Impact of Light-Activated Nanocomposite with Erythrosine B on agr Quorum Sensing System in Staphylococcus aureus
Abstract
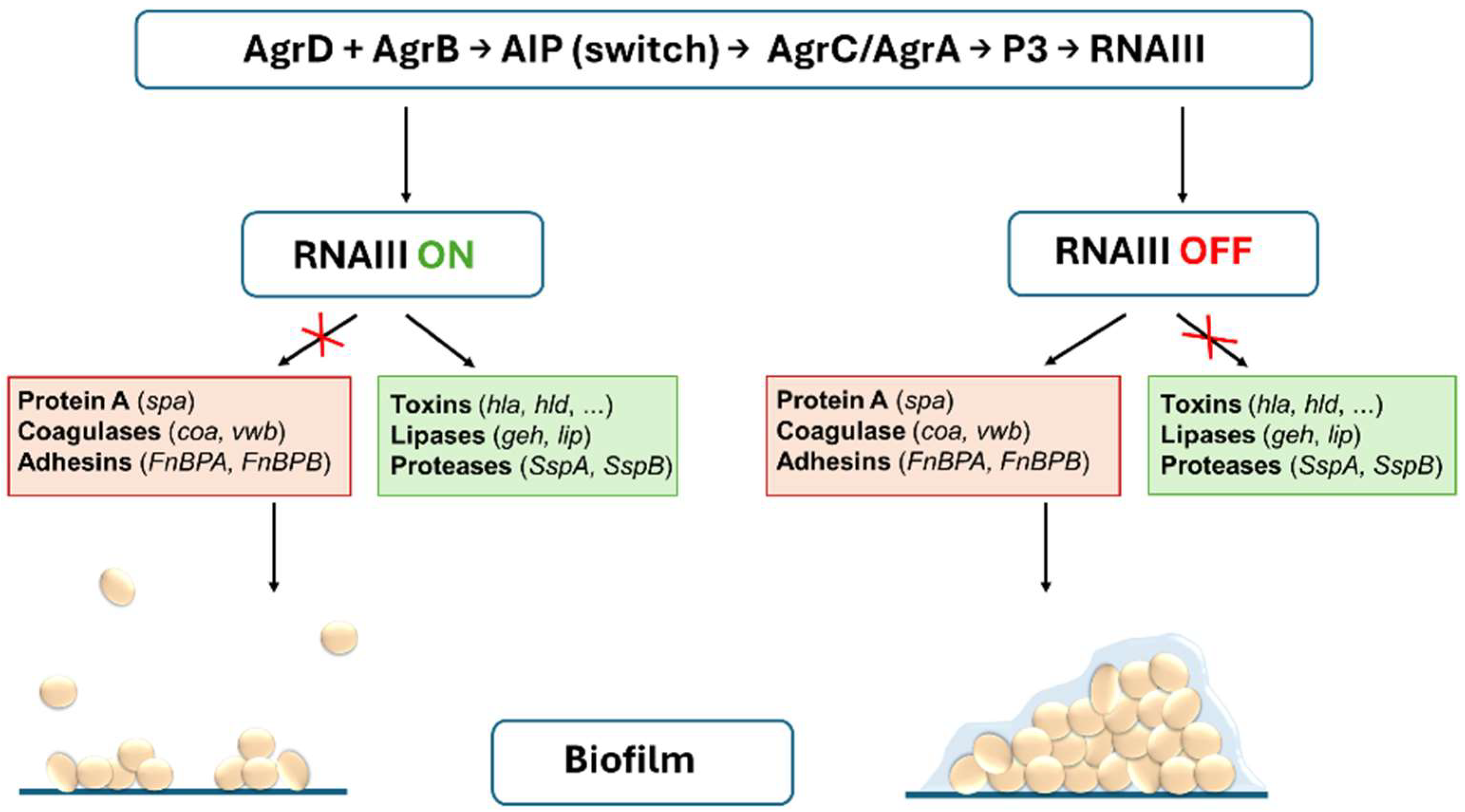
1. Introduction
2. Results
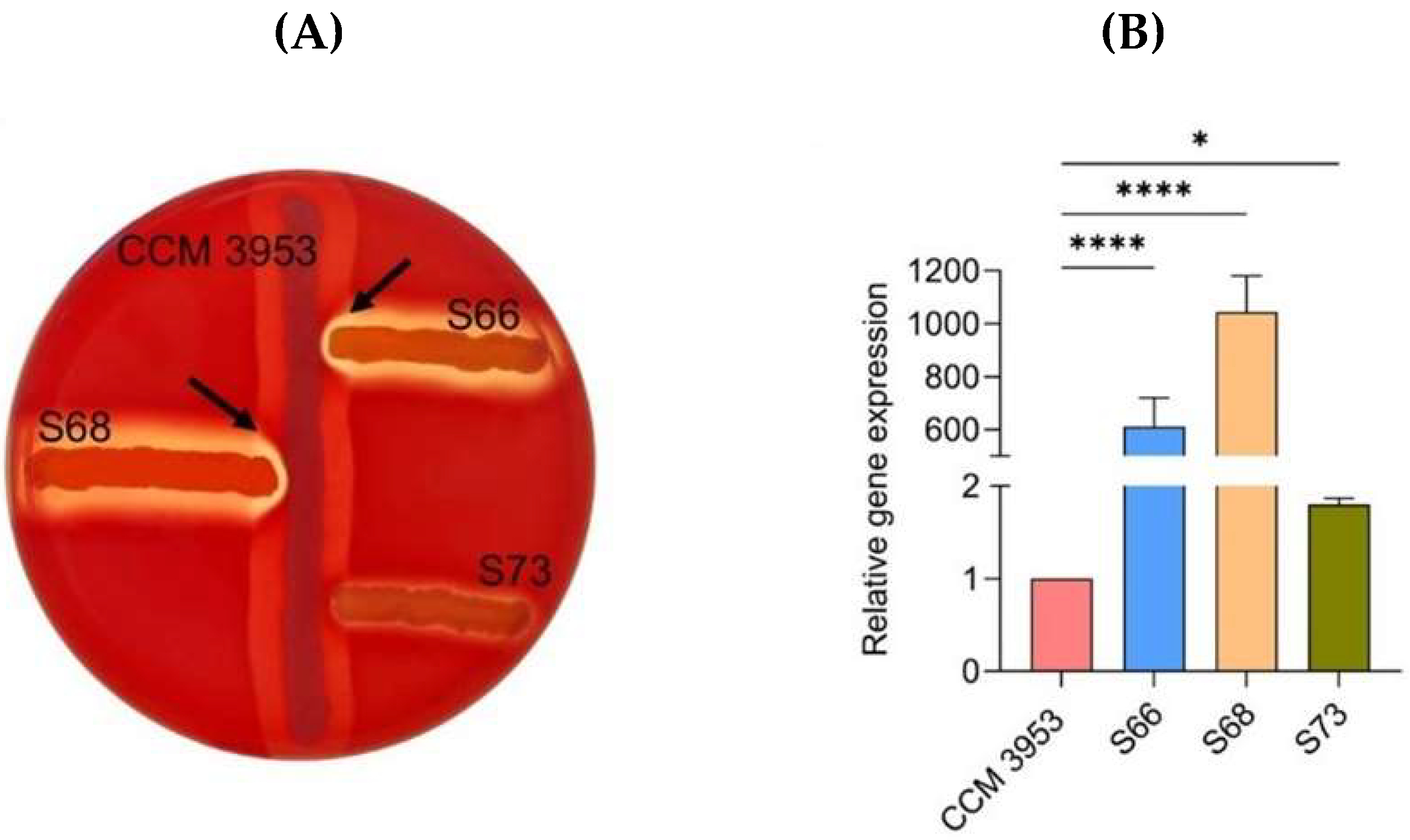
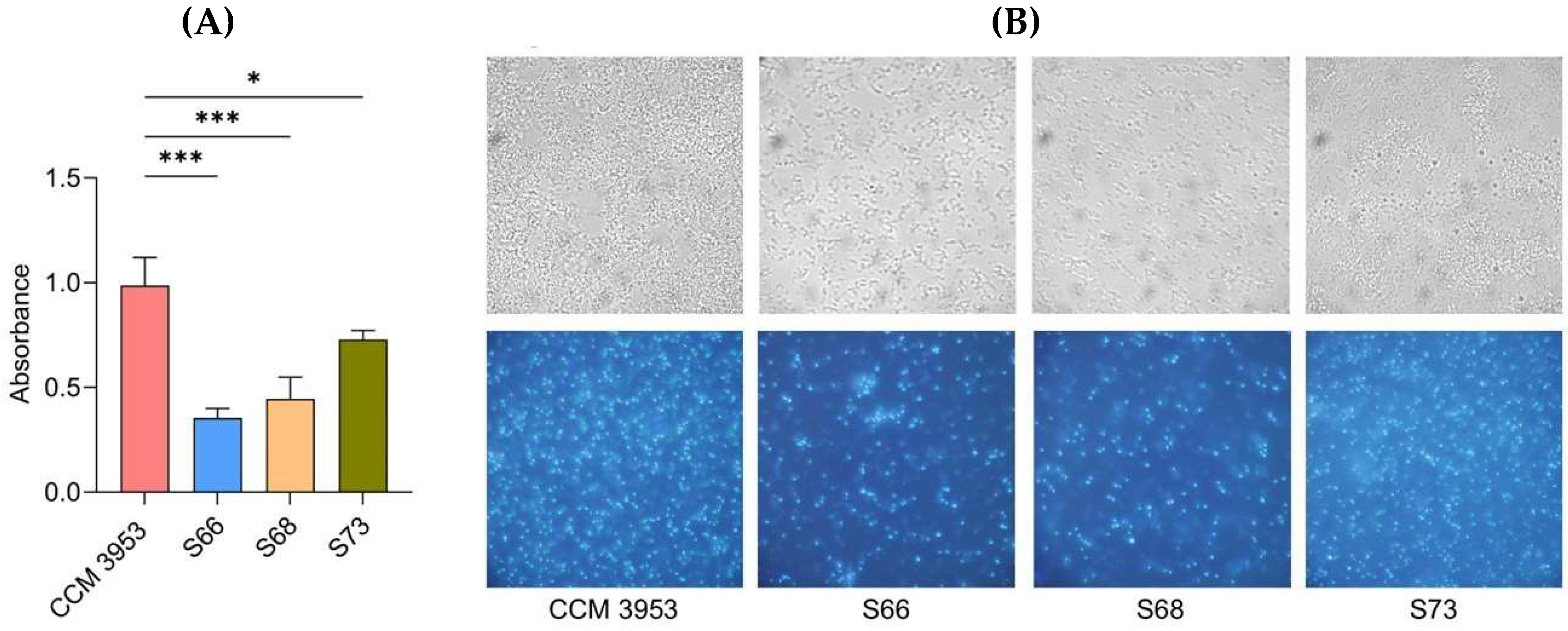
2.1. Activity of the agr QS System and Biofilm Formation
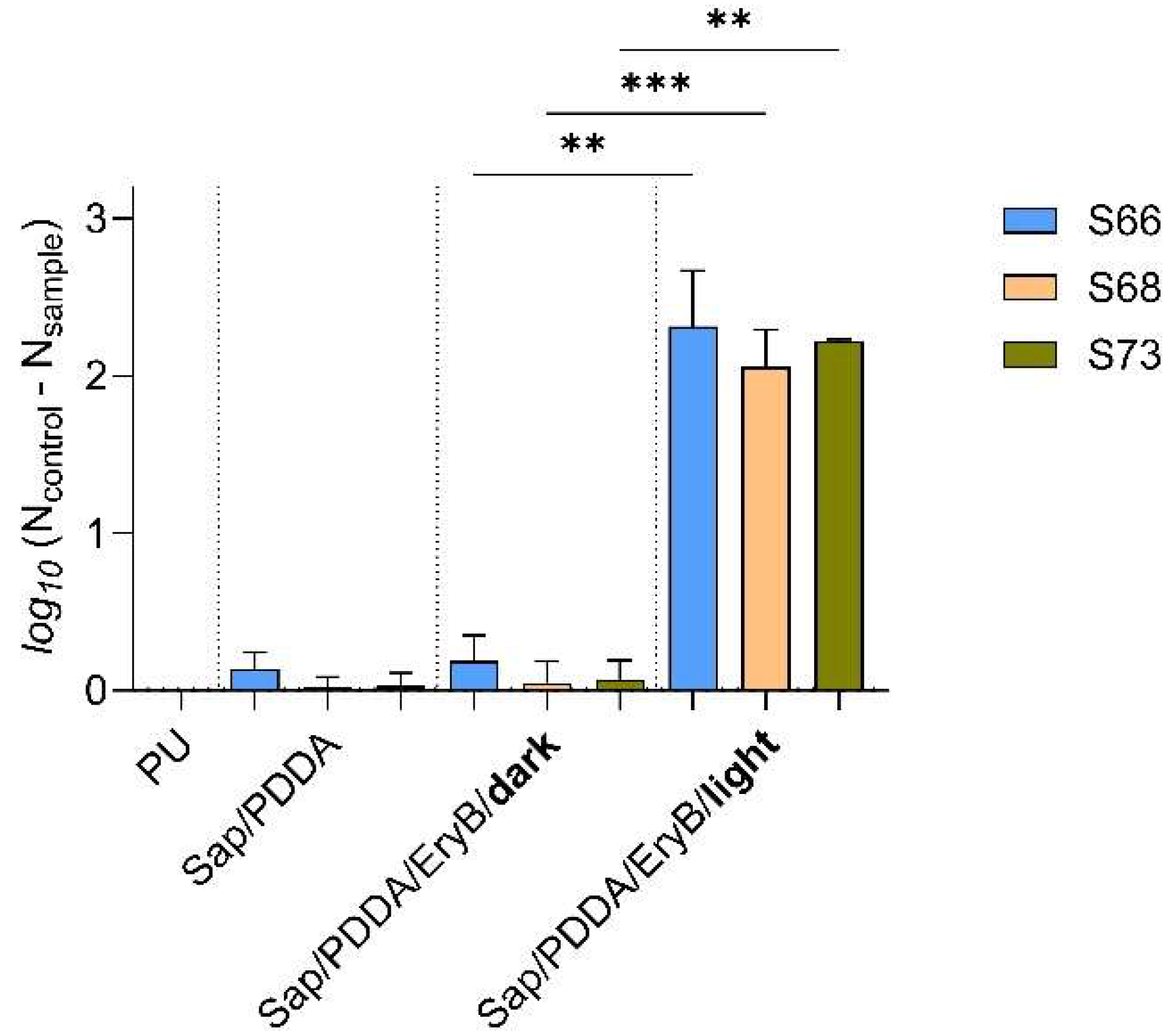
2.2. Anti-Biofilm Effectiveness of Nanocomposite Functionalized with EryB
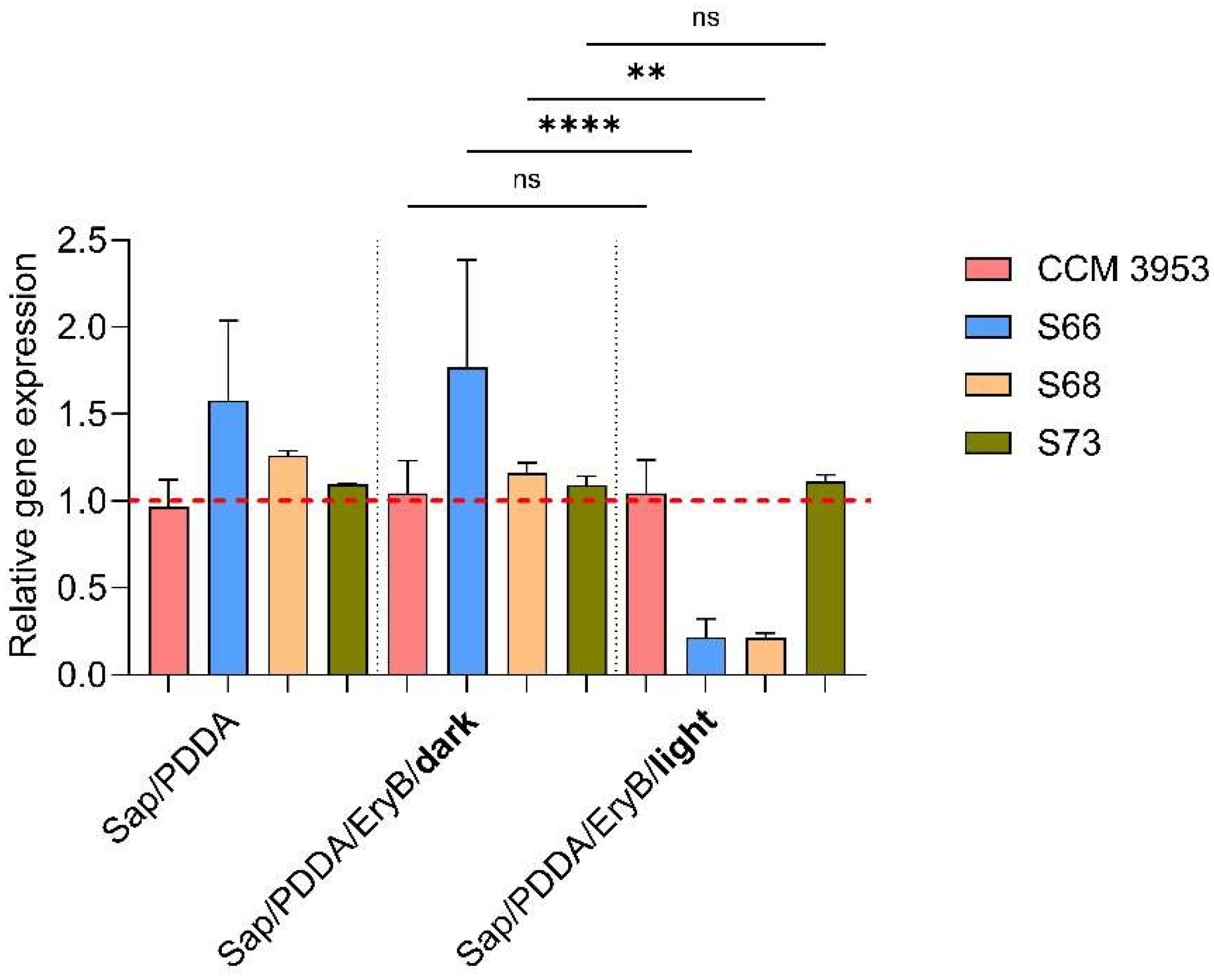
2.3. Analysis of the Effect of Photoactive Nanomaterial on hld Gene Expression Changes
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Microplate-Based Assessment of Biofilm Formation
4.2. Analysis of AGR System Functionality Using the CAMP Test
4.3. Preparation of Nanocomposites and PDI
4.4. Isolation of RNA and qPCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.-S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- McCarthy, H.; Rudkin, J.K.; Black, N.S.; Gallagher, L.; O’Neill, E.; O’Gara, J.P. Methicillin Resistance and the Biofilm Phenotype in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Stryjewski, M.E.; Corey, G.R. Methicillin-Resistant Staphylococcus aureus: An Evolving Pathogen. Clin. Infect. Dis. 2014, 58, S10–S19. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Dayan, G.H.; Mohamed, N.; Scully, I.L.; Cooper, D.; Begier, E.; Eiden, J.; Jansen, K.U.; Gurtman, A.; Anderson, A.S. Staphylococcus aureus: The Current State of Disease, Pathophysiology and Strategies for Prevention. Expert Rev. Vaccines 2016, 15, 1373–1392. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.-E.-A.; Fatima, M.; Zaheer, C.-N.F.; Muneer, A.; Murtaza, M.; et al. MRSA Compendium of Epidemiology, Transmission, Pathophysiology, Treatment, and Prevention within One Health Framework. Front. Microbiol. 2022, 13, 1067284. [Google Scholar] [CrossRef]
- Rasheed, N.; Hussein, N. Staphylococcus aureus: An Overview of Discovery, Characteristics, Epidemiology, Virulence Factors and Antimicrobial Sensitivity. Eur. J. Mol. Clin. Med. 2021, 8, 1160–1183. [Google Scholar]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of Antimicrobial Resistance in Biofilms. npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of Healthcare-Associated Infections Connected to Medical Devices-An Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. MMBR 2020, 84, e00026. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.-F.; Vuong, C.; Otto, M. Staphylococcus Quorum Sensing in Biofilm Formation and Infection. Int. J. Med. Microbiol. 2006, 296, 133–139. [Google Scholar] [CrossRef]
- Podkowik, M.; Perault, A.I.; Putzel, G.; Pountain, A.; Kim, J.; DuMont, A.L.; Zwack, E.E.; Ulrich, R.J.; Karagounis, T.K.; Zhou, C.; et al. Quorum-Sensing Agr System of Staphylococcus aureus Primes Gene Expression for Protection from Lethal Oxidative Stress. eLife 2024, 12, RP89098. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, V.; Kavitha, M. Deciphering Agr Quorum Sensing in Staphylococcus aureus: Insights and Therapeutic Prospects. Mol. Biol. Rep. 2024, 51, 155. [Google Scholar] [CrossRef]
- Boles, B.R.; Horswill, A.R. Agr-Mediated Dispersal of Staphylococcus aureus Biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef]
- Verbeke, F.; De Craemer, S.; Debunne, N.; Janssens, Y.; Wynendaele, E.; Van de Wiele, C.; De Spiegeleer, B. Peptides as Quorum Sensing Molecules: Measurement Techniques and Obtained Levels In Vitro and In Vivo. Front. Neurosci. 2017, 11, 183. [Google Scholar] [CrossRef]
- Le, K.Y.; Otto, M. Quorum-Sensing Regulation in Staphylococci-an Overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef]
- Thoendel, M.; Horswill, A.R. Identification of Staphylococcus aureus AgrD Residues Required for Autoinducing Peptide Biosynthesis. J. Biol. Chem. 2009, 284, 21828–21838. [Google Scholar] [CrossRef]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-Independent Target Gene Control by the Agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef]
- Ji, G.; Beavis, R.C.; Novick, R.P. Cell Density Control of Staphylococcal Virulence Mediated by an Octapeptide Pheromone. Proc. Natl. Acad. Sci. USA 1995, 92, 12055–12059. [Google Scholar] [CrossRef]
- Novick, R.P.; Ross, H.F.; Projan, S.J.; Kornblum, J.; Kreiswirth, B.; Moghazeh, S. Synthesis of Staphylococcal Virulence Factors Is Controlled by a Regulatory RNA Molecule. EMBO J. 1993, 12, 3967–3975. [Google Scholar] [CrossRef]
- Novick, R.P. Autoinduction and Signal Transduction in the Regulation of Staphylococcal Virulence. Mol. Microbiol. 2003, 48, 1429–1449. [Google Scholar] [CrossRef]
- Novick, R.P.; Geisinger, E. Quorum Sensing in Staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef] [PubMed]
- Morfeldt, E.; Taylor, D.; von Gabain, A.; Arvidson, S. Activation of Alpha-Toxin Translation in Staphylococcus aureus by the Trans-Encoded Antisense RNA, RNAIII. EMBO J. 1995, 14, 4569–4577. [Google Scholar] [CrossRef]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Janzon, L.; Löfdahl, S.; Arvidson, S. Identification and Nucleotide Sequence of the Delta-Lysin Gene, Hld, Adjacent to the Accessory Gene Regulator (Agr) of Staphylococcus aureus. Mol. Gen. Genet. 1989, 219, 480–485. [Google Scholar] [CrossRef]
- Janzon, L.; Arvidson, S. The Role of the Delta-Lysin Gene (Hld) in the Regulation of Virulence Genes by the Accessory Gene Regulator (agr) in Staphylococcus aureus. EMBO J. 1990, 9, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Gagnaire, J.; Dauwalder, O.; Boisset, S.; Khau, D.; Freydière, A.-M.; Ader, F.; Bes, M.; Lina, G.; Tristan, A.; Reverdy, M.-E.; et al. Detection of Staphylococcus aureus Delta-Toxin Production by Whole-Cell MALDI-TOF Mass Spectrometry. PLoS ONE 2012, 7, e40660. [Google Scholar] [CrossRef] [PubMed]
- Tegmark, K.; Morfeldt, E.; Arvidson, S. Regulation of Agr-Dependent Virulence Genes in Staphylococcus aureus by RNAIII from Coagulase-Negative Staphylococci. J. Bacteriol. 1998, 180, 3181–3186. [Google Scholar] [CrossRef]
- Benito, Y.; Kolb, F.A.; Romby, P.; Lina, G.; Etienne, J.; Vandenesch, F. Probing the Structure of RNAIII, the Staphylococcus aureus Agr Regulatory RNA, and Identification of the RNA Domain Involved in Repression of Protein A Expression. RNA 2000, 6, 668–679. [Google Scholar] [CrossRef]
- Huntzinger, E.; Boisset, S.; Saveanu, C.; Benito, Y.; Geissmann, T.; Namane, A.; Lina, G.; Etienne, J.; Ehresmann, B.; Ehresmann, C.; et al. Staphylococcus aureus RNAIII and the Endoribonuclease III Coordinately Regulate Spa Gene Expression. EMBO J. 2005, 24, 824–835. [Google Scholar] [CrossRef]
- Painter, K.L.; Krishna, A.; Wigneshweraraj, S.; Edwards, A.M. What Role Does the Quorum-Sensing Accessory Gene Regulator System Play during Staphylococcus aureus Bacteremia? Trends Microbiol. 2014, 22, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Joo, H.-S.; Chatterjee, S.S.; Otto, M. Phenol-Soluble Modulins--Critical Determinants of Staphylococcal Virulence. FEMS Microbiol. Rev. 2014, 38, 698–719. [Google Scholar] [CrossRef]
- Traber, K.E.; Lee, E.; Benson, S.; Corrigan, R.; Cantera, M.; Shopsin, B.; Novick, R.P. Agr Function in Clinical Staphylococcus aureus Isolates. Microbiology 2008, 154, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Fernandes, M.; Laabei, M.; Pagan, N.; Hidalgo, J.; Molinos, S.; Villar Hernandez, R.; Domínguez-Villanueva, D.; Jenkins, A.T.A.; Lacoma, A.; Prat, C. Accessory Gene Regulator (Agr) Functionality in Staphylococcus aureus Derived from Lower Respiratory Tract Infections. PLoS ONE 2017, 12, e0175552. [Google Scholar] [CrossRef]
- Kavanaugh, J.S.; Horswill, A.R. Impact of Environmental Cues on Staphylococcal Quorum Sensing and Biofilm Development. J. Biol. Chem. 2016, 291, 12556–12564. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, I.P.; Dumont, A.L.; James, D.B.A.; Yoong, P.; Saville, B.R.; Soper, N.; Torres, V.J.; Creech, C.B. Children with Invasive Staphylococcus aureus Disease Exhibit a Potently Neutralizing Antibody Response to the Cytotoxin LukAB. Infect. Immun. 2014, 82, 1234–1242. [Google Scholar] [CrossRef]
- He, L.; Le, K.Y.; Khan, B.A.; Nguyen, T.H.; Hunt, R.L.; Bae, J.S.; Kabat, J.; Zheng, Y.; Cheung, G.Y.C.; Li, M.; et al. Resistance to Leukocytes Ties Benefits of Quorum-Sensing Dysfunctionality to Biofilm Infection. Nat. Microbiol. 2019, 4, 1114–1119. [Google Scholar] [CrossRef]
- Pérez, C.; Zúñiga, T.; Palavecino, C.E. Photodynamic Therapy for Treatment of Staphylococcus aureus Infections. Photodiagnosis Photodyn. Ther. 2021, 34, 102285. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Raorane, C.J.; Ishaque, F.; Ahn, Y.-H. Antimicrobial Photodynamic Inactivation of Wastewater Microorganisms by Halogenated Indole Derivative Capped Zinc Oxide. Environ. Res. 2022, 214, 113905. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Szpara, J.; Bartusik-Aebisher, D. Advances in Medicine: Photodynamic Therapy. Int. J. Mol. Sci. 2024, 25, 8258. [Google Scholar] [CrossRef]
- Wood, S.; Metcalf, D.; Devine, D.; Robinson, C. Erythrosine Is a Potential Photosensitizer for the Photodynamic Therapy of Oral Plaque Biofilms. J. Antimicrob. Chemother. 2006, 57, 680–684. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Jiang, L.; Huang, H. Photodynamic Therapy with Photodegradable Photosensitizers. Chem. Commun. 2025, 61, 2627–2635. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Fuchs, B.B.; Coleman, J.J.; Prates, R.A.; Astrakas, C.; St. Denis, T.G.; Ribeiro, M.S.; Mylonakis, E.; Hamblin, M.R.; Tegos, G.P. Concepts and Principles of Photodynamic Therapy as an Alternative Antifungal Discovery Platform. Front. Microbiol. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Light, C.; Salehi, B.; Rogel-Castillo, C.; Victoriano, M.; Martorell, M.; Sharifi-Rad, J.; Martins, N.; Rodrigues, C.F. Novel Therapies for Biofilm-Based Candida Spp. Infections. Adv. Exp. Med. Biol. 2019, 1214, 93–123. [Google Scholar] [CrossRef]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 8, 642609. [Google Scholar] [CrossRef]
- FD&C Red No. 3. 2025. Available online: https://www.fda.gov/food/hfp-constituent-updates/fda-revoke-authorization-use-red-no-3-food-and-ingested-drugs (accessed on 30 May 2025).
- Bugyna, L.; Bilská, K.; Boháč, P.; Pribus, M.; Bujdák, J.; Bujdáková, H. Anti-Biofilm Effect of Hybrid Nanocomposite Functionalized with Erythrosine B on Staphylococcus aureus Due to Photodynamic Inactivation. Molecules 2024, 29, 3917. [Google Scholar] [CrossRef] [PubMed]
- Bilská, K.; Bujdák, J.; Bujdáková, H. Nanocomposite System with Photoactive Phloxine B Eradicates Resistant Staphylococcus aureus. Heliyon 2024, 10, e33660. [Google Scholar] [CrossRef]
- Dadi, N.C.t.; Dohál, M.; Medvecká, V.; Bujdák, J.; Koči, K.; Zahoranová, A.; Bujdáková, H. Physico-Chemical Characterization and Antimicrobial Properties of Hybrid Film Based on Saponite and Phloxine B. Molecules 2021, 26, 325. [Google Scholar] [CrossRef]
- Skoura, E.; Boháč, P.; Barlog, M.; Pálková, H.; Mautner, A.; Bugyna, L.; Bujdáková, H.; Bujdák, J. Structure, Photoactivity, and Antimicrobial Properties of Phloxine B/Poly(Caprolactone) Nanocomposite Thin Films. Appl. Clay Sci. 2023, 242, 107037. [Google Scholar] [CrossRef]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Xiong, J.; Yang, G.-X.; Hu, J.-F.; Zhu, Q.; Chen, Z. Staphylococcus aureus Biofilm: Formulation, Regulatory, and Emerging Natural Products-Derived Therapeutics. Biofilm 2024, 7, 100175. [Google Scholar] [CrossRef] [PubMed]
- Bronesky, D.; Wu, Z.; Marzi, S.; Walter, P.; Geissmann, T.; Moreau, K.; Vandenesch, F.; Caldelari, I.; Romby, P. Staphylococcus aureus RNAIII and Its Regulon Link Quorum Sensing, Stress Responses, Metabolic Adaptation, and Regulation of Virulence Gene Expression. Annu. Rev. Microbiol. 2016, 70, 299–316. [Google Scholar] [CrossRef]
- Boisset, S.; Geissmann, T.; Huntzinger, E.; Fechter, P.; Bendridi, N.; Possedko, M.; Chevalier, C.; Helfer, A.C.; Benito, Y.; Jacquier, A.; et al. Staphylococcus aureus RNAIII Coordinately Represses the Synthesis of Virulence Factors and the Transcription Regulator Rot by an Antisense Mechanism. Genes Dev. 2007, 21, 1353–1366. [Google Scholar] [CrossRef]
- Murray, E.J.; Williams, P. Detection of Agr-Type Autoinducing Peptides Produced by Staphylococcus aureus. In Quorum Sensing; Leoni, L., Rampioni, G., Eds.; Springer: New York, NY, USA, 2018; Volume 1673, pp. 89–96. ISBN 978-1-4939-7308-8. [Google Scholar]
- Garapati, C.; Clarke, B.; Zadora, S.; Burney, C.; Cameron, B.D.; Fournier, R.; Baugh, R.F.; Boddu, S.H.S. Development and Characterization of Erythrosine Nanoparticles with Potential for Treating Sinusitis Using Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2015, 12, 9–18. [Google Scholar] [CrossRef]
- Yarwood, J.M.; Bartels, D.J.; Volper, E.M.; Greenberg, E.P. Quorum Sensing in Staphylococcus aureus Biofilms. J. Bacteriol. 2004, 186, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Chen, J.; Drlica, K.; Shopsin, B. Tuning of the Lethal Response to Multiple Stressors with a Single-Site Mutation during Clinical Infection by Staphylococcus aureus. mBio 2017, 8, e01476-17. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Liang, H.; Kong, X.; Xie, S.; Cho, H.; Deng, X.; Ji, Q.; Zhang, H.; Alvarez, S.; Hicks, L.M.; et al. Quorum-Sensing Agr Mediates Bacterial Oxidation Response via an Intramolecular Disulfide Redox Switch in the Response Regulator AgrA. Proc. Natl. Acad. Sci. USA 2012, 109, 9095–9100. [Google Scholar] [CrossRef]
- Baković, J.; Yu, B.Y.K.; Silva, D.; Baczynska, M.; Peak-Chew, S.Y.; Switzer, A.; Burchell, L.; Wigneshweraraj, S.; Vandanashree, M.; Gopal, B.; et al. Redox Regulation of the Quorum-Sensing Transcription Factor AgrA by Coenzyme A. Antioxidants 2021, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Hendiani, S.; Pornour, M.; Kashef, N. Sub-Lethal Antimicrobial Photodynamic Inactivation: An in Vitro Study on Quorum Sensing-Controlled Gene Expression of Pseudomonas Aeruginosa Biofilm Formation. Lasers Med. Sci. 2019, 34, 1159–1165. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Mahmoudi, H.; Rezaei-soufi, L.; Alikhani, M.Y.; Bahador, A. Potentiation Effects of Antimicrobial Photodynamic Therapy on Quorum Sensing Genes Expression: A Promising Treatment for Multi-Species Bacterial Biofilms in Burn Wound Infections. Photodiagn. Photodyn. Ther. 2020, 30, 101717. [Google Scholar] [CrossRef]
- Park, H.J.; Moon, Y.; Yoon, H.; Park, Y.M.; Yoon, J.; Bang, I.S. Agr Function Is Upregulated by Photodynamic Therapy for Staphylococcus aureus and Is Related to Resistance to Photodynamic Therapy. Microbiol. Immunol. 2013, 57, 547–552. [Google Scholar] [CrossRef]
- George, S.E.; Hrubesch, J.; Breuing, I.; Vetter, N.; Korn, N.; Hennemann, K.; Bleul, L.; Willmann, M.; Ebner, P.; Götz, F.; et al. Oxidative Stress Drives the Selection of Quorum Sensing Mutants in the Staphylococcus aureus Population. Proc. Natl. Acad. Sci. USA 2019, 116, 19145–19154. [Google Scholar] [CrossRef]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2005, 22, 1B-1. [Google Scholar] [CrossRef]
- Gaálová-Radochová, B.; Kendra, S.; Jordao, L.; Kursawe, L.; Kikhney, J.; Moter, A.; Bujdáková, H. Effect of Quorum Sensing Molecule Farnesol on Mixed Biofilms of Candida Albicans and Staphylococcus aureus. Antibiotics 2023, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Theis, T.; Skurray, R.A.; Brown, M.H. Identification of Suitable Internal Controls to Study Expression of a Staphylococcus aureus Multidrug Resistance System by Quantitative Real-Time PCR. J. Microbiol. Methods 2007, 70, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugyna, L.; Švantner, Ľ.; Bilská, K.; Pribus, M.; Bujdáková, H. Impact of Light-Activated Nanocomposite with Erythrosine B on agr Quorum Sensing System in Staphylococcus aureus. Antibiotics 2025, 14, 1010. https://doi.org/10.3390/antibiotics14101010
Bugyna L, Švantner Ľ, Bilská K, Pribus M, Bujdáková H. Impact of Light-Activated Nanocomposite with Erythrosine B on agr Quorum Sensing System in Staphylococcus aureus. Antibiotics. 2025; 14(10):1010. https://doi.org/10.3390/antibiotics14101010
Chicago/Turabian StyleBugyna, Larysa, Ľubomír Švantner, Katarína Bilská, Marek Pribus, and Helena Bujdáková. 2025. "Impact of Light-Activated Nanocomposite with Erythrosine B on agr Quorum Sensing System in Staphylococcus aureus" Antibiotics 14, no. 10: 1010. https://doi.org/10.3390/antibiotics14101010
APA StyleBugyna, L., Švantner, Ľ., Bilská, K., Pribus, M., & Bujdáková, H. (2025). Impact of Light-Activated Nanocomposite with Erythrosine B on agr Quorum Sensing System in Staphylococcus aureus. Antibiotics, 14(10), 1010. https://doi.org/10.3390/antibiotics14101010






