.F.G.; Verbrugh, H.A.; Ad Hoc Advisory Committee on Disinfectants of the Health Council of The Netherlands. Resisting disinfectants. Commun. Med. 2022, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Mao, D.; Wang, X.; Luo, Y. NaClO Co-selects antibiotic and disinfectant resistance in Klebsiella pneumonia: Implications for the potential risk of extensive disinfectant use during COVID-19 pandemic. J. Hazard. Mater. 2024, 470, 134102. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qi, S.; Wang, W.; Lu, H. Synergistic effects of quaternary ammonium compounds and antibiotics on the evolution of antibiotic resistance. Water Res. 2025, 275, 123206. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, L.; Liu, Z.; Han, J.; Zhao, Y.; Jin, Y.; Sheng, Y.; Zhu, L.; Hu, B. Excessive disinfection aggravated the environmental prevalence of antimicrobial resistance during COVID-19 pandemic. Sci. Total Environ. 2023, 882, 163598. [Google Scholar] [CrossRef]
- Gharbi, M.; Moore, L.S.P.; Castro-Sánchez, E.; Spanoudaki, E.; Grady, C.; Holmes, A.H.; Drumright, L.N. A needs assessment study for optimising prescribing practice in secondary care junior doctors: The Antibiotic Prescribing Education among Doctors (APED). BMC Infect. Dis. 2016, 16, 456. [Google Scholar] [CrossRef]
- Pandolfo, A.M.; Horne, R.; Jani, Y.; Reader, T.W.; Bidad, N.; Brealey, D.; Enne, V.I.; Livermore, D.M.; Gant, V.; Brett, S.J. Understanding decisions about antibiotic prescribing in ICU: An application of the Necessity Concerns Framework. BMJ Qual. Saf. 2022, 31, 199–210. [Google Scholar] [CrossRef]
- O’Reilly, D.; Livada, A.; Steiner, L.; Drew, R.J.; Mc Callion, N. Beyond the incubator: Applying a “one health” approach in the NICU. Pediatr. Res. 2024, 96, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Al Sawafi, F.; Aldwaha, H.; Almusalhi, H.; Alnairi, K.; Alshukairi, S.; AlHabsi, R.; Alrashdi, B.; Manhas, Y.; Hamadalnil, Y.; Nair, A. Insights Into Pathogen Diversity and Antimicrobial Resistance Profiles in the Intensive Care Units of a Regional Hospital in Oman. Cureus 2025, 17, e81014. [Google Scholar] [CrossRef] [PubMed]
- McGill, E.; Neitzel, A.; Bartoszko, J.J.; Buchanan-Chell, M.; Grant, J.; Leal, J.; Smith, S.; Titoria, R.; Varsaneux, O.; Frenette, C. Antimicrobial stewardship programs in a network of Canadian acute care hospitals: A cross-sectional survey. Antimicrob. Steward. Healthc. Epidemiol. 2025, 5, e122. [Google Scholar] [CrossRef]
- Hersh, A.L.; King, L.M.; Shapiro, D.J.; Hicks, L.A.; Fleming-Dutra, K.E. Unnecessary Antibiotic Prescribing in US Ambulatory Care Settings, 2010–2015. Clin. Infect. Dis. 2021, 72, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions—United States. 2022. Available online: https://www.cdc.gov/antibiotic-use/data/report-2022.html (accessed on 5 September 2025).
- Obolensky, L.; Wambugu, E.; Kubai, E.K.; Doig, I.; Beattie, M.; Dillon, M.J. Antibiotic use in rural Kenyan livestock: Navigating misuse, experience gaps and AMR risks. Microbiology 2025, 17, 001582. [Google Scholar] [CrossRef]
- Zhang, T.; Nickerson, R.; Zhang, W.; Peng, X.; Shang, Y.; Zhou, Y.; Luo, Q.; Wen, G.; Cheng, Z. The impacts of animal agriculture on One Health-Bacterial zoonosis, antimicrobial resistance, and beyond. One Health 2024, 18, 100748. [Google Scholar] [CrossRef]
- Ma, Z.; Lee, S.; Jeong, K.C. Mitigating antibiotic resistance at the livestock environment interface: A review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations, Structural Data From Agricultural Censuses. 2023. Available online: https://www.fao.org/faostat/en/#data/WCAD/visualize (accessed on 6 September 2025).
- Wen, R.; Li, C.; Zhao, M.; Wang, H.; Tang, Y. Withdrawal of antibiotic growth promoters in China and its impact on the foodborne pathogen Campylobacter coli of swine origin. Front. Microbiol. 2022, 13, 1004725. [Google Scholar] [CrossRef]
- Liu, J.; Jin, C.; Ghafarifarsani, H. Dietary Supplementation of the Rainbow Trout, Oncorhynchus mykiss by Red Ginseng, Panax Ginseng” Powder: Enhancing Effects on Growth, Immunity and Disease Resistance. Vet. Res. Commun. 2025, 49, 299. [Google Scholar] [CrossRef]
- Thilakarathne, M.; Sridhar, V.; Kline, K. Dissemination of antibiotic resistance in receiving environments under a changing climate: A modeling exercise. J. Environ. Manag. 2025, 392, 126563. [Google Scholar] [CrossRef]
- Li, W.; Huang, T.; Liu, C.; Wushouer, H.; Yang, X.; Wang, R.; Xia, H.; Li, X.; Qiu, S.; Chen, S. Changing climate and socioeconomic factors contribute to global antimicrobial resistance. Nat. Med. 2025, 31, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- van Bavel, B.; Berrang-Ford, L.; Moon, K.; Gudda, F.; Thornton, A.J.; Robinson, R.F.S.; King, R. Intersections between climate change and antimicrobial resistance: A systematic scoping review. Lancet Planet. Health 2024, 8, e1118–e1128. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.; Corley, M.; Wright, N.; Andrewin, A.; Georges, R.; Ramroop, S.; Greenaway, S.; Gent, N.; Astwood, N.; Hardy, W. The United Nations’ General Assembly High-Level Meeting on antimicrobial resistance: A joint statement from the Heads of Government and Chief Medical Officers of the United Kingdom’s Overseas Territories. J. Antimicrob. Chemother. 2024, 79, 2729–2730. [Google Scholar] [CrossRef]
- Abate, M.G.; Wondimagegne, Z.T.; Belachew, T. Optimal complementary feeding practice and associated factors among children in Konso Zone, South Ethiopia. Front. Nutr. 2025, 12, 1568887. [Google Scholar] [CrossRef]
- Nogueira, K.D.S.; Tomaz, A.P.O.; Kubis, G.C.; Marques, R.Z.; Witt, N.G.P.M.; Borba, A.L.B.; Leite, B.Z.; Gomes, M.P. Occurrence and Risk Assessment of Antimicrobials and Resistant Bacteria in Treated Sewage Effluents in South Brazil. Antibiotics 2025, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.; Basher, N.S.; Kirat, H.; Meradji, S.; Ibrahim, N.A.; Idres, T.; Touati, A. The Role of the Environment (Water, Air, Soil) in the Emergence and Dissemination of Antimicrobial Resistance: A One Health Perspective. Antibiotics 2025, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Samhouri, D.; Mahrous, H.; Saidouni, A.; El Kholy, A.; Ghazy, R.M.; Sadek, M.; Kodama, C.; Tayler, E.; Holm, M.; Al Eryani, S.M. Review on progress, challenges, and recommendations for implementing the One Health approach in the Eastern Mediterranean Region. One Health 2025, 20, 101057. [Google Scholar] [CrossRef]
- Sams-Dodd, J.; Sams-Dodd, F. The contribution of antimicrobials and antimicrobial resistance to climate change and a possible way to reverse it whilst still offering high quality healthcare-a conceptual analysis. Front. Public Health 2025, 13, 1644086. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Engelstädter, J.; Zhang, S.; Ding, P.; Mao, L.; Yuan, Z.; Bond, P.L.; Guo, J. Non-antibiotic pharmaceuticals enhance the transmission of exogenous antibiotic resistance genes through bacterial transformation. ISME J. 2020, 14, 2179–2196. [Google Scholar] [CrossRef] [PubMed]
- World Population Prospects 2022: Summary of Results | Population Division. Available online: https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022 (accessed on 5 September 2025).
- Aslam, B.; Asghar, R.; Muzammil, S.; Shafique, M.; Siddique, A.B.; Khurshid, M.; Ijaz, M.; Rasool, M.H.; Chaudhry, T.H.; Aamir, A. AMR and Sustainable Development Goals: At a crossroads. Global Health 2024, 20, 73. [Google Scholar] [CrossRef]
- Diani, E.; Bianco, G.; Gatti, M.; Gibellini, D.; Gaibani, P. Colistin: Lights and Shadows of an Older Antibiotic. Molecules 2024, 29, 2969. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Hmidi, I.; Souguir, M.; Métayer, V.; Drapeau, A.; François, P.; Madec, J.Y.; Haenni, M.; Mansour, W. Characterization of Enterobacterales resistant to extended-spectrum cephalosporins isolated from meat in Tunisia. J. Food Prot. 2025, 88, 100610. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a potential hotspot for antibiotic resistance dissemination by horizontal gene transfer events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Chen, Q.L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.Z. Transfer of antibiotic resistance from manure-amended soils to vegetable microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef]
- Xiong, L.; Sun, Y.; Shi, L.; Yan, H. Characterization of antimicrobial resistance genes and class 1 integrase gene in raw meat and aquatic product, fresh vegetable and fruit, and swine manure in southern China. Food Control. 2019, 104, 240–246. [Google Scholar] [CrossRef]
- Fernández Salgueiro, M.; Cernuda Martínez, J.A.; Gan, R.K.; Arcos González, P. Climate change and antibiotic resistance: A scoping review. Environ. Microbiol. Rep. 2024, 16, e70008. [Google Scholar] [CrossRef]
- Akello, W. Climate Change and Veterinary Medicine: A Call to Action for a Healthier Planet. F1000Research 2024, 13, 1360. [Google Scholar] [CrossRef]
- Yu, H. Climate change unveils hidden microbial dangers. Environ. Sci. Ecotechnol. 2025, 24, 100544. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Sellera, F.P.; Lincopan, N.; Debone, D.; Miraglia, S.G.E.K.; Tavella, R.A. Catastrophic floods and antimicrobial resistance: Interconnected threats with wide-ranging impacts. One Health 2024, 19, 100891. [Google Scholar] [CrossRef] [PubMed]
- Mahieddine, F.C.; Mathieu-Denoncourt, A.; Duperthuy, M. Temperature Influences Antimicrobial Resistance and Virulence of Vibrio parahaemolyticus Clinical Isolates from Quebec, Canada. Pathogens 2025, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- CDC. Cholera and Other Vibrio Illness Surveillance: Annual Summary. 2019. Available online: https://www.cdc.gov/vibrio/php/surveillance/annual-summary-2019.html (accessed on 6 September 2025).
- Imoli, D.; Maingi, J.M.; Mbae, C.; Kavai, S.M.; Wairimu, C.; Mundalo, S.; Odityo, G.; Wairimu, M.; Mekuria, Z.; Gebreyes, W. Genomic characterization of multidrug-resistant extended-spectrum β-lactamase-producing Vibrio cholerae O1 strains from 2022 cholera outbreak in Kenya. J. Antimicrob. Chemother. 2025, 80, 2399–2407. [Google Scholar] [CrossRef]
- El-Liethy, M.A.; Selvarajan, R.; Dakhil, M.A.; Ayukafangha, E.; Marimuthu, P.; Abia, A.L.K. A review of the occurrence, antimicrobial resistance and health implications of Vibrio cholerae in African aquatic milieus, and the analysis of the impact of climate change on cholera outbreaks in Southern Africa. Sci. Total Environ. 2025, 994, 180057. [Google Scholar] [CrossRef]
- Koua, E.L.; Moussana, F.H.; Sodjinou, V.D.; Kambale, F.; Kimenyi, J.P.; Diallo, S.; Okeibunor, J.; Gueye, S.S. Exploring the burden of cholera in the WHO African region: Patterns and trends from 2000 to 2023 cholera outbreak data. BMJ Glob. Health 2025, 10, e016491. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Ma, T.; Lu, W.; Sa, N.; Gong, L.; Wang, X.; Tian, J.; Xiao, Y.; Jiang, L. Genomic Characteristics and Antibiotic Resistance Evolution of Vibrio cholerae O139—Anhui Province, China, 2013–2024. China CDC Wkly. 2025, 7, 1057–1063. [Google Scholar] [CrossRef]
- Thajudeen, J.; Venkatachalam, S.; Vipindas, P.V. Antibiotic resistome in the glacier forelands of polar regions. Appl. Environ. Microbiol. 2025, 91, e0076225. [Google Scholar] [CrossRef]
- Abbas, A.; Barkhouse, A.; Hackenberger, D.; Wright, G.D. Antibiotic resistance: A key microbial survival mechanism that threatens public health. Cell Host Microbe 2024, 32, 837–851. [Google Scholar] [CrossRef] [PubMed]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Kaba, H.E.J.; Kuhlmann, E.; Scheithauer, S. Thinking outside the box: Association of antimicrobial resistance with climate warming in Europe—A 30 country observational study. Int. J. Hyg. Environ. Health 2020, 223, 151–158. [Google Scholar] [CrossRef]
- Bloomfield, L.E.; Coombs, G.W.; Tempone, S.; Armstrong, P.K. Marked increase in community-associated methicillin-resistant Staphylococcus aureus infections, Western Australia, 2004–2018. Epidemiol. Infect. 2020, 148, e153. [Google Scholar] [CrossRef]
- Ramsay, J.P.; Parahitiyawa, N.; Mowlaboccus, S.; Mullally, C.A.; Yee, N.W.T.; Shoby, P.; Colombi, E.; Tan, H.L.; Pearson, J.C.; Coombs, G.W. Genomic characterization of a unique Panton-Valentine leucocidin-positive community-associated methicillin-resistant Staphylococcus aureus lineage increasingly impacting on Australian indigenous communities. Microb. Genom. 2023, 9, 001172. [Google Scholar] [CrossRef]
- Chandipwisa, C.; Uwishema, O.; Debebe, A.; Abdalmotalib, M.M.; Barakat, R.; Oumer, A.; John, M.; Taa, L.; Onyeaka, H. Climate change and the global food chain: A catalyst for emerging infectious diseases? Int. J. Emerg. Med. 2025, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Debellut, F.; Friedrich, A.; Baral, R.; Pecenka, C.; Mugisha, E.; Neuzil, K.M. The cost of typhoid illness in low- and middle-income countries, a scoping review of the literature. PLoS ONE 2024, 19, e0305692. [Google Scholar] [CrossRef]
- Fagunwa, O.E.; Ashiru-Oredope, D.; Gilmore, B.F.; Doherty, S.; Oyama, L.B.; Huws, S.A. Climate change as a challenge for pharmaceutical storage and tackling antimicrobial resistance. Sci. Total Environ. 2024, 956, 177367. [Google Scholar] [CrossRef]
- Ali Khan, E.; Rizwan, M.; Wang, Y.; Munir, F.; Hua, J. Challenges and Future Prospects of Pakistan’s Animal Industry: Economic Potential, Emerging Trends, and Strategic Directions. Vet. Sci. 2025, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Segala, F.V.; Guido, G.; Stroffolini, G.; Masini, L.; Cattaneo, P.; Moro, L.; Motta, L.; Gobbi, F.; Nicastri, E.; Vita, S. Insights into the ecological and climate crisis: Emerging infections threatening human health. Acta Trop. 2025, 262, 107531. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.J.; Jurga, E.; Wajnberg, G.; Shay, J.; Cameron, R.; Barclay, C.; Sehar, A.; Dooley, D.; John, N.; Scott, A. Crossing the streams: Improving data quality and integration across the One Health genomics continuum with data standards and implementation strategies. Can. J. Microbiol. 2025, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, W.A.; Hamer, D.H.; Shioda, K. The potential impact of climate change on medication access and quality deserves far more attention. One Health 2024, 20, 100957. [Google Scholar] [CrossRef]
- Omuse, E.R.; Machekano, H.; Sokame, B.M.; Mutyambai, D.M.; Dubois, T.; Subramanian, S.; Chidawanyika, F. One Health interventions and challenges under rural African smallholder farmer settings: A scoping review. One Health 2025, 20, 100959. [Google Scholar] [CrossRef]
- Bernardin, A.; Masrour, T.; Partridge, B.; Martin, A.J.M.; Kelly, A.; Perez-Acle, T. Proper pandemic preparedness requires an integrated cross-regional effort, the case of the ECLIPSE consortium in America: A narrative review. J. Health Popul. Nutr. 2025, 44, 211. [Google Scholar] [CrossRef]
- Chung, P.Y. One Health strategies in combating antimicrobial resistance: A Southeast Asian perspective. J. Glob. Health 2025, 15, 03025. [Google Scholar] [CrossRef]
- Kou, R.; Li, X.; Wang, H.; Wu, Y.; Zou, D.; Hu, J.; Qiu, S.; Yang, L.; Zhang, X. Panel data analysis of the effect of ambient temperature on antimicrobial resistance in China. Sci. Rep. 2025, 15, 18391. [Google Scholar] [CrossRef]
- Rodríguez Del Río, Á.; Scheu, S.; Rillig, M.C. Soil microbial responses to multiple global change factors as assessed by metagenomics. Nat. Commun. 2025, 16, 5058. [Google Scholar] [CrossRef]
- Sharma, M.; Akhter, M.S.; Roy, S.; Srejon, R. Future Issues in Global Health: Challenges and Conundrums. Int. J. Environ. Res. Public Health 2025, 22, 325. [Google Scholar] [CrossRef]
- Balta, I.; Lemon, J.; Murnane, C.; Pet, I.; Vintila, T.; McCleery, D.; Callaway, T.; Douglas, A.; Stef, L.; Corcionivoschi, N. The One Health aspect of climate events with impact on foodborne pathogens transmission. One Health 2024, 19, 100926. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, Q.; Lei, C.; Lu, T.; Qian, H. Atmospheric antibiotic resistome driven by air pollutants. Sci. Total Environ. 2023, 902, 165942. [Google Scholar] [CrossRef]
- Zhou, Z.; Shuai, X.; Lin, Z.; Yu, X.; Ba, X.; Holmes, M.A.; Xiao, Y.; Gu, B.; Chen, H. Association between particulate matter (PM)(2.5) air pollution and clinical antibiotic resistance: A global analysis. Lancet Planet. Health 2023, 7, e649–e659. [Google Scholar] [CrossRef]
- Xiu, L.; Hu, S. Air pollution might contribute to antimicrobial resistance: A One Health perspective. Sci. One Health 2025, 4, 00118. [Google Scholar] [CrossRef]
- Sparrow, A.; Smith-Torino, M.; Shamamba, S.M.; Chirakarhula, B.; Lwaboshi, M.A.; Benn, C.S.; Chumakov, K. A Risk Management Approach to Global Pandemics of Infectious Disease and Anti-Microbial Resistance. Trop. Med. Infect. Dis. 2024, 9, 280. [Google Scholar] [CrossRef]
- Kato, T.; Yagi, Y.; Maruyama, T.; Hamada, Y. Pharmacokinetics/Pharmacodynamics-Based Repositioning of Cefmetazole and Flomoxef in Extended-Spectrum β-Lactamase-Producing Enterobacterales Treatment: An Injectable Carbapenem-Sparing and Outpatient Strategy. Antibiotics 2025, 14, 737. [Google Scholar] [CrossRef]
- Alqahtani, M.S.M.; Shahin, G.; Abdelalim, I.T.I.; Khalaf, S.M.H. Evaluation of ecological consequences on the global distribution of Staphylococcus aureus Rosenbach 1884 due to climate change, using Maxent modeling. Sci. Rep. 2025, 15, 11457. [Google Scholar] [CrossRef] [PubMed]
- Deza-Cruz, I.; de Menezes, A.; Gardner, B.; Aktan, Í.; Alnajjar, S.; Betson, M.; Cabal Rosel, A.; Caniça, M.; Chambers, M.A.; Tarrant, G.; et al. Mapping the evidence of the effects of environmental factors on the prevalence of antibiotic resistance in the non-built environment. Environ. Int. 2025, 202, 109634. [Google Scholar] [CrossRef]
- Ablakimova, N.; Rachina, S.; Smagulova, G.; Vlasenko, A.; Mussina, A.; Zhylkybekova, A.; Yessenzhulova, A.; Koshmaganbetova, G.K.; Iztleuov, Y. Impact of complex interventions on antibacterial therapy and etiological diagnostics in community-acquired pneumonia: A 12-month pre- and post-intervention study. Front. Pharmacol. 2025, 16, 1627858. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Simos, P.; Sivabalan, P.; Escolà-Vergé, L.; Garnham, K.; Isler, B.; ESGBIES Study Group. An Update on Recent Clinical Trial Data in Bloodstream Infection. Antibiotics 2024, 13, 1035. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, N.A.; Silvester, R.; Cross, G.; Weightman, A.J.; Jones, D.L.; Kasprzyk-Hordern, B. Assessing the risk of antimicrobial resistance and potential environmental harm through national-scale surveillance of antimicrobials in hospital and community wastewater. Environ. Int. 2025, 202, 109606. [Google Scholar] [CrossRef]
- Tetteh, K.K.A. Climate change as a catalyst for antimicrobial resistance. Nat. Med. 2025, 31, 1751–1752. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Tartari, E.; Kilpatrick, C.; Storr, J.; Bellare, N.; Bana, J.; Santos, A.F.; Charnaud, S.; Ross, A.L.; Schwaber, M.J. WHO global research agenda for hand hygiene improvement in health care: A Delphi consensus study. Infect. Control Hosp. Epidemiol. 2025, 46, 449–464. [Google Scholar] [CrossRef]
- Lin, H.; Wang, D.; Wang, Q.; Mao, J.; Yang, L.; Bai, Y.; Qu, J. Epigenetic modifications and metabolic gene mutations drive resistance evolution in response to stimulatory antibiotics. Mol. Syst. Biol. 2025, 21, 294–314. [Google Scholar] [CrossRef]
- Tigges, P.; Greser, A.; Gágyor, I.; Kraft, J.; Maun, A.; Schmiemann, G.; Schwienhorst-Stich, E.M.; Heintze, C.; Schuster, A. Addressing AMR and planetary health in primary care: The potential of general practitioners as change agents. Front. Public Health 2024, 12, 1383423. [Google Scholar] [CrossRef]
- Borgianni, L.; Cardinali, G.; Cassetti, C.; Cavalieri, D.; De Filippo, C.; De Giuseppe, R.; Di Leonardo, R.; Druzhinina, I.S.; Duprex, W.P.; Egamberdieva, D. The power of microbial life for the transformation towards a sustainable planet: Key messages from the 2024 IUMS Congress in Florence, the city of the Renaissance. Microlife 2025, 6, uqaf018. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yao, X.; Hou, Y.; Zhang, D.; Xie, R.; Shi, C.; Shang, Y.; Bi, H.; Song, W.; Hua, L. Global trends of antimicrobial resistance and virulence of Klebsiella pneumoniae from different host sources. Commun. Med. 2025, 5, 383. [Google Scholar] [CrossRef] [PubMed]
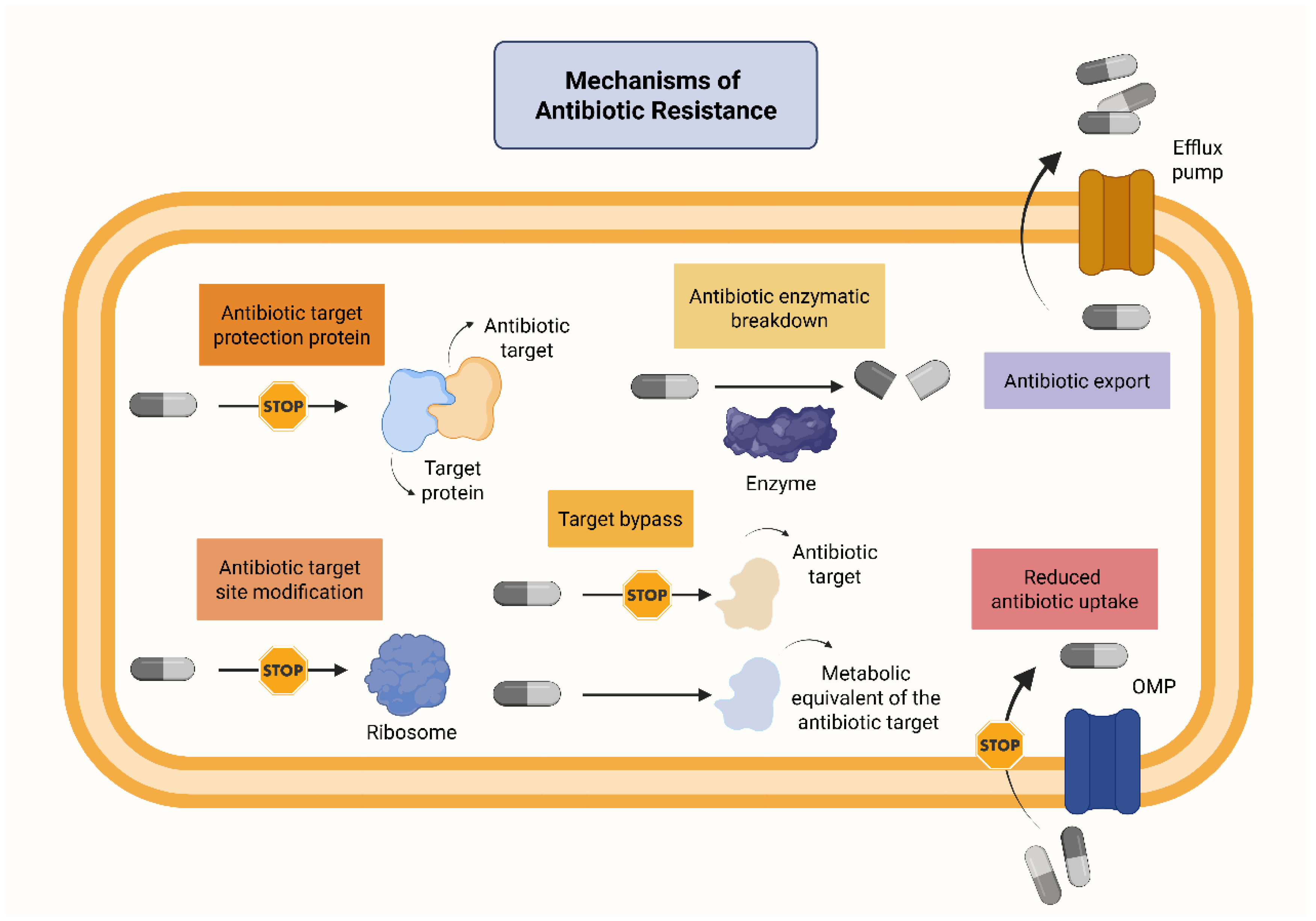
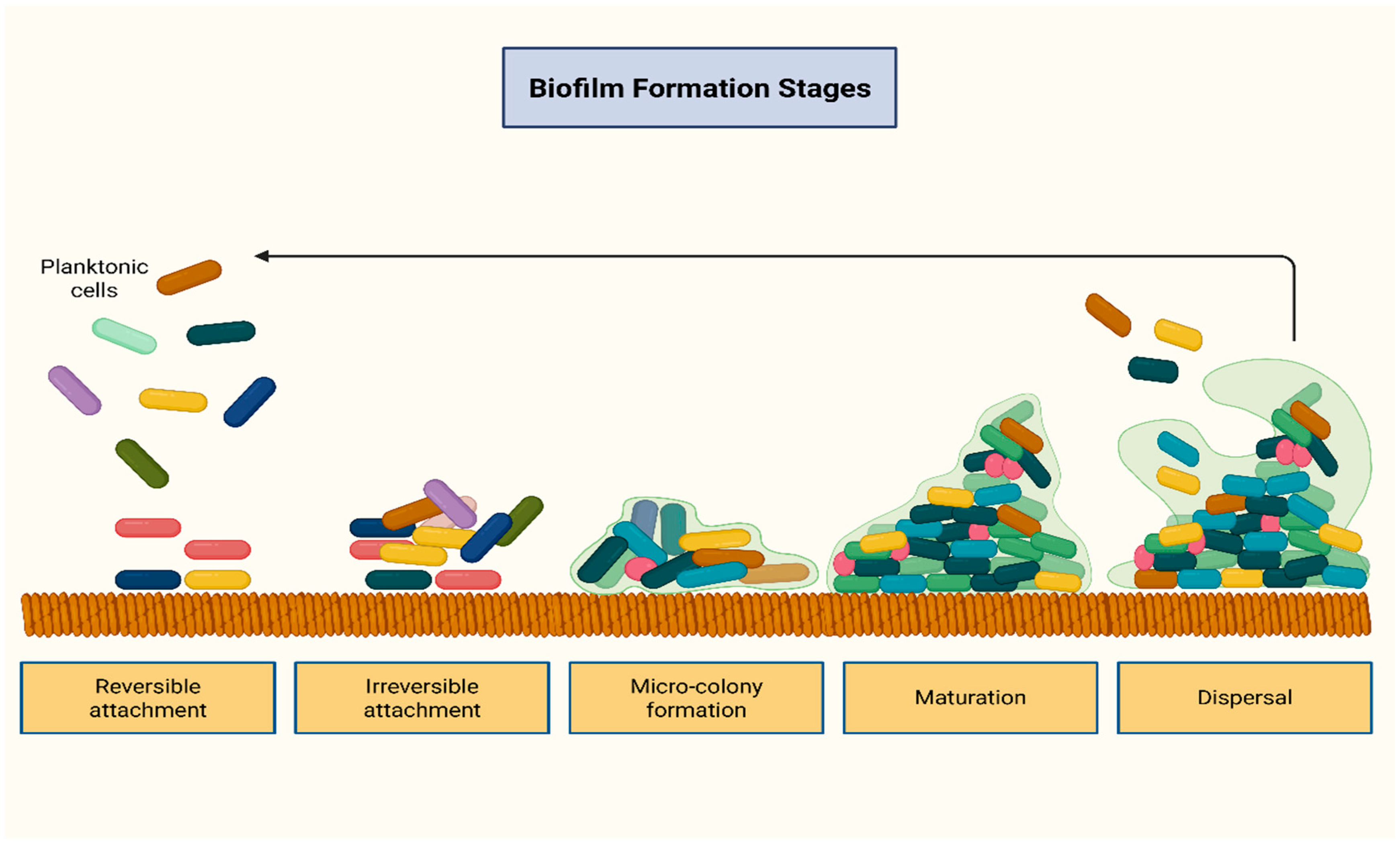
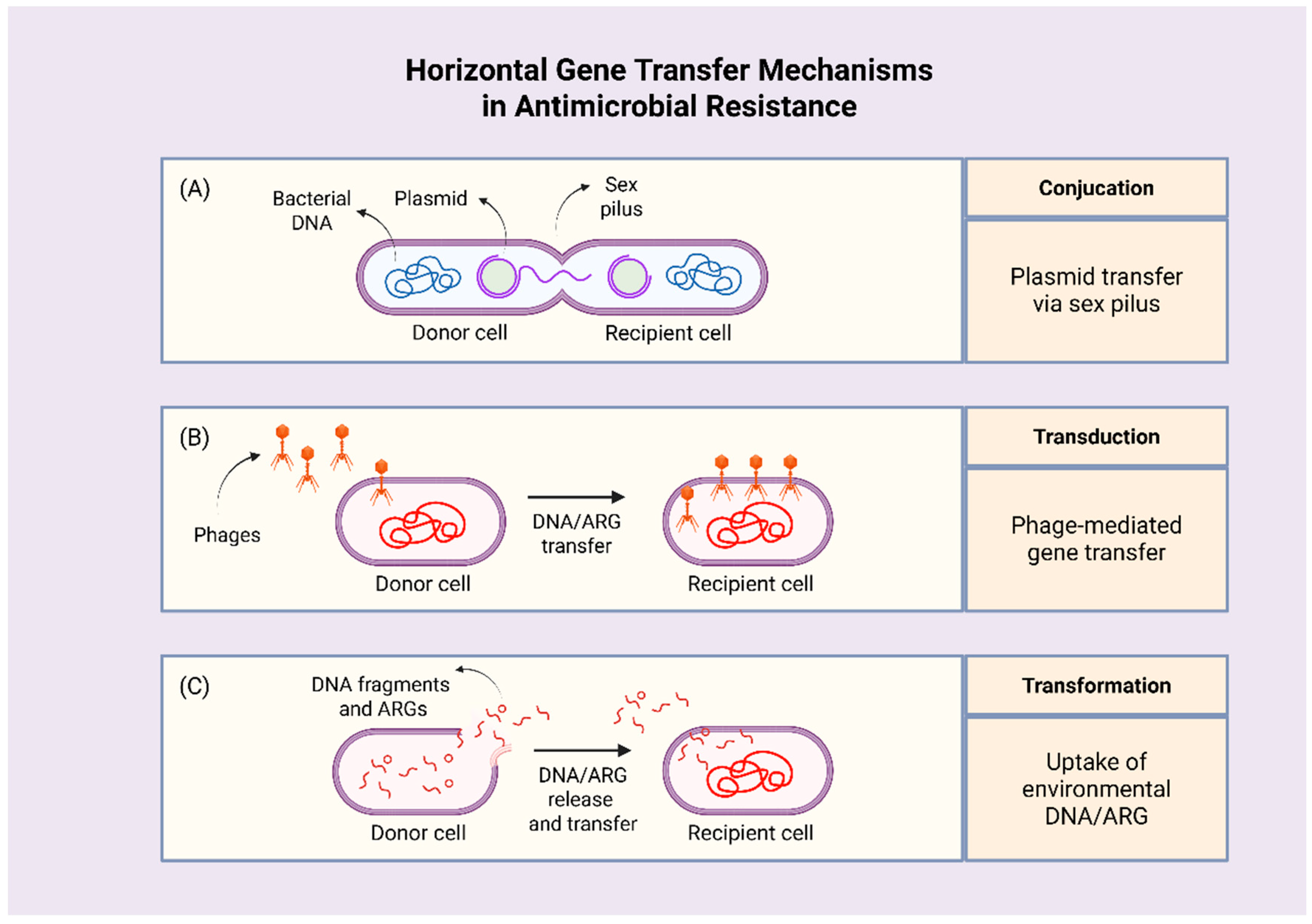


| Drivers of AMR | Setting/Paradigm |
|---|---|
| Inappropriate use of disinfectants [17,18,19,20,21] | Healthcare-associated or in the community |
| Inappropriate use of antiseptics [17,18,19,20,21] | Healthcare-associated or in the community |
| Overuse and misuse of antibiotics in healthcare settings [22,23,24,25,26,27,28] | Hospitals or other healthcare-associated settings (i.e., nursing homes) |
| Aging population [29,30] | More prone to epigenetic modifications and AMR |
| Overuse and misuse of antibiotics in the community [31,32] | By primary care physicians in the community |
| Overuse and misuse of antibiotics for the growth of animals by farmers [33,34,35] | Animal sector |
| Misuse of antibiotics in agriculture [36] | Plant workers |
| Lack of Legislation [37,38,39,40] | Policies, such as those regarding farming, growing crops and antibiotics surveillance |
| Socioeconomic factors [41] | Poor sanitation in low-income countries |
| Inappropriate handling of food [42,43] | Food supplies in low-income countries |
| Inappropriate handling of sewages [44,45,46] | From hospitals, pharmaceutical companies, industries |
| Climate change [18,46,47,48,49] | Increasing temperatures, humidity, heavy rainfalls, flooding, droughts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounatidis, D.C.; Evangelopoulos, A.; Geladari, E.V.; Evangelopoulos, A.A.; Adamou, A.; Kargioti, S.; Geladari, C.V.; Dalamaga, M.; Sevastianos, V.; Vallianou, N.G. Antimicrobial Resistance in the Era of Climate Change: Why We Should All Embrace and Integrate the One Health Approach in Clinical Practice? Antibiotics 2025, 14, 1042. https://doi.org/10.3390/antibiotics14101042
Kounatidis DC, Evangelopoulos A, Geladari EV, Evangelopoulos AA, Adamou A, Kargioti S, Geladari CV, Dalamaga M, Sevastianos V, Vallianou NG. Antimicrobial Resistance in the Era of Climate Change: Why We Should All Embrace and Integrate the One Health Approach in Clinical Practice? Antibiotics. 2025; 14(10):1042. https://doi.org/10.3390/antibiotics14101042
Chicago/Turabian StyleKounatidis, Dimitris C., Apostolos Evangelopoulos, Eleni V. Geladari, Angelos A. Evangelopoulos, Andreas Adamou, Sofia Kargioti, Charalampia V. Geladari, Maria Dalamaga, Vasileios Sevastianos, and Natalia G. Vallianou. 2025. "Antimicrobial Resistance in the Era of Climate Change: Why We Should All Embrace and Integrate the One Health Approach in Clinical Practice?" Antibiotics 14, no. 10: 1042. https://doi.org/10.3390/antibiotics14101042
APA StyleKounatidis, D. C., Evangelopoulos, A., Geladari, E. V., Evangelopoulos, A. A., Adamou, A., Kargioti, S., Geladari, C. V., Dalamaga, M., Sevastianos, V., & Vallianou, N. G. (2025). Antimicrobial Resistance in the Era of Climate Change: Why We Should All Embrace and Integrate the One Health Approach in Clinical Practice? Antibiotics, 14(10), 1042. https://doi.org/10.3390/antibiotics14101042









