The Effect of Chlorhexidine Mouthwashes on the Microbiota Associated with Peri-Implantitis Lesions: A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Subject Recruitment and Characteristics
2.2. Effect of CHX Treatment on Microbial Diversity
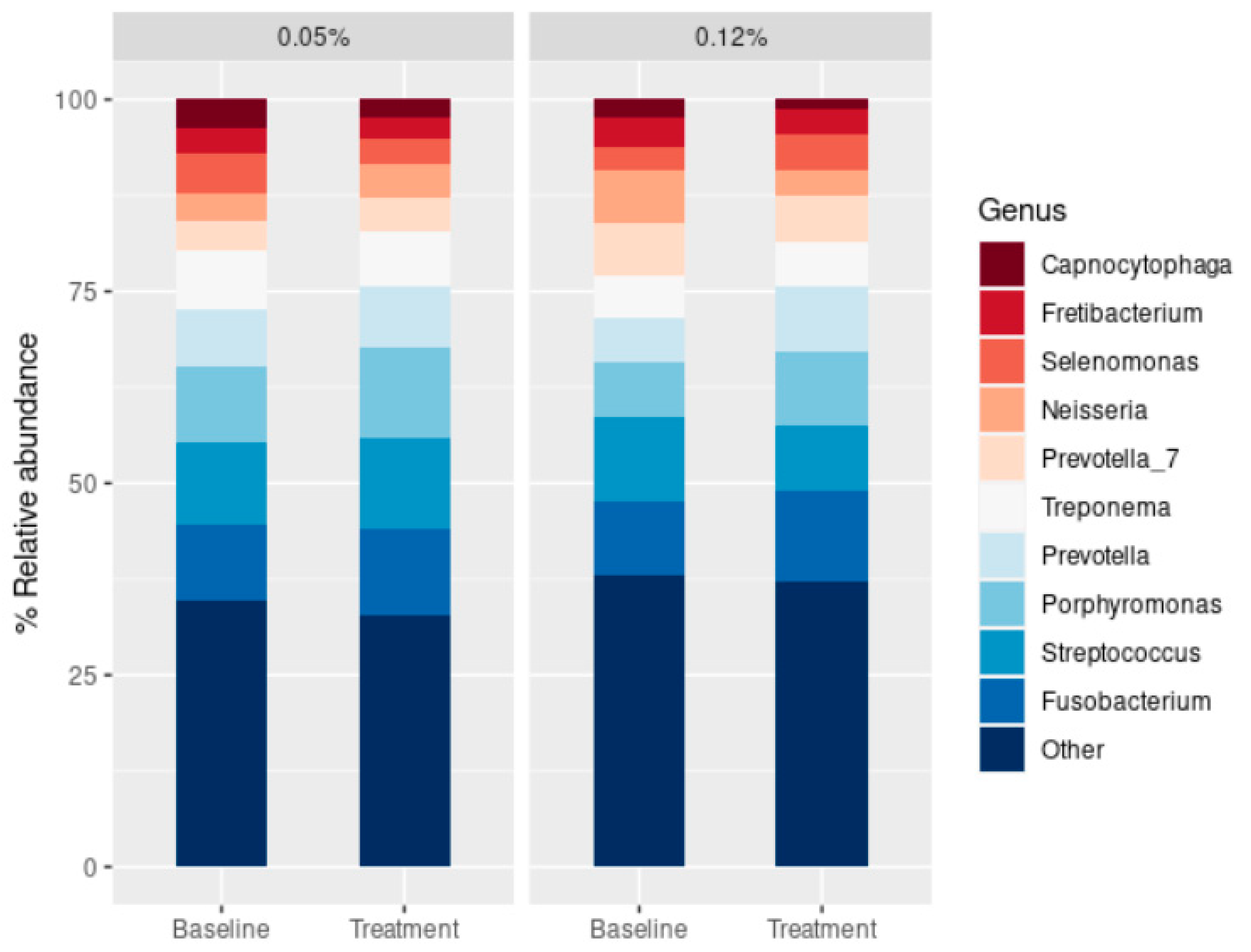
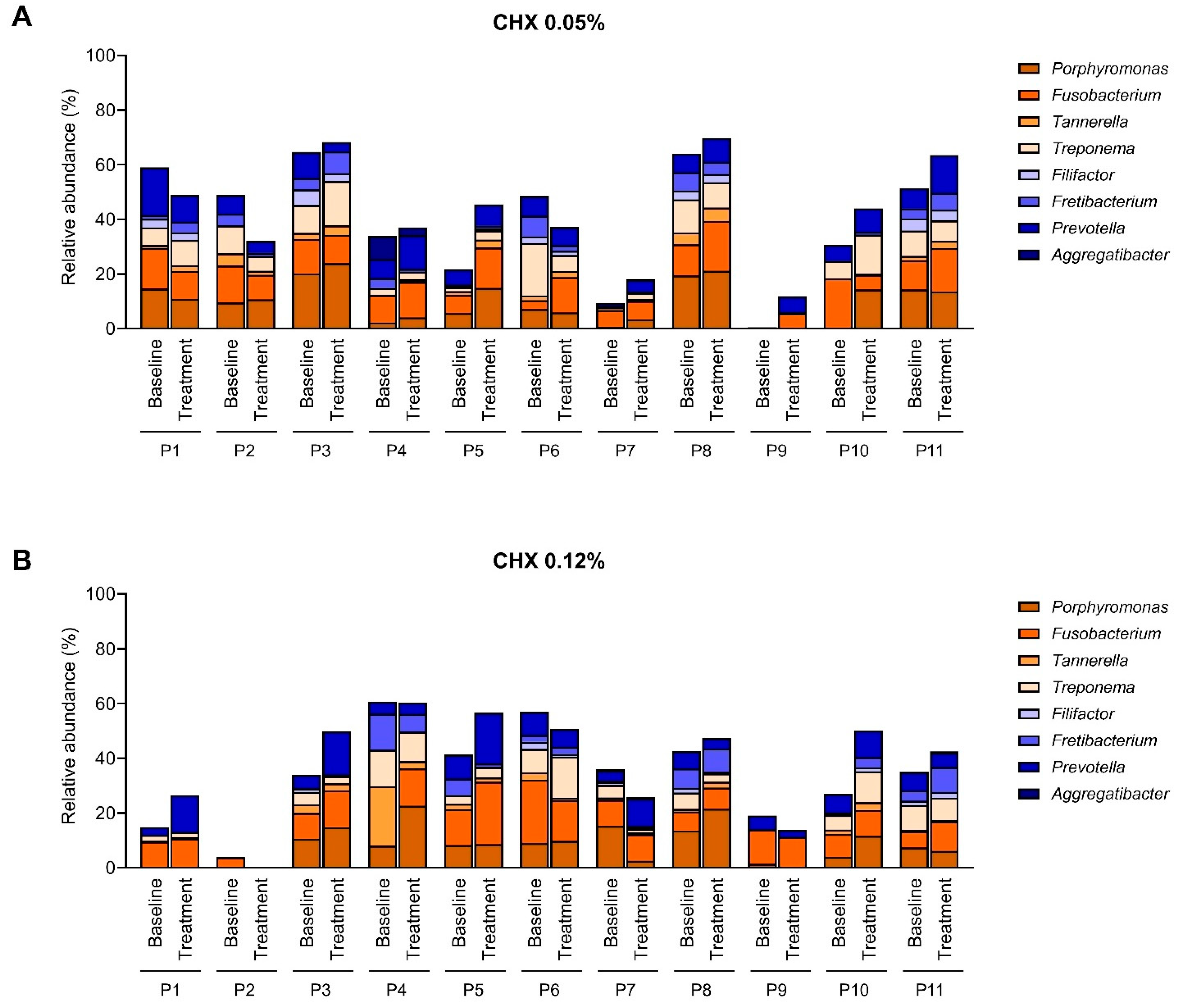
2.3. Effect of the CHX Treatment on the Abundance of Main Periodontopathogens
3. Discussion
4. Study Limitations
- Only 22 patients were included, equally divided between two treatment groups. This limited cohort reduces statistical power and makes it difficult to detect subtle but potentially relevant differences in microbiota composition. Moreover, the microbial response to CHX was strongly patient-dependent, with marked differences in baseline microbiota composition and treatment outcomes. This variability complicates the interpretation of group-level effects. Larger-scale studies are necessary to confirm these preliminary findings.
- The intervention lasted only 15 days; therefore, the results cannot be extrapolated to the longer durations of treatment often prescribed in clinical practice. While long-term treatment may have other undesirable effects, short-term exposure may underestimate both beneficial and adverse effects on peri-implant microbiota.
- The commercial mouthwashes used contained not only CHX but also cetylpyridinium chloride. The combined effect of these agents cannot be disentangled, limiting the ability to attribute observed changes specifically to CHX.
- The limited or absent microbiological effect observed probably reflects the restricted penetration of mouthwashes into deep peri-implant pockets. This issue should be considered when evaluating antiseptic rinses as treatment modalities.
- Factors such as smoking habits, systemic conditions (e.g., epilepsy in one participant), and oral hygiene practices may have influenced microbiota composition, but their impact could not be fully assessed due to the small cohort size.
5. Materials and Methods
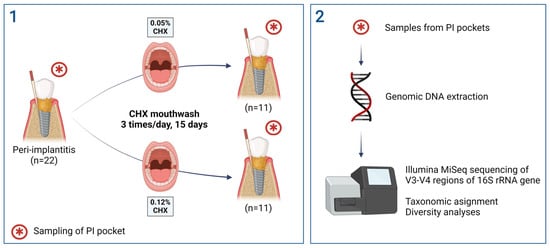
5.1. Subject Recruitment
5.2. Sample Collection and Preparation
5.3. Microbial DNA Extraction
5.4. Library Preparation
5.5. Bioinformatics and Microbial Diversity Analysis
6. Conclusions
- Even though the study of a larger number of patients is required in order to withdraw solid conclusions, this pilot study indicates that short-time, intensive prescription of CHX mouthwashes should be recommended with caution for the treatment of PI, since it does not have any statistically significant effect on the lesion-associated microbiota, with a large variability in the patients’ response, and may have undesirable side effects.
- The lowest CHX concentration (0.05%) should be considered for combined or long-term therapies, since no statistically significant differences were observed in comparison with 0.12% CHX.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamed, M.T.; Mously, H.A. Investigating Economic and Clinical Implications of Tooth Implant Supported Prosthesis among Patients and Practitioners. Int. J. Pharm. Res. Allied Sci. 2019, 8, 116–121. [Google Scholar] [CrossRef]
- Kochar, S.P.; Reche, A.; Paul, P. The Etiology and Management of Dental Implant Failure: A Review. Cureus 2022, 14, e30455. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S246–S266. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef]
- Elemek, E.; Agrali, O.B.; Kuru, B.; Kuru, L. Peri-implantitis and Severity Level. Eur. J. Dent. 2020, 14, 24–30. [Google Scholar] [CrossRef][Green Version]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar][Green Version]
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral microbiome and peri-implant diseases: Where are we now? Ther. Clin. Risk Manag. 2017, 13, 1529–1542. [Google Scholar] [CrossRef]
- Chun Giok, K.; Menon, R.K. The Microbiome of Peri-Implantitis: A Systematic Review of Next-Generation Sequencing Studies. Antibiotics 2023, 12, 1610. [Google Scholar]
- Di Spirito, F.; Giordano, F.; Di Palo, M.P.; D’Ambrosio, F.; Scognamiglio, B.; Sangiovanni, G.; Caggiano, M.; Gasparro, R. Microbiota of Peri-Implant Healthy Tissues, Peri-Implant Mucositis, and Peri-Implantitis: A Comprehensive Review. Microorganisms 2024, 12, 1137. [Google Scholar] [CrossRef]
- Leonhardt, A.; Renvert, S.; Dahlen, G. Microbial findings at failing implants. Clin. Oral Implant. Res. 1999, 10, 339–345. [Google Scholar] [CrossRef]
- Mombelli, A.; Decaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38 (Suppl. S11), 203–213. [Google Scholar] [CrossRef]
- Maruyama, N.; Maruyama, F.; Takeuchi, Y.; Aikawa, C.; Izumi, Y.; Nakagawa, I. Intraindividual variation in core microbiota in peri-implantitis and periodontitis. Sci. Rep. 2014, 4, 6602. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, S.; Staufenbiel, I.; Scherer, R.; Schilhabel, M.; Winkel, A.; Stumpp, S.N.; Eberhard, J.; Stiesch, M. Pyrosequencing of supra- and subgingival biofilms from inflamed peri-implant and periodontal sites. BMC Oral Health 2014, 14, 157. [Google Scholar] [CrossRef]
- Yu, X.-L.; Chan, Y.; Zhuang, L.; Lai, H.C.; Lang, N.P.; Keung Leung, W.; Watt, R.M. Intra-oral single-site comparisons of periodontal and peri-implant microbiota in health and disease. Clin. Oral Implant. Res. 2019, 30, 760–776. [Google Scholar]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162 Pt A, 22–38. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar]
- Manoil, D.; Belibasakis, G.N. Microbial Principles of Peri-Implant Infections. In Dental Implants and Oral Microbiome Dysbiosis; Neelakantan, P., Princy Solomon, A., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Liñares, A.; Sanz-Sánchez, I.; Dopico, J.; Molina, A.; Blanco, J.; Montero, E. Efficacy of adjunctive measures in the non-surgical treatment of peri-implantitis: A systematic review. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 224–243. [Google Scholar]
- Gunsolley, J.C. Clinical efficacy of antimicrobial mouthrinses. J. Dent. 2010, 38 (Suppl. S1), S6–S10. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Romanos, G.; Kang, P.; Travan, S.; Schmidt, S.; Papathanasiou, E.; Tatarakis, N.; Tandlich, M.; Liberman, L.H.; Horwitz, J.; et al. Repeated delivery of chlorhexidine chips for the treatment of peri-implantitis: A multicenter, randomized, comparative clinical trial. J. Periodontol. 2021, 92, 11–20. [Google Scholar]
- Gennai, S.; Bollain, J.; Ambrosio, N.; Marruganti, C.; Graziani, F.; Figuero, E. Efficacy of adjunctive measures in peri-implant mucositis. A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 161–187. [Google Scholar] [CrossRef]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and treatment of peri-implant diseases-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 4–76. [Google Scholar] [CrossRef]
- Dumitriu, A.S.; Păunică, S.; Nicolae, X.A.; Bodnar, D.C.; Albu, Ș.D.; Suciu, I.; Ciongaru, D.N.; Giurgiu, M.C. The Effectiveness of the Association of Chlorhexidine with Mechanical Treatment of Peri-Implant Mucositis. Healthcare 2023, 11, 1918. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Santagati, M.; Alibrandi, A.; Iorio-Siciliano, V.; Ramaglia, L. Effect of Nonsurgical Mechanical Debridement With or Without Chlorhexidine Formulations in the Treatment of Peri-Implant Mucositis. A Randomized Placebo-Controlled Clinical Trial. Clin. Oral Implant. Res. 2025, 36, 566–577. [Google Scholar] [CrossRef]
- Liu, S.; Limiñana-Cañal, J.; Yu, J. Does chlorhexidine improve outcomes in non-surgical management of peri-implant mucositis or peri-implantitis?: A systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e608–e615. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, A.; Perussolo, J.; Ercal, P.; Gkranias, N.; Donos, N. The Effect of Non-Surgical Periodontal Therapy on Subgingival Microbiota: A Systematic Review and Meta-Analysis. J. Periodontal. Res. 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Verspecht, T.; Rodriguez Herrero, E.; Khodaparast, L.; Khodaparast, L.; Boon, N.; Bernaerts, K.; Quirynen, M.; Teughels, W. Development of antiseptic adaptation and cross-adapatation in selected oral pathogens in vitro. Sci. Rep. 2019, 9, 8326. [Google Scholar] [CrossRef]
- Palka, L.; Nowakowska-Toporowska, A.; Dalewski, B. Is Chlorhexidine in Dentistry an Ally or a Foe? A Narrative Review. Healthcare 2022, 10, 764. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Narayana, A.; Balkrishanan, D. Chlorhexidine for the Treatment of Peri-Implantitis: Is it a Benison? J. Long. Term. Eff. Med. Implant. 2022, 32, 19–23. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Bazo, A.P.; da Silva Franchi, C.A.; Marques, M.E.; Salvadori, D.M. Chlorhexidine induces DNA damage in rat peripheral leukocytes and oral mucosal cells. J. Periodontal Res. 2004, 39, 358–361. [Google Scholar] [CrossRef]
- Heasman, P.A.; Seymour, R.A. Pharmacological control of periodontal diseaseI. Antiplaque agent. J. Dent. 1994, 22, 323–335. [Google Scholar] [CrossRef]
- Calsina-Gomis, G.; Serrano-Granger, J. ¿Existen realmente diferencias clínicas entre las distintas concentraciones de clorhexidina?: Comparación de colutorios. RCOE 2005, 10, 457–464. [Google Scholar] [CrossRef]
- Haydari, M.; Bardakci, A.G.; Koldsland, O.C.; Aass, A.M.; Sandvik, L.; Preus, H.R. Comparing the effect of 0.06–0.12% and 0.2% Chlorhexidine on plaque, bleeding and side effects in an experimental gingivitis model: A parallel group, double masked randomized clinical trial. BMC Oral Health 2017, 17, 118. [Google Scholar] [CrossRef]
- Guggenheim, B.; Giertsen, E.; Schüpbach, P.; Shapiro, S. Validation of an in vitro biofilm model of supragingival plaque. J. Dent. Res. 2001, 80, 363–370. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Llama-Palacios, A.; Blanc, V.; León, R.; Herrera, D.; Sanz, M. Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J. Periodontal Res. 2011, 46, 252–260. [Google Scholar] [CrossRef]
- Kommerein, N.; Stumpp, S.N.; Müsken, M.; Ehlert, N.; Winkel, A.; Häussler, S.; Behrens, P.; Buettner, F.F.R.; Stiesch, M. An oral multispecies biofilm model for high content screening applications. PLoS ONE 2017, 12, e0173973. [Google Scholar] [CrossRef]
- Zayed, N.; Boon, N.; Bernaerts, K.; Chatzigiannidou, I.; Van Holm, W.; Verspecht, T.; Teughels, W. Differences in chlorhexidine mouthrinses formulations influence the quantitative and qualitative changes in in-vitro oral biofilms. J. Periodont. Res. 2022, 57, 52–62. [Google Scholar] [CrossRef]
- Fernandez y Mostajo, M.; Exterkate, R.A.M.; Buijs, M.J.; Crielaard, W.; Zaura, E. Effect of mouthwashes on the composition and metabolic activity of oral biofilms grown in vitro. Clin. Oral Investig. 2017, 21, 1221–1230. [Google Scholar] [CrossRef]
- Chatzigiannidou, I.; Teughels, W.; Van de Wiele, T.; Boon, N. Oral biofilms exposure to chlorhexidine results in altered microbial composition and metabolic profile. NPJ Biofilms Microbiomes 2020, 6, 13. [Google Scholar] [CrossRef]
- Al-Kamel, A.; Baraniya, D.; Al-Hajj, W.A.; Halboub, E.; Abdulrab, S.; Chen, T.; Al-Hebshi, N.N. Subgingival microbiome of experimental gingivitis: Shifts associated with the use of chlorhexidine and N. -acetyl cysteine mouthwashes. J. Oral Microbiol. 2019, 11, 1608141. [Google Scholar] [CrossRef]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef]
- Tribble, G.D.; Angelov, N.; Weltman, R.; Wang, B.-Y.; Eswaran, S.V.; Gay, I.C.; Parthasarathy, K.; Dao, D.-H.V.; Richardson, K.N.; Ismail, N.M.; et al. Frequency of Tongue Cleaning Impacts the Human Tongue Microbiome Composition and Enterosalivary Circulation of Nitrate. Front. Cell. Infect. Microbiol. 2019, 9, 39. [Google Scholar] [CrossRef]
- Philip, J.; Buijs, M.J.; Pappalardo, V.Y.; Crielaard, W.; Brandt, B.W.; Zaura, E. The microbiome of dental and peri-implant subgingival plaque during peri-implant mucositis therapy: A randomized clinical trial. J. Clin. Periodontol. 2022, 49, 28–38. [Google Scholar] [CrossRef]
- do Amaral, G.C.L.S.; Hassan, M.A.; Sloniak, M.C.; Pannuti, C.M.; Romito, G.A.; VillarCC. Effects of antimicrobial mouthwashes on the human oral microbiome: Systematic review of controlled clinical trials. Int. J. Dent. Hyg. 2023, 21, 128–140. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- He, X.; Hu, W.; He, J.; Guo, L.; Lux, R.; Shi, W. Community-based interference against integration of Pseudomonas aeruginosa into human salivary microbial biofilm. Mol. Oral Microbiol. 2011, 26, 337–352. [Google Scholar] [CrossRef]
- Goh, C.E.; Bohn, B.; Marotz, C.; Molinsky, R.; Roy, S.; Paster, B.J.; Chen, C.; Rosenbaum, M.; Yuzefpolskaya, M.; Colombo, P.C.; et al. Nitrite Generating and Depleting Capacity of the Oral Microbiome and Cardiometabolic Risk: Results from ORIGINS. J. Am. Heart Assoc. 2022, 11, e023038. [Google Scholar] [CrossRef]
- Tsigarida, A.A.; Dabdoub, S.M.; Nagaraja, H.N.; Kumar, P.S. The Influence of Smoking on the Peri-Implant Microbiome. J. Dent. Res. 2015, 94, 1202–1217. [Google Scholar] [CrossRef]
- Costa, A.L.; Yasuda, C.L.; Shibasaki, W.; Nahás-Scocate, A.C.R.; de Freitas, C.F.; Carvalho, P.E.G.; Cendes, F. The association between periodontal disease and seizure severity in refractory epilepsy patients. Seizure 2014, 23, 227–230. [Google Scholar] [CrossRef]
- Serrano, J.; Escribano, M.; Roldán, S.; Martín, C.; Herrera, D. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, S106–S138. [Google Scholar] [CrossRef]
- Mao, X.; Hiergeist, A.; Auer, D.L.; Scholz, K.J.; Muehler, D.; Hiller, K.-A.; Maisch, T.; Buchalla, W.; Hellwig, E.; Gessner, A.; et al. Ecological Effects of Daily Antiseptic Treatment on Microbial Composition of Saliva-Grown Microcosm Biofilms and Selection of Resistant Phenotypes. Front. Microbiol. 2022, 13, 934525. [Google Scholar] [CrossRef]
- Exterkate, R.A.; Zaura, E.; Brandt, B.W.; Buijs, M.J.; Koopman, J.E.; Crielaard, W.; Cate, J.M.T. The effect of propidium monoazide treatment on the measured bacterial composition of clinical samples after the use of a mouthwash. Clin. Oral Investig. 2015, 19, 813–822. [Google Scholar] [CrossRef]
- Shapiro, S.; Giertsen, E.; Guggenheim, B. An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouthrinses. Caries Res. 2002, 36, 93–100. [Google Scholar] [CrossRef]
- Touzel, R.E.; Sutton, J.M.; Wand, M.E. Establishment of a multi-species biofilm model to evaluate chlorhexidine efficacy. J. Hosp. Infect. 2016, 92, 154–160. [Google Scholar] [CrossRef]
- Sánchez, G.A.; Miozza, V.A.; Delgado, A.; Busch, L. Total salivary nitrates and nitrites in oral health and periodontal disease. Nitric Oxide 2014, 36, 31–35. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Carlström, M.; Ghasemi, A. Inorganic nitrate: A potential prebiotic for oral microbiota dysbiosis associated with type 2 diabetes. Nitric Oxide 2021, 116, 38–46. [Google Scholar] [CrossRef]
- Sanz-Martin, I.; Doolittle-Hall, J.; Teles, R.P.; Patel, M.; Belibasakis, G.N.; Hämmerle, C.H.F.; Jung, R.E.; Teles, F.R.F. Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing. J. Clin. Periodontol. 2017, 44, 1274–1284. [Google Scholar] [CrossRef]
- Mark Welch, J.L.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef]
- Cieplik, F.; Zaura, E.; Brandt, B.W.; Buijs, M.J.; Buchalla, W.; Crielaard, W.; Laine, M.L.; Deng, D.M.; Exterkate, R.A.M. Microcosm biofilms cultured from different oral niches in periodontitis patients. J. Oral Microbiol. 2019, 11, 1551596. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, J.; de la Fuente-Núñez, C.; Wang, Z.; Hancock, R.E.W.; Roberts, C.R.; Ma, J.; Li, J.; Haapasalo, M.; Wang, Q. Experimental and Theoretical Investigation of Multispecies Oral Biofilm Resistance to Chlorhexidine Treatment. Sci. Rep. 2016, 6, 27537. [Google Scholar] [CrossRef]
- Hoare, A.; Marsh, P.D.; Diaz, P.I. Ecological Therapeutic Opportunities for Oral Diseases. Microbiol. Spectr. 2017, 5, 4. [Google Scholar] [CrossRef]
- López-López, A.; Camelo-Castillo, A.; Ferrer, M.D.; Simon-Soro, Á.; Mira, A. Health-Associated Niche Inhabitants as Oral Probiotics: The Case of Streptococcus dentisani. Front. Microbiol. 2017, 8, 379. [Google Scholar] [CrossRef]
- Muras, A.; Mallo, N.; Otero-Casal, P.; Pose-Rodríguez, J.M.; Otero, A. Quorum sensing systems as a new target to prevent biofilm-related oral diseases. Oral Dis. 2022, 28, 307–313. [Google Scholar]
- Parga, A.; Muras, A.; Otero-Casal, P.; Arredondo, A.; Soler-Ollé, A.; Àlvarez, G.; Alcaraz, L.D.; Mira, A.; Blanc, V.; Otero, A. The quorum quenching enzyme Aii20J modifies in vitro periodontal biofilm formation. Front. Cell. Infect. Microbiol. 2023, 13, 1118630. [Google Scholar] [CrossRef]
- Sanz, M.; Chapple, I.L. Working Group 4 of the VEWoP: Clinical research on peri-implant diseases: Consensus report of Working Group 4. J. Clin. Periodontol. 2012, 39 (Suppl. S12), 202–206. [Google Scholar]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef]
- Parga, A.; Pose-Rodríguez, J.M.; Muras, A.; Baus-Domínguez, M.; Otero-Casal, P.; Ortega-Quintana, M.L.; Torres-Lagares, D.; Otero, A. Do concurrent peri-implantitis and periodontitis share their microbiotas? A pilot study. Dent. J. 2024, 12, 113. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.1.3; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Allaire, J.J.; Horner, J.; Marti, V.; Porte, N. markdown: Markdown Rendering for R, Version 0.7.4; R Foundation for Statistical Computing: Vienna, Austria, 2014.
- Boettiger, C. knitcitations: Citations for Knitr Markdown Files, Version 1.0.5; R Foundation for Statistical Computing: Vienna, Austria, 2014.
- Xie, Y. Dynamic Documents with R and Knitr; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Solymos, P.; Stevens, M.H.; Szoecs, E.; et al. Vegan: Community Ecology Package, Version 2.6-2. CRAN; 2022. Available online: https://cran.r-project.org/package=vegan (accessed on 7 October 2025).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]

| 0.05% CHX Group | 0.12% CHX Group | |
|---|---|---|
| Age (years) | 65 ± 7.4 | 65 ± 12.6 |
| Sex, n (%) | ||
| Female | 6 (54.54%) | 6 (54.54%) |
| Male | 5 (45.45%) | 5 (45.45%) |
| Average implant age before PI onset (years) | 3.13 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pose-Otero, F.; Arredondo, A.; Parga, A.; Muras, A.; Gallas, M.; Otero-Casal, P.; Pose-Rodríguez, J.M.; Otero, A. The Effect of Chlorhexidine Mouthwashes on the Microbiota Associated with Peri-Implantitis Lesions: A Pilot Study. Antibiotics 2025, 14, 1032. https://doi.org/10.3390/antibiotics14101032
Pose-Otero F, Arredondo A, Parga A, Muras A, Gallas M, Otero-Casal P, Pose-Rodríguez JM, Otero A. The Effect of Chlorhexidine Mouthwashes on the Microbiota Associated with Peri-Implantitis Lesions: A Pilot Study. Antibiotics. 2025; 14(10):1032. https://doi.org/10.3390/antibiotics14101032
Chicago/Turabian StylePose-Otero, Félix, Alexandre Arredondo, Ana Parga, Andrea Muras, Mercedes Gallas, Paz Otero-Casal, José Manuel Pose-Rodríguez, and Ana Otero. 2025. "The Effect of Chlorhexidine Mouthwashes on the Microbiota Associated with Peri-Implantitis Lesions: A Pilot Study" Antibiotics 14, no. 10: 1032. https://doi.org/10.3390/antibiotics14101032
APA StylePose-Otero, F., Arredondo, A., Parga, A., Muras, A., Gallas, M., Otero-Casal, P., Pose-Rodríguez, J. M., & Otero, A. (2025). The Effect of Chlorhexidine Mouthwashes on the Microbiota Associated with Peri-Implantitis Lesions: A Pilot Study. Antibiotics, 14(10), 1032. https://doi.org/10.3390/antibiotics14101032