Green Solutions to a Growing Problem: Harnessing Plants for Antibiotic Removal from the Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Design and Objectives
2.2. Search Strategy
2.3. Eligibility Criteria
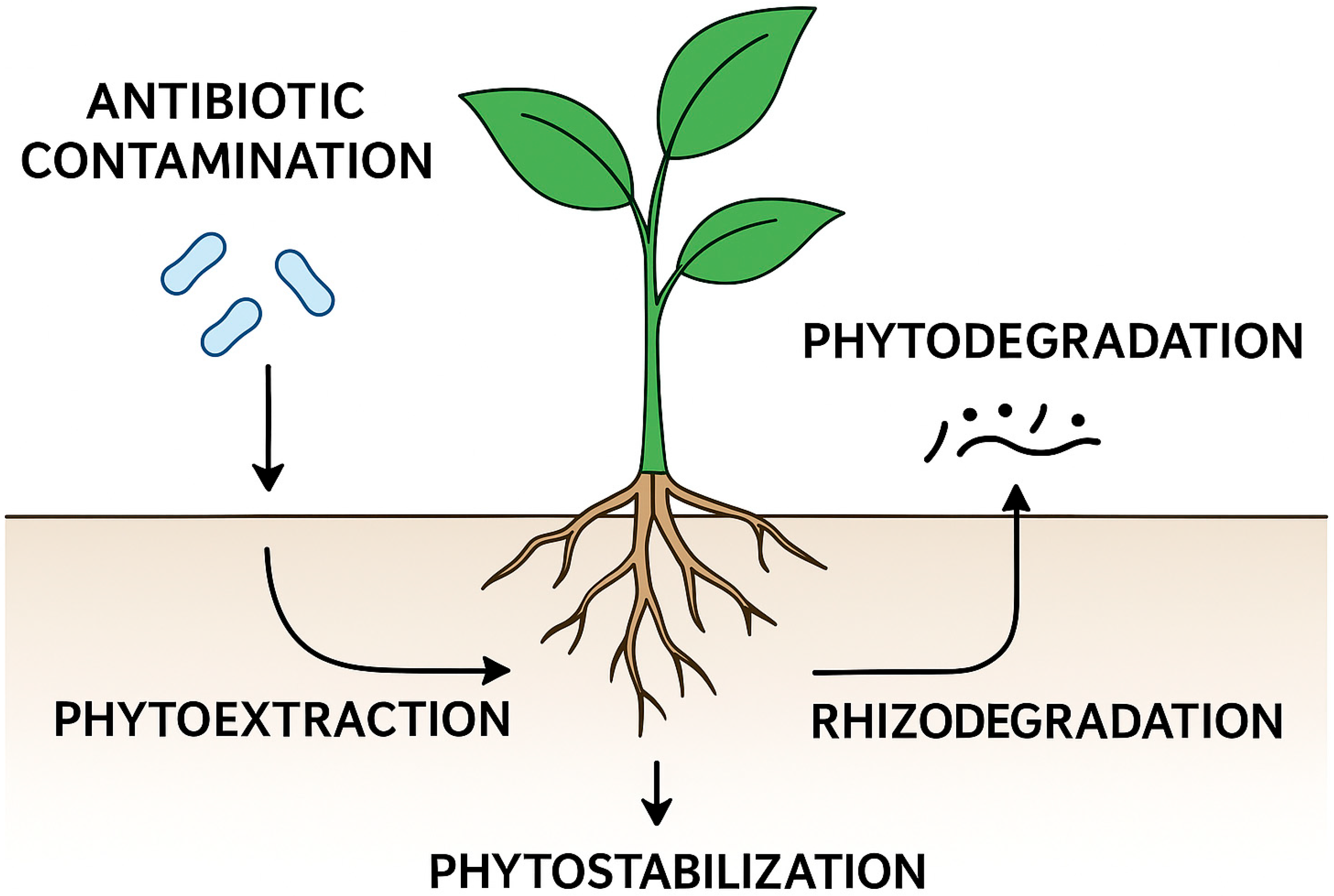
3. Why Plants? A Green Alternative for a Global Challenge
3.1. Phytoextraction: Uptake and Accumulation
3.2. Phytodegradation: Metabolic Breakdown
3.3. Rhizodegradation: Microbial Degradation in the Rhizosphere
3.4. Phytostabilization and Immobilization
3.5. Phytovolatilization: Rare in Antibiotic Remediation
3.6. Conclusions
4. Plant Players: Species with High Remediation Potential
4.1. Aquatic Macrophytes
4.2. Terrestrial Plants
4.3. Criteria for Plant Selection
5. Beyond the Roots: Rhizosphere Dynamics and Microbial Allies
5.1. Rhizosphere Microbiota: Catalysts of Bioremediation and Plant Immunity
5.2. Microbial Communities Involved
5.3. Mechanisms of Plant–Microbe Synergy
5.4. Engineering the Rhizosphere
5.5. Challenges and Future Needs
5.6. Conclusions
6. Nature-Inspired Engineering: Constructed Wetlands and Integrated Systems
6.1. Constructed Wetlands: Types and Principles
6.2. Performance for Antibiotic Removal
6.3. Design Factors and Optimization Strategies
6.4. Integration with Other Technologies
6.5. Case Studies and Real-World Applications
| Region | Target Antibiotic(s) | Main Findings/Application Details | Reference(s) |
|---|---|---|---|
| China | Tetracyclines (TC, OTC, CTC) | Vertical up-flow CWs treating swine wastewater achieved high removal (69–99.9%) of tetracyclines and reduction in tet genes. | Huang et al. [120] |
| China | Oxytetracycline, Tetracycline, Doxycycline, Chlortetracycline | CWs filled with coke + plants (CP-CW) showed removal rates of ~91% for OTC, ~90% for TC, ~85% for DOX. | Bai et al. [13] |
| Europe (Poland & Czechia) | Sulfonamides (e.g., sulfamethoxazole) | In full-scale CWs, sulfamethoxazole was removed with ~86–99% efficiency; sul1 genes persisted with little change. | Felis et al. [121] |
| Lab-scale study | Oxytetracycline | Vertical-flow CWs with zeolite + activated carbon achieved up to 97% removal of OTC. | Yuan et al. [122] |
7. Challenges and Knowledge Gaps
7.1. Incomplete or Variable Removal Efficiency
7.2. Phytotoxicity and Growth Inhibition
7.3. Accumulation in Plant Tissues and Biomass Management
7.4. Unknown Transformation Products and Ecotoxicity
7.5. Complexity of Mixed Contaminant Environments
7.6. Limited Field-Scale Validation and Long-Term Studies
7.7. Regulatory and Standardization Gaps
| Aspect | Summary | References |
|---|---|---|
| Addressed Problem | Environmental dissemination of antibiotics → ecological imbalances, spread of resistance genes (ARGs), inefficiency/high cost of conventional treatment technologies. | Bielen et al. [1]; Xu et al. [2]; Gomes [3]; Grenni et al. [4]; Tang et al. [5]; La Rosa et al. [6]; Huang et al. [7]. |
| Proposed Solution | Phytoremediation: use of plants (and associated microbes) to remove, transform, or immobilize antibiotics in soils and waters. | Singh et al. [8]; Aryal [9]; Kafle et al. [10]. |
| Main Mechanisms |
| Chen et al. [19]; Zhao et al. [20]; Zhou et al. [21]; Yang et al. [22]; Li et al. [23]; Yi et al. [24]. |
| Key Plant Species |
| Alfarsi et al. [62]; Von Salzen et al. [63]; Aydin et al. [64]; Xu et al. [66]; Huang et al. [67]; Borowik et al. [72]; Madikizela [77]; Milke et al. [78]. |
| Role of the Rhizosphere | Root-associated microbes (bacteria, fungi, actinomycetes) enhance degradation; plant–microbe synergies are crucial for efficiency. | Kraemer et al. [48]; Martínez-Martínez et al. [49]; Zambelli et al. [50]; Angelini [51]; Akrout et al. [94]; Mohanram & Kumar [98]. |
| Engineered Systems | Constructed wetlands (CWs), floating treatment wetlands (FTWs), integration with biochar, photocatalysis, microbial fuel cells. | He et al. [12]; Bai et al. [13]; Sabri et al. [110]; Tarigan et al. [111]; Ajibade et al. [115]; Chen et al. [116]; Alcaide et al. [118]. |
| Advantages | Nature-based, low-cost, eco-friendly solution; co-benefits: increased biodiversity, soil stabilization, improved water quality. | Haque et al. [18]; Chowdhury et al. [31]. |
| Limitations |
| Narciso et al., 2023 [124]; Minden et al., 2018 [125]; Polianciuc et al., 2020 [126]; Zhang et al., 2025 [127]; Moreno et al., 2022 [131] |
| Future Perspectives |
| Mallari et al., 2025 [134]; Diwan et al., 2022 [135]; Janga et al., 2023 [136]; Agrahari et al., 2024 [137]; Zaman et al., 2024 [138]; Longo et al., 2024 [139]. |
8. The Road Ahead: Innovation and Future Research Directions
8.1. Genetic Engineering and Synthetic Biology
8.2. Microbiome Engineering and Rhizosphere Optimization
8.3. Smart Monitoring and AI-Driven System Design
8.4. Hybrid and Modular Remediation Systems
8.5. Policy, Education, and Circular Economy Integration
9. Future Directions and Conclusions: Rooting for Green Remediation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AMR | Antimicrobial Resistance |
| ARG | Antibiotic Resistance Gene |
| CO2 | Carbon Dioxide |
| CWs | Constructed Wetlands |
| FTWs | Floating Treatment Wetlands |
| FWS | Free Water Surface |
| GEMs | Genetically Engineered Microorganisms |
| HRT | Hydraulic Retention Time |
| ISR | Induced Systemic Resistance |
| LPs | Liposaccharides |
| MFCs | Microbial Fuel Cells |
| P450s | Cytocrome P450 enzymes |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PAL | Phenylalanine Ammonia-Lyase |
| PFAS | Perfluoroalkyl and Polyfluoroalkyl Substance |
| PGPB | Plant Growth-Promoting Bacteria |
| PGPMs | Plant Growth-Promoting Microorganisms |
| POD | Peroxidase |
| PPO | Polyphenol Oxidase |
| PR | Pathogenesis-Related |
| PRISMA | Preferred Reporting Item for Systematic Reviews and Meta-Analyses |
| ROS | Reactive Oxygen Species |
| SAR | Systemic Acquired Resistance |
| SOD | Superoxide Dismutase |
| SSF | Subsurface Flow |
| SynComs | Synthetic Microbial Communities |
| TiO2 | Titanium Dioxide |
| TPs | Transformation |
| UV | Ultraviolet |
| VOCS | Volatile Organic Compounds |
| VSSF | Vertical Subsurface Flow |
References
- Bielen, A.; Šimatović, A.; Kosić Vukšić, J.; Senta, I.; Ahel, M.; Babić, S.; Jurina, T.; González Plaza, J.J.; Milaković, M.; Udiković Kolić, N. Negative environmental impacts of antibiotic contaminated effluents from pharmaceutical industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Yuan, Y.; Fang, F.; Xi, B.; Tan, W. Antibiotic Resistance Occurrence and Ecological Impact in Landfill Leachate: A Review on Compound Effect of Antibiotics and non-Antibiotics. Emerg. Contam. 2025, 11, 100508. [Google Scholar] [CrossRef]
- Gomes, M.P. The convergence of antibiotic contamination, resistance, and climate dynamics in freshwater ecosystems. Water 2024, 16, 2606. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; La Mastra, C.; Magnano San Lio, R.; Barchitta, M.; Agodi, A. The Impact of Wastewater on Antimicrobial Resistance: A Scoping Review of Transmission Pathways and Contributing Factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Singh, D.; Joshi, H.; Maurya, S.; Shukla, S.; Madheshiya, K.; Gupta, G. Bioremediation of Antibiotics and Their Metabolites from Wastewater. In Biotechnological Removal of Emerging Pollutants from Wastewater Systems; Dey, S., Shah, M.P., Eds.; Advances in Wastewater Research Series; Springer: Singapore, 2025; p. 743. ISBN 978-9819639458. [Google Scholar]
- Aryal, M. Phytoremediation Strategies for Mitigating Environmental Toxicants. Heliyon 2024, 10, e38683. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. The Combined Rhizoremediation by a Triad: Plant Microorganism Functional Materials. Environ. Sci. Pollut. Res. 2023, 30, 90500–90521. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Jiang, L.; Wagner, T.; Sutton, N.B.; Ji, R.; Langenhoff, A.A.M. Improving Removal of Antibiotics in Constructed Wetland Treatment Systems Based on Key Design and Operational Parameters: A Review. J. Hazard. Mater. 2021, 407, 124386. [Google Scholar] [CrossRef]
- Bai, S.; Wang, X.; Zhang, Y.; Liu, F.; Shi, L.; Ding, Y.; Wang, M.; Lyu, T. Constructed Wetlands as Nature-Based Solutions for the Removal of Antibiotics: Performance, Microbial Response, and Emergence of Antimicrobial Resistance (AMR). Sustainability 2022, 14, 14989. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ji, J.; Li, H.; Liu, S. Current Natural Degradation and Artificial Intervention Removal Techniques for Antibiotics in the Aquatic Environment: A Review. Appl. Sci. 2025, 15, 5182. [Google Scholar] [CrossRef]
- Anand, U.; Carpena, M.; Kowalska-Góralska, M.; Garcia-Perez, P.; Sunita, K.; Bontempi, E.; Dey, A.; Prieto, M.A.; Proćków, J.; Simal-Gandara, J. Safer Plant-Based Nanoparticles for Combating Antibiotic Resistance in Bacteria: A Comprehensive Review on Its Potential Applications, Recent Advances, and Future Perspective. Sci. Total Environ. 2022, 821, 153472. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. The Evolving Landscape of Advanced Oxidation Processes in Wastewater Treatment: Challenges and Recent Innovations. Processes 2025, 13, 987. [Google Scholar] [CrossRef]
- Haque, M.A.; Rogerson, L.; Nath, N.D.; Haruna, S.; Ahn, J.; Johnston, T.V.; Lin, C.S.K.; Chong, L.; Na, L.; Jang, M.J.; et al. Sustainable Management and Valorization of Antibiotic Waste. Chem. Eng. J. 2024, 498, 155372. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.-S.; Wu, Q.; Huang, W.-J.; Yang, F.; Wang, Y.-J.; He, L.-X.; Ying, G.-G.; Chen, W.-L.; Chen, C.-E. Removal, Fate, and Bioavailability of Fluoroquinolone Antibiotics in a Phytoremediation System with Four Wetland Plants: Combining Dynamic DGT and Traditional Methods. Sci. Total Environ. 2023, 881, 163464. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Yao, X.; Wan, W.; Xu, L.; Guo, L.; Bai, J.; Hu, C.; Yu, H. Transformation of Antibiotics to Non-Toxic and Non-Bactericidal Products by Laccases Ensure the Safety of Stropharia rugosoannulata. J. Hazard. Mater. 2024, 476, 135099. [Google Scholar] [CrossRef]
- Zhou, T.; An, Q.; Zhang, L.; Wen, C.; Yan, C. Phytoremediation for Antibiotics Removal from Aqueous Solutions: A Meta-Analysis. Environ. Res. 2024, 240, 117516. [Google Scholar] [CrossRef]
- Yang, K.M.; Poolpak, T.; Pokethitiyook, P. Rhizodegradation: The Plant Root Exudate and Microbial Community Relationship. In Phytoremediation; Newman, L., Ansari, A.A., Gill, S.S., Naeem, M., Gill, R., Eds.; Springer: Cham, Switzerland, 2023; pp. 225–242. [Google Scholar] [CrossRef]
- Li, J.F.; Chen, L.; Jin, S.; Huang, L.X.; Chen, H.H. Influences of Plants and Soil Microbes on Antibiotics in the Rhizosphere: A Review. Plant Soil Environ. 2025, 71, 67–92. [Google Scholar] [CrossRef]
- Yi, X.; Wen, P.; Liang, J.-L.; Jia, P.; Yang, T.; Feng, S.; Liao, B.; Shu, W.; Li, J. Phytostabilization Mitigates Antibiotic Resistance Gene Enrichment in a Copper Mine Tailings Pond. J. Hazard. Mater. 2023, 443, 130255. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Z.; Wang, D.; Ma, Y.; Guo, P. Root Surface Microbial Biofilms in Phytoremediation: Formation Processes, Regulatory Mechanisms, Influencing Factors and Roles. Environ. Technol. Innov. 2025, 40, 104406. [Google Scholar] [CrossRef]
- Jassal, P.S.; Kudave, P.S.; Wani, A.K.; Yadav, T. Prospects of Phytoremediation in Degradation of Environmental Contaminants: Recent Advances, Challenges and Way Forward. Int. J. Phytoremediat. 2025, 27, 1442–1459. [Google Scholar] [CrossRef]
- Feng, Q.; Guo, K.; Liu, B.; Wang, C.; Yan, B.; Yue, Q.; Gao, Y.; Gao, B. Recent Advances and Prospects in Antibiotics Removal by Coagulation Technique: A Systematic Review. J. Clean. Prod. 2025, 514, 145825. [Google Scholar] [CrossRef]
- Dong, W.; Song, Y.; Wang, L.; Jian, W.; Zhou, Q. Phyto- and Microbial-Based Remediation of Rare-Earth-Element-Polluted Soil. Microorganisms 2025, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, K.; Brzychczy-Włoch, M.; Pamuła, E. Antibiotics Modified by Hydrophobic Ion-Pairing—A Solution to the World’s Problems with Resistant Bacteria? Sustain. Mater. Technol. 2023, 37, e00662. [Google Scholar] [CrossRef]
- Wang, J.; Aghajani Delavar, M. Techno-Economic Analysis of Phytoremediation: A Strategic Rethinking. Sci. Total Environ. 2023, 902, 165949. [Google Scholar] [CrossRef]
- Chowdhury, K.F.; Hall, R.J.; McNally, A.; Carter, L.J. Phytoremediation as a Tool to Remove Drivers of Antimicrobial Resistance in the Aquatic Environment. Rev. Environ. Contam. Toxicol. 2023, 261, 16. [Google Scholar] [CrossRef]
- Frascaroli, G.; Hunter, C.; Roberts, J.; Escudero, A. Antibiotic Removal by Three Promising Microalgae Strains: Biotic, Abiotic Routes, and Response Mechanisms. Water Air Soil Pollut. 2024, 235, 600. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Cho, K.-S.; Yun, J. Phytoremediation Strategies for Co-Contaminated Soils: Overcoming Challenges, Enhancing Efficiency, and Exploring Future Advancements and Innovations. Processes 2025, 13, 132. [Google Scholar] [CrossRef]
- Marques, R.Z.; Oliveira, P.G.D.; Barbato, M.L.; Kitamura, R.S.A.; Maranho, L.T.; Brito, J.C.M.; Nogueira, K.d.S.; Juneau, P.; Gomes, M.P. Green Solutions for Antibiotic Pollution: Assessing the Phytoremediation Potential of Aquatic Macrophytes in Wastewater Treatment Plants. Environ. Pollut. 2024, 357, 124376. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Gauba, P. Harnessing Plants for Ciprofloxacin Pollution: A Green Approach. Def. Life Sci. J. 2025, 10, 57–64. [Google Scholar]
- Kitamura, R.S.A.; Fusaro, T.; Marques, R.Z.; Brito, J.C.M.; Juneau, P.; Gomes, M.P. The Use of Aquatic Macrophytes as a Nature-Based Solution to Prevent Ciprofloxacin Deleterious Effects on Microalgae. Water 2023, 15, 2143. [Google Scholar] [CrossRef]
- Pietrini, F.; Passatore, L.; Carloni, S.; Zacchini, M. Non-Standard Physiological Endpoints to Evaluate the Toxicity of Emerging Contaminants in Aquatic Plants: A Case Study on the Exposure of Lemna minor L. and Spirodela polyrhiza (L.) Schleid. to Dimethyl Phthalate (DMP). In Emerging Contaminants and Plants; Aftab, T., Ed.; Emerging Contaminants and Associated Treatment Technologies; Springer: Cham, Switzerland, 2023; pp. 87–108. [Google Scholar] [CrossRef]
- Gomes, M.P. Nanophytoremediation: Advancing Phytoremediation Efficiency Through Nanotechnology Integration. Discov. Plants 2025, 2, 8. [Google Scholar] [CrossRef]
- Shanmugapriya, S.; Manivannan, G.; Selvakumar, G.; Sivakumar, N. Extracellular Fungal Peroxidases and Laccases for Waste Treatment: Recent Improvement. In Recent Advancement in White Biotechnology Through Fungi; Yadav, A., Singh, S., Mishra, S., Gupta, A., Eds.; Fungal Biology; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Sharma, M.; Rawat, S.; Rautela, A. Phytoremediation in Sustainable Wastewater Management: An Eco-Friendly Review of Current Techniques and Future Prospects. AQUA—Water Infrastruct. Ecosyst. Soc. 2024, 73, 1946–1975. [Google Scholar] [CrossRef]
- Geng, J.; Liu, X.; Wang, J.; Li, S. Accumulation and Risk Assessment of Antibiotics in Edible Plants Grown in Contaminated Farmlands: A Review. Sci. Total Environ. 2022, 853, 158616. [Google Scholar] [CrossRef]
- Abu-Tahon, M.A.; Housseiny, M.M.; Aboelmagd, H.I.; Daifalla, N.; Khalili, M.; Isichei, A.C.; Ramadan, A.; Abu El-Saad, A.M.; Seddek, N.H.; Ebrahim, D.; et al. A Holistic Perspective on the Efficiency of Microbial Enzymes in Bioremediation Process: Mechanism and Challenges: A Review. Int. J. Biol. Macromol. 2025, 308, 142278. [Google Scholar] [CrossRef]
- Alnasser, S.M. The Role of Glutathione S-Transferases in Human Disease Pathogenesis and Their Current Inhibitors. Genes Dis. 2025, 12, 101482. [Google Scholar] [CrossRef]
- Vašková, J.; Kocan, L.; Vaško, L.; Perjési, P. Glutathione-Related Enzymes and Proteins: A Review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13, 95. [Google Scholar] [CrossRef]
- Soana, E.; Vincenzi, F.; Gavioli, A.; Castaldelli, G. Different Denitrification Capacity in Phragmites australis and Typha latifolia Sediments: Does the Availability of Surface Area for Biofilm Colonization Matter? Water 2025, 17, 560. [Google Scholar] [CrossRef]
- Yang, C.-X.; Chen, S.-J.; Hong, X.-Y.; Wang, L.-Z.; Wu, H.-M.; Tang, Y.-Y.; Gao, Y.-Y.; Hao, G.-F. Plant Exudates-Driven Microbiome Recruitment and Assembly Facilitates Plant Health Management. FEMS Microbiol. Rev. 2025, 49, fuaf008. [Google Scholar] [CrossRef]
- Kraemer, S.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, J.G.; Rosales-Loredo, S.; Hernández-Morales, A.; Arvizu-Gómez, J.L.; Carranza-Álvarez, C.; Macías-Pérez, J.R.; Rolón-Cárdenas, G.A.; Pacheco-Aguilar, J.R. Bacterial Communities Associated with the Roots of Typha spp. and its Relationship in Phytoremediation Processes. Microorganisms 2023, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, A.; Nocito, F.F.; Araniti, F. Unveiling the Multifaceted Roles of Root Exudates: Chemical Interactions, Allelopathy, and Agricultural Applications. Agronomy 2025, 15, 845. [Google Scholar] [CrossRef]
- Angelini, P. Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics 2024, 13, 746. [Google Scholar] [CrossRef]
- McCorquodale-Bauer, K.; Grosshans, R.; Zvomuya, F.; Cicek, N. Critical Review of Phytoremediation for the Removal of Antibiotics and Antibiotic Resistance Genes in Wastewater. Sci. Total Environ. 2023, 870, 161876. [Google Scholar] [CrossRef]
- Limmer, M.; Burken, J. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef]
- Bi, B.; Wang, K.; Zhang, H.; Wang, Y.; Fei, H.; Pan, R.; Han, F. Plants Use Rhizosphere Metabolites to Regulate Soil Microbial Diversity. Land Degrad. Dev. 2021, 32, 5267–5280. [Google Scholar] [CrossRef]
- Paladino, G.L.; Dupaul, G.; Jonsson, A.; Haller, H.; Eivazi, A.; Hedenström, A. Selecting Effective Plant Species for The Phytoremediation of Persistent Organic Pollutants and Multielement Contaminated Fibrous Sediments. Environ. Sci. Eur. 2025, 37, 117. [Google Scholar] [CrossRef]
- Mukherjee, S.; Leri, A.C.; Bandaranayaka, C.; Vázquez-Núñez, E.; Barros, R.; Khan, A.H.A.; Zhou, P.; Zhang, T.; Bernal, M.P.; Clemente, R.; et al. Sustainable Management of Post-Phytoremediation Biomass. Energy Ecol. Environ. 2025, 35, 103241. [Google Scholar] [CrossRef]
- Rasool, S.; Ahmad, I.; Jamal, A.; Saeed, M.F.; Zakir, A.; Abbas, G.; Seleiman, M.F.; Caballero-Calvo, A. Evaluation of Phytoremediation Potential of An Aquatic Macrophyte (Eichhornia crassipes) In Wastewater Treatment. Sustainability 2023, 15, 11533. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Huang, M.; Zou, Z.; Guo, A.; Wang, J.; Zou, J. Sulfonamide Antibiotics Alter Gaseous Nitrogen Emissions in the Soil–Plant System: A Mesocosm Experiment and Meta-Analysis. Sci. Total Environ. 2022, 828, 154230. [Google Scholar] [CrossRef]
- Mocek-Płóciniak, A.; Mencel, J.; Zakrzewski, W.; Roszkowski, S. Phytoremediation as An Effective Remedy for Removing Trace Elements from Ecosystems. Plants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Chao, C.; Gong, S.; Xie, Y. The Performance of a Multi-Stage Surface Flow Constructed Wetland for the Treatment of Aquaculture Wastewater and Changes in Epiphytic Biofilm Formation. Microorganisms 2025, 13, 494. [Google Scholar] [CrossRef]
- Singh, P.; Singh, G.; Singh, A.; Mishra, V.K.; Shukla, R. Macrophytes for Utilization in Constructed Wetland as Efficient Species for Phytoremediation of Emerging Contaminants from Wastewater. Wetlands 2024, 44, 22. [Google Scholar] [CrossRef]
- Alfarsi, A.; Werid, G.M.; Kumar, A.; Nugegoda, D. Multigenerational Toxicity Effects and Impact of Antibiotics Exposed to Duckweed, Lemna minor. Sci. Total Environ. 2025, 977, 179324. [Google Scholar] [CrossRef]
- Von Salzen, J.; Petersen, F.; Ulbrich, A.; Streif, S. Modeling Growth Dynamics of Lemna minor: Process Optimization Considering the Influence of Plant Density and Light Intensity. Plants 2025, 14, 1722. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Arabacı, D.N.; Shahi, A.; Fakhri, H.; Ovez, S. Enhanced Removal of Antibiotics Using Eichhornia crassipes Root Biomass in an Aerobic Hollow-Fiber Membrane Bioreactor. Biofouling 2022, 38, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Cui, B.; Fan, X.; Li, S.; Gao, F. Ryegrass (Lolium multiflorum L.) and Indian Mustard (Brassica juncea L.) Intercropping Can Improve the Phytoremediation of Antibiotics and Antibiotic Resistance Genes but Not Heavy Metals. Sci. Total Environ. 2021, 784, 147093. [Google Scholar] [CrossRef]
- Xu, L.S.; Wang, W.Z.; Deng, J.B.; Zhao, X.; Liu, X.; Wang, J.; Lin, H. The Residue of Tetracycline Antibiotics in Soil and Brassica juncea var gemmifera, and the Diversity of Soil Bacterial Community under Different Livestock Manure Treatments. Environ. Geochem. Health 2023, 45, 7–17. [Google Scholar] [CrossRef]
- Huang, S.; Chen, X.; Yan, R.; Huang, M.; Chen, D. Isolation, Identification and Antibacterial Mechanism of the Main Antibacterial Component from Pickled and Dried Mustard (Brassica juncea Coss. var. Foliosa Bailey). Molecules 2022, 27, 2418. [Google Scholar] [CrossRef]
- Thepbandit, W.; Athinuwat, D. Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Feng, Y.; Li, X.; Xu, J.; Jiang, J. Adaptation of Rhizosphere Microbial Communities to Continuous Exposure to Multiple Residual Antibiotics in Vegetable Farms. Int. J. Environ. Res. Public Health 2023, 20, 3137. [Google Scholar] [CrossRef]
- Guo, S.; Ge, Y.; Xiao, J. A Review of Phytochemistry, Metabolite Changes, and Medicinal Uses of the Common Sunflower Seed and Sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Hunce, S.Y.; Clemente, R.; Bernal, M.P. Energy Production Potential of Phytoremediation Plant Biomass: Helianthus annuus and Silybum marianum. Ind. Crops Prod. 2019, 135, 206–216. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Energy Potential of Zea mays Grown in Cadmium-Contaminated Soil. Energies 2025, 18, 2402. [Google Scholar] [CrossRef]
- Wang, P.; Xie, W.; Ding, L.; Zhuo, Y.; Gao, Y.; Li, J.; Zhao, L. Effects of Maize–Crop Rotation on Soil Physicochemical Properties, Enzyme Activities, Microbial Biomass and Microbial Community Structure in Southwest China. Microorganisms 2023, 11, 2621. [Google Scholar] [CrossRef] [PubMed]
- Oubohssaine, M.; Dahmani, I. Phytoremediation: Harnessing Plant Power and Innovative Technologies for Effective Soil Remediation. Plant Stress 2024, 14, 100578. [Google Scholar] [CrossRef]
- Ammu, K.P.; Malathi, P.; Sellamuthu, K.M.; Jayashree, R.; Senthil Kumar, G. Phytoremediation of Salt-Affected Soils: Mechanistic Insights and Criteria for Plant Selection. Arid Land Res. Manag. 2025, 39, 455–475. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Cappelli, S.L.; Shrestha, R.; Gerin, S.; Lohila, A.K.; Heinonsalo, J.; Nelson, D.B.; Kahmen, A.; Duan, P.; Sebag, D.; et al. diversity drives positive microbial associations in the rhizosphere enhancing carbon use efficiency in agricultural soils. Nat. Commun. 2024, 15, 8065. [Google Scholar] [CrossRef]
- Madikizela, L.M. Removal of Organic Pollutants in Water Using Water Hyacinth (Eichhornia crassipes). J. Environ. Manag. 2021, 295, 113153. [Google Scholar] [CrossRef]
- Milke, J.; Gałczyńska, M.; Wróbel, J. The Importance of Biological and Ecological Properties of Phragmites australis (Cav.) Trin. Ex Steud., in Phytoremediation of Aquatic Ecosystems—A Review. Water 2020, 12, 1770. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Bahar, M.M.; Naidu, R. Diffuse Soil Pollution from Agriculture: Impacts and Remediation. Sci. Total Environ. 2025, 962, 178398. [Google Scholar] [CrossRef] [PubMed]
- Nybom, I.; Bucheli, T.D.; Garlanda, G. Antibiotics Uptake from Soil and Translocation in the Plants—Meta-Analysis. Chimia 2024, 78, 209–214. [Google Scholar] [CrossRef]
- Joshi, D.; Kaushik, A.; Kumar, R.; Arya, A.; Santoyo, G.; Singh, V.K.; Kashyap, N.; Solanki, M.K.; Kumari, M.; Bhardwaj, N.; et al. Improving Plant Performance through Microbiome Manipulation: The Potential Role of Current Bioengineering Approaches. Bacteria 2025, 4, 12. [Google Scholar] [CrossRef]
- Asghar, W.; Craven, K.D.; Swenson, J.R.; Kataoka, R.; Mahmood, A.; Farias, J.G. Enhancing the Resilience of Agroecosystems through Improved Rhizosphere Processes: A Strategic Review. Int. J. Mol. Sci. 2025, 26, 109. [Google Scholar] [CrossRef]
- Laishram, B.; Devi, O.R.; Dutta, R.; Senthilkumar, T.; Goyal, G.; Paliwal, D.K.; Panotra, N.; Rasool, A. Plant-Microbe Interactions: PGPM as Microbial Inoculants/Biofertilizers for Sustaining Crop Productivity and Soil Fertility. Curr. Res. Microb. Sci. 2024, 16, 100333. [Google Scholar] [CrossRef]
- Sahoo, S.; Panda, S.S.; Kumar, S.; Nigam, R.; Serangi, S.; Choudhary, M.; Yuvarani, R.; Shivam. A Review on Plant-Microbe Interactions and Its Defence Mechanism. Plant Cell Biotechnol. Mol. Biol. 2024, 25, 159–175. [Google Scholar] [CrossRef]
- Hönig, M.; Roeber, V.M.; Schmülling, T.; Cortleven, A. Chemical Priming of Plant Defense Responses to Pathogen Attacks. Front. Plant Sci. 2023, 14, 1146577. [Google Scholar] [CrossRef]
- Zhu, F.; Cao, M.-Y.; Zhang, Q.-P.; Mohan, R.; Schar, J.; Mitchell, M.; Chen, H.; Liu, F.; Wang, D.; Fu, Z.Q. Join the Green Team: Inducers of Plant Immunity in the Plant Disease Sustainable Control Toolbox. J. Adv. Res. 2024, 57, 15–42. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Mir, Z.A. Plant Immunity: At the Crossroads of Pathogen Perception and Defense Response. Plants 2024, 13, 1434. [Google Scholar] [CrossRef]
- Haghpanah, M.; Namdari, A.; Kaleji, M.K.; Nikbakht-Dehkordi, A.; Arzani, A.; Araniti, F. Interplay between ROS and Hormones in Plant Defense against Pathogens. Plants 2025, 14, 1297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pandey, S.; Kumar Pati, P. Interaction between Pathogenesis-Related (PR) Proteins and Phytohormone Signaling Pathways in Conferring Disease Tolerance in Plants. Physiol. Plant. 2025, 177, e70174. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mahawar, L.; Mishra, A.; Albrectsen, B.R. Microbial Contributions to Plant Growth and Stress Tolerance: Mechanisms for Sustainable Plant Production. Plant Stress 2025, 17, 100966. [Google Scholar] [CrossRef]
- Wei, J.; Song, K.; Zang, Z.; Yang, H.; Gao, Y.; Zhang, J.; Wang, Z.; Liu, C. Influence of Specific Tobacco Endophytic Bacillus on Tobacco Leaf Quality Enhancement during Fermentation. Front. Microbiol. 2024, 15, 1468492. [Google Scholar] [CrossRef]
- Thepbandit, W.; Srisuwan, A.; Siriwong, S.; Nawong, S.; Athinuwat, D. Bacillus vallismortis TU-Orga21 Blocks Rice Blast through Both Direct Effect and Stimulation of Plant Defense. Front. Plant Sci. 2023, 14, 1103487. [Google Scholar] [CrossRef]
- Nilmat, A.; Thepbandit, W.; Chuaboon, W.; Athinuwat, D. Pseudomonas fluorescens SP007S Formulations in Controlling Soft Rot Disease and Promoting Growth in Kale. Agronomy 2023, 13, 1856. [Google Scholar] [CrossRef]
- Akrout, I.; Staita, K.; Zouari-Mechichi, H.; Ghariani, B.; Khmaissa, M.; Navarro, D.; Doan, A.; Albert, Q.; Faulds, C.; Sciara, G.; et al. Valorizing Fungal Diversity for the Degradation of Fluoroquinolones. Heliyon 2024, 10, e30611. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, I.; Sahraoui, L.H.; Fontaine, I. Arbuscular Mycorrhizal Fungal-Assisted Phytoremediation of Soil Contaminated with Persistent Organic Pollutants: A Review. Eur. J. Soil Sci. 2016, 67, 624–640. [Google Scholar] [CrossRef]
- Pascual-García, A.; Bonhoeffer, S.; Bell, T. Metabolically Cohesive Microbial Consortia and Ecosystem Functioning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190245. [Google Scholar] [CrossRef]
- Courti, I.; Muja, C.; Maho, T.; Sainct, F.P.; Guillot, P. Degradation of Bacterial Antibiotic Resistance Genes during Exposure to Non-Thermal Atmospheric Pressure Plasma. Antibiotics 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Mohanram, S.; Kumar, P. Rhizosphere Microbiome: Revisiting the Synergy of Plant-Microbe Interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Veríssimo, A.C.S.; Pinto, D.C.G.A.; Sierra-Garcia, I.N.; Granada, C.E.; Cremades, J.; Silva, H.; Cunha, Â. Engineering the Rhizosphere Microbiome with Plant Growth Promoting Bacteria for Modulation of the Plant Metabolome. Plants 2024, 13, 2309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dubey, A. Rhizosphere Microbiome: Engineering Bacterial Competitiveness for Enhancing Crop Production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef]
- Niegowska, M.; Sanseverino, I.; Navarro, A.; Lettieri, T. Knowledge Gaps in the Assessment of Antimicrobial Resistance in Surface Waters. FEMS Microbiol. Ecol. 2021, 97, fiab140. [Google Scholar] [CrossRef]
- Grabarczyk, L.; Mulkiewicz, E.; Stepnowski, P.; Stolte, S.; Puckowski, A.; Białk-Bielińska, A. Ecotoxicity Screening Evaluation of Selected Pharmaceuticals and Their Transformation Products towards Various Organisms. Environ. Sci. Pollut. Res. 2020, 27, 26103–26114. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Ramlal, A.; Rani, A.; Nautiyal, A.; Kalra, C.; Kumari, R.; Kumar, J.; Veeranna, S.; Mishra, V. Importance of Omics Approaches in Plant-Microbe Interaction for Plant Disease Control. Physiol. Mol. Plant Pathol. 2023, 128, 102153. [Google Scholar] [CrossRef]
- Wankhade, A.; Wilkinson, E.; Britt, D.W.; Kaundal, A. A Review of Plant–Microbe Interactions in the Rhizosphere and the Role of Root Exudates in Microbiome Engineering. Appl. Sci. 2025, 15, 7127. [Google Scholar] [CrossRef]
- Rani, A.; Chauhan, M.; Kumar Sharma, P.; Kumari, M.; Mitra, D.; Joshi, S. Microbiological Dimensions and Functions in Constructed Wetlands: A Review. Curr. Res. Microb. Sci. 2024, 7, 100311. [Google Scholar] [CrossRef]
- Hassan, A.I.; Saleh, H.M. Production of Amino Acids and Nucleic Acids from Genetically Engineered Microbial Cells and Their Relevance to Biodegradation. Green Energy Environ. Technol. 2023, 2, 1–47. [Google Scholar] [CrossRef]
- Langergrabe, G. Modelling of Processes in Subsurface Flow Constructed Wetlands: A Review. J. Water Process Eng. 2025, 70, 107078. [Google Scholar]
- Ijoma, G.N.; Lopes, T.; Mannie, T.; Mhlongo, T.N. Exploring Macrophytes’ Microbial Populations Dynamics to Enhance Bioremediation in Constructed Wetlands for Industrial Pollutants Removal in Sustainable Wastewater Treatment. Symbiosis 2024, 92, 323–354. [Google Scholar] [CrossRef]
- Sabri, N.A.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Performance of Full Scale Constructed Wetlands in Removing Antibiotics and Antibiotic Resistance Genes. Sci. Total Environ. 2021, 786, 147368. [Google Scholar] [CrossRef]
- Tarigan, M.; Raji, S.; Al-Fatesh, H.; Czermak, P.; Ebrahimi, M. The Occurrence of Micropollutants in the Aquatic Environment and Technologies for Their Removal. Processes 2025, 13, 843. [Google Scholar] [CrossRef]
- Aswad, Z.S.; Ali, A.H.; Al-Mhana, N.M. Vertical Subsurface Flow and Free Surface Flow Constructed Wetlands for Sustainable Power Generation and Real Wastewater Selective Pollutants Removal. J. Eng. Sustain. Dev. 2020, 24, 91–102. [Google Scholar] [CrossRef]
- Robinson, G.; Caldwell, G.; Wade, M.; Free, A.; Jones, C.L.W.; Stead, S.M. Profiling Bacterial Communities Associated with Sediment-Based Aquaculture Bioremediation Systems under Contrasting Redox Regimes. Sci. Rep. 2016, 6, 38850. [Google Scholar] [CrossRef]
- Kumar, V.; Lakkaboyana, S.K.; Sharma, N.; Chakraborty, P.; Umesh, M.; Pasrija, R.; Thomas, J.; Kalebar, V.U.; Jayaraj, I.; Awasthi, M.K.; et al. A Critical Assessment of Technical Advances in Pharmaceutical Removal from Wastewater—A Critical Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100363. [Google Scholar] [CrossRef]
- Ajibade, S.; Nnadozie, E.C.; Iwai, C.B.; Ghotekar, S.; Chang, S.W.; Ravindran, B.; Kumar Awasthi, M. Biochar-Based Compost: A Bibliometric And Visualization Analysis. Bioengineered 2022, 13, 15013–15032. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Sahreen, S.; Mukhtar, H. Development of Bacterial Augmented Floating Treatment Wetlands System (FTWs) for Eco-Friendly Degradation of Malachite Green Dye in Water. Sustainability 2023, 15, 4541. [Google Scholar] [CrossRef]
- Alcaide, F.; Sirés, I.; Brillas, E.; Cabot, P.L. Coupling Wastewater Treatment with Fuel Cells and Hydrogen Technology. Curr. Opin. Electrochem. 2024, 45, 101530. [Google Scholar] [CrossRef]
- Domínguez-Solís, D.; Martínez-Rodríguez, M.C.; Ramírez-Escamilla, H.G.; Campos-Villegas, L.E.; Domínguez-Solís, R. Constructed Wetlands as a Decentralized Treatment Option for Domestic Wastewater: A Systematic Review (2015–2024). Water 2025, 17, 1451. [Google Scholar] [CrossRef]
- Huang, X.; Liu, C.; Li, K.; Su, J.; Zhu, G.; Liu, L. Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Res. 2015, 70, 109–117. [Google Scholar] [CrossRef]
- Felis, E.; Sochacki, A.; Bajkacz, S.; Łuczkiewicz, A.; Jóźwiakowski, K.; García, J.; Vymazal, J. Removal of selected sulfonamides and sulfonamide resistance genes from wastewater in full-scale constructed wetlands. Sci. Total Environ. 2024, 912, 169195. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, Y.; Shang, Y.; Bai, M.; Li, L.; Li, X.; Deng, P.; Riaz, L.; Guo, Y.; Lu, J. Fate and removal of oxytetracycline and antibiotic resistance genes in vertical-flow constructed wetland with different substrates. Water 2025, 17, 1412. [Google Scholar] [CrossRef]
- Chen, J.; Tong, T.; Jiang, X.; Xie, S. Biodegradation of Sulfonamides in Both Oxic and Anoxic Zones of Vertical Flow Constructed Wetland and the Potential Degraders. Environ. Pollut. 2020, 265, 115040. [Google Scholar] [CrossRef] [PubMed]
- Narciso, A.; Barra Caracciolo, A.; De Carolis, C. Overview of Direct and Indirect Effects of Antibiotics on Terrestrial Organisms. Antibiotics 2023, 12, 1471. [Google Scholar] [CrossRef]
- Minden, V.; Schnetger, B.; Pufal, G.; Leonhardt, S.D. Antibiotic-Induced Effects on Scaling Relationships and on Plant Element Contents in Herbs and Grasses. Ecol. Evol. 2018, 8, 6699–6713. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, J.; Feng, M.; Ye, C. Transformation Products of Antibiotics: Overlooked Drivers for Enhancing the Environmental Spread of Antibiotic Resistance. J. Environ. Expo. Assess. 2025, 4, 10. [Google Scholar] [CrossRef]
- Bub, S.; Petschick, L.L.; Stehle, S.; Wolfram, J.; Schulz, R. Limitations of Chemical Monitoring Hinder Aquatic Risk Evaluations on the Macroscale. Science 2025, 388, 1301–1305. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Linnea-Niemi, J.V.; Kudłak, B.; Williams, M.J.; Jönsson, J.; Schiöth, H.B. Role of the Synergistic Interactions of Environmental Pollutants in the Development of Cancer. Geohealth 2022, 6, e2021GH000552. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Sha, N.; Tang, S.; Peng, Y.; Zhao, Y. Changes in Microbial Community Structure and Co-Metabolism During the Domestication of Ofloxacin-Degrading Bacteria. Environ. Sci. Eur. 2022, 34, 117. [Google Scholar] [CrossRef]
- Moreno, H.D.; Köring, M.; Di Pane, J.; Tremblay, N.; Wiltshire, K.H.; Boersma, M.; Meunier, C.L. An Integrated Multiple Driver Mesocosm Experiment Reveals the Effect of Global Change on Planktonic Food Web Structure. Commun. Biol. 2022, 5, 179. [Google Scholar] [CrossRef]
- Cleary, D.W.; Bishop, A.H.; Zhang, L.; Topp, E.; Wellington, E.M.H.; Gaze, W.H. Long-Term Antibiotic Exposure in Soil is Associated with Changes in Microbial Community Structure and Prevalence of Class 1 Integrons. FEMS Microbiol. Ecol. 2016, 92, fiw159. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding Human Health Risks Caused by Antibiotic Resistant Bacteria (ARB) and Antibiotic Resistance Genes (ARG) in Water Environments: Current Knowledge and Questions to be Answered. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2016–2059. [Google Scholar] [CrossRef]
- Mallari, P.; Rostami, L.D.; Alanko, I.; Howaili, F.; Ran, M.; Bansal, K.K.; Rosenholm, J.M.; Salo-Ahen, O.M.H. The Next Frontier: Unveiling Novel Approaches for Combating Multidrug-Resistant Bacteria. Pharm. Res. 2025, 42, 859–889. [Google Scholar] [CrossRef]
- Diwan, D.; Rashid, M.M.; Vaishnav, A. Current Understanding of Plant-Microbe Interaction Through the Lenses of Multi-Omics Approaches and Their Benefits in Sustainable Agriculture. Microbiol. Res. 2022, 265, 127180. [Google Scholar] [CrossRef]
- Janga, J.K.; Reddy, R.R.; Raviteja, K.V.N.S. Integrating Artificial Intelligence, Machine Learning, and Deep Learning Approaches into Remediation of Contaminated Sites: A Review. Chemosphere 2023, 345, 140476. [Google Scholar] [CrossRef]
- Agrahari, S.; Kumar, S. Emerging and futuristic phyto-technologies for sustainable wastewater treatment with resource recovery and economical aspects. J. Water Process. Eng. 2024, 65, 105753. [Google Scholar] [CrossRef]
- Zaman, W.; Ali, S.; Akhtar, M.S. Harnessing the Power of Plants: Innovative Approaches to Pollution Prevention and Mitigation. Sustainability 2024, 16, 10587. [Google Scholar] [CrossRef]
- Longo, S.; Cellura, M.; Luu, L.Q.; Thanh Nguyen, T.Q.; Rincione, R.; Guarino, F. Circular Economy and Life Cycle Thinking Applied to the Biomass Supply Chain: A Review. Renew. Energy 2024, 220, 119598. [Google Scholar] [CrossRef]
- Wang, X.; Ryu, D.; Houtkooper, R.H.; Auwerx, J. Antibiotic Use and Abuse: A Threat to Mitochondria and Chloroplasts with Impact on Research, Health, and Environment. BioEssays 2015, 37, 1045–1053. [Google Scholar] [CrossRef]
- Krzywonos, M.; Piwowar-Sulej, K. Plant-Based Innovations for the Transition to Sustainability: A Bibliometric and In-Depth Content Analysis. Foods 2022, 11, 3137. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Tomé, L.M.; Dornelles Parise, M.T.; Parise, D.; de Carvalho Azevedo, V.A.; Brenig, B.; Badotti, F.; Góes-Neto, A. Pure Lignin Induces Overexpression of Cytochrome P450 (CYP) Encoding Genes and Brings Insights into the Lignocellulose Depolymerization by Trametes villosa. Heliyon 2024, 10, e28449. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Newberger, D.; Vivanco, J.M. The Rhizosphere Microbiome: Plant-Microbial Interactions for Resource Acquisition. J. Appl. Microbiol. 2022, 133, 2864–2876. [Google Scholar] [CrossRef]
- Tiwari, P.; Khare, T.; Shriram, V.; Bae, H.; Kumar, V. Plant Synthetic Biology for Producing Potent Phyto-Antimicrobials to Combat Antimicrobial Resistance. Biotechnol. Adv. 2021, 48, 107729. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Song, H.; Ding, M.; Du, J.; Ma, Q.; Yuan, Y. Design, Analysis and Application of Synthetic Microbial Consortia. Synth. Syst. Biotechnol. 2016, 1, 109–117. [Google Scholar] [CrossRef]
- Arias-Sánchez, F.I.; Vessman, B.; Mitri, S. Artificially Selecting Microbial Communities: If We Can Breed Dogs, Why Not Microbiomes? PLoS Biol. 2019, 17, e3000356. [Google Scholar] [CrossRef]
- Nosratabad, N.A.; Yan, Q.; Cai, Z.; Wan, C. Exploring Nanomaterial-Modified Biochar for Environmental Remediation Applications. Heliyon 2024, 10, e37123. [Google Scholar] [CrossRef] [PubMed]
- Silerio-Vázquez, F.J.; González-Burciaga, L.A.; Antileo, C.; Núñez-Núñez, C.M.; Proal-Nájera, J.B. Photocatalytic Degradation of Antibiotics in Water Via TiO2−X: Research Needs for Technological Advancements. J. Hazard. Mater. Adv. 2024, 16, 100506. [Google Scholar] [CrossRef]
- Arivukkarasu, D.; Sathyanathan, R. Floating wetland treatment an ecological approach for the treatment of water and wastewater—A review. Mater. Today Proc. 2023, 77, 176–181. [Google Scholar] [CrossRef]
- Manolis, E.N.; Manoli, E.N. Raising Awareness of The Sustainable Development Goals Through Ecological Projects in Higher Education. J. Clean. Prod. 2021, 279, 123614. [Google Scholar] [CrossRef]
- Kowalska, A.; Biczak, R. Phytoremediation and Environmental Law: Harnessing Biomass and Microbes to Restore Soils and Advance Biofuel Innovation. Energies 2025, 18, 1860. [Google Scholar] [CrossRef]
- Lavanya, M.B.; Viswanath, D.S.; Sivapullaiah, P.V. Phytoremediation: An Eco-Friendly Approach for Remediation of Heavy Metal-Contaminated Soils—A Comprehensive Review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100975. [Google Scholar] [CrossRef]
- Pastor-López, E.J.; Escolà, M.; Kisielius, V.; Arias, C.A.; Carvalho, P.N.; Gorito, A.M.; Ramos, S.; Freitas, V.; Guimaraes, L.; Almeida, C.M.R.; et al. Potential of nature-based solutions to reduce antibiotics, antimicrobial resistance, and pathogens in aquatic ecosystems: A critical review. Sci. Total Environ. 2024, 946, 174273. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M.N. Genetically Engineered Microorganisms For Environmental Remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef] [PubMed]
- Waly, M.M.; Ahmed, T.; Abunada, Z.; Mickovski, S.B.; Thomson, C. Constructed Wetland for Sustainable and Low-Cost Wastewater Treatment: Review Article. Land 2022, 11, 1388. [Google Scholar] [CrossRef]
- Adu, O.; Ma, X.; Sharma, V.K. Bioavailability, Phytotoxicity and Plant Uptake of per-and Polyfluoroalkyl Substances (PFAS): A Review. J. Hazard. Mater. 2023, 447, 130805. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lin, X.; Xiao, Y. Integration of Smart Sensors and Phytoremediation for Real-Time Pollution Monitoring and Ecological Restoration in Agricultural Waste Management. Front. Plant Sci. 2025, 16, 1550302. [Google Scholar] [CrossRef] [PubMed]
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Language | English | Non-English publications |
| Type of publication | Peer-reviewed articles (original research, reviews, meta-analyses, case studies) | Conference abstracts, editorials, preprints |
| Time frame | Published between 2015 and August 2025 | Published before 2015 |
| Topic relevance | Focus on plant-based removal or degradation of antibiotics from environment | Articles focused exclusively on metals, pesticides, or non-antibiotic pollutants |
| Context | Aquatic and terrestrial environmental settings | Purely clinical or pharmacological studies |
| Mechanisms | Studies exploring plant uptake, degradation, rhizosphere activity, or system design | Studies lacking mechanistic or empirical data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusumano, G.; Angeles Flores, G.; Venanzoni, R.; Angelini, P.; Zengin, G. Green Solutions to a Growing Problem: Harnessing Plants for Antibiotic Removal from the Environment. Antibiotics 2025, 14, 1031. https://doi.org/10.3390/antibiotics14101031
Cusumano G, Angeles Flores G, Venanzoni R, Angelini P, Zengin G. Green Solutions to a Growing Problem: Harnessing Plants for Antibiotic Removal from the Environment. Antibiotics. 2025; 14(10):1031. https://doi.org/10.3390/antibiotics14101031
Chicago/Turabian StyleCusumano, Gaia, Giancarlo Angeles Flores, Roberto Venanzoni, Paola Angelini, and Gokhan Zengin. 2025. "Green Solutions to a Growing Problem: Harnessing Plants for Antibiotic Removal from the Environment" Antibiotics 14, no. 10: 1031. https://doi.org/10.3390/antibiotics14101031
APA StyleCusumano, G., Angeles Flores, G., Venanzoni, R., Angelini, P., & Zengin, G. (2025). Green Solutions to a Growing Problem: Harnessing Plants for Antibiotic Removal from the Environment. Antibiotics, 14(10), 1031. https://doi.org/10.3390/antibiotics14101031









