Establishing Priority Pediatric Antimicrobial Stewardship Interventions in the US: Findings from a Delphi Consensus Study
Abstract
1. Introduction
2. Results
2.1. Round 1—Initial Idea Generation
2.2. Round 2
2.3. Round 3
2.4. Round 4
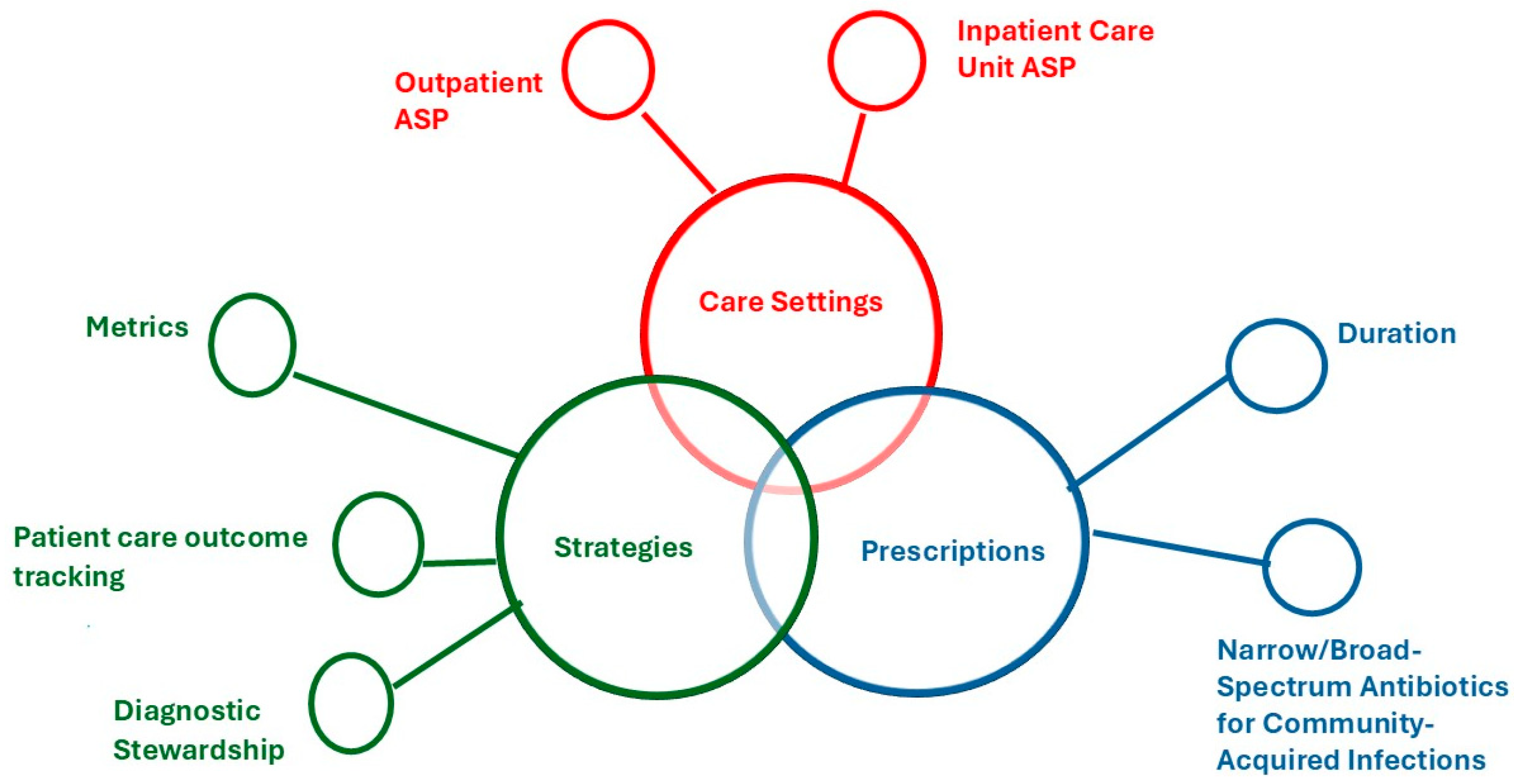
2.4.1. Care Settings
ASP in the ICU
Outpatient ASP
2.4.2. Prescriptions
Duration
Narrow/Broad-Spectrum Antibiotics for Community-Acquired Infections
2.4.3. Strategies
Metrics
Patient Care Outcome Tracking
Diagnostic Stewardship
3. Discussion
3.1. Strategies—Meaningful Metrics and Patient Outcomes
3.2. Diagnostic Stewardship
3.3. Prescribing Targets—Treatment Duration and Community-Acquired Infections
3.4. Care Settings—ICU and Outpatient ASP
3.5. Limitations
4. Methods
4.1. Overview
4.2. Recruitment of Participants
4.3. Data Collection
4.3.1. First Round Delphi—Initial Idea Generation
4.3.2. Second Round Delphi—Prioritization
4.3.3. Third Round of Delphi—Refinement
4.3.4. Round 4—Final Consensus and Discussion
5. Conclusions
6. Contributions to Literature
- This study has the potential to significantly influence stewardship practices and enhance outcomes for children facing the increasing threat of antimicrobial resistance, as it is among the first to systematically prioritize pediatric ASP interventions through expert consensus.
- The recommendations that follow will bridge the gap between the conceptual underpinnings of antimicrobial stewardship and their application in pediatric healthcare settings.
- By establishing key priorities, this research lays the groundwork for future empirical studies, including interventional trials and implementation science research, to assess the effectiveness of identified ASP strategies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| ASP | Antimicrobial Stewardship Program |
| ID | Infectious Disease |
| ICU | Intensive Care Unit |
| CAP | Community-Acquired Pneumonia |
| OPerAtiC | Optimizing PERioperative AnTibiotICs |
| DOT | Days of Therapy |
| ECMO | Extracorporeal Membrane Oxygenation |
References
- Bamford, A.; Masini, T.; Williams, P.; Sharland, M.; Gigante, V.; Dixit, D.; Sati, H.; Huttner, B.; Bin Nisar, Y.; Cappello, B.; et al. Tackling the threat of antimicrobial resistance in neonates and children: Outcomes from the first WHO-convened Paediatric Drug Optimisation exercise for antibiotics. Lancet Child Adolesc. Health 2024, 8, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Sridhar, D.; Blaser, M.; Wang, M.; Woolhouse, M. Achieving global targets for antimicrobial resistance. Science 2016, 353, 874–875. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations (2016). Review on Antimicrobial Resistance. Available online: https://amr-review.org/sites/default/files/160518_Final paper_with cover.pdf (accessed on 3 July 2025).
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Hu, Y.; Harwell, J. Global trends and impact of antimicrobial resistance in paediatric populations: An analysis using WHO AWaRe classification and priority pathogens. In Proceedings of the ESCMID Global 2025, Vienna, Austria, 11–15 April 2025. [Google Scholar]
- Gandra, S.; Mojica, N.; Klein, E.Y.; Ashok, A.; Nerurkar, V.; Kumari, M.; Ramesh, U.; Dey, S.; Vadwai, V.; Das, B.R.; et al. Trends in antibiotic resistance among major bacterial pathogens isolated from blood cultures tested at a large private laboratory network in India, 2008–2014. Int. J. Infect. Dis. 2016, 50, 75–82. [Google Scholar] [CrossRef]
- Bielicki, J.; Lundin, R.; Patel, S.; Paulus, S. Antimicrobial stewardship for neonates and children: A global approach. Pediatr. Infect. Dis. J. 2015, 34, 311–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chai, G.; Governale, L.; McMahon, A.W.; Trinidad, J.P.; Staffa, J.; Murphy, D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics 2012, 130, 23–31. [Google Scholar] [CrossRef] [PubMed]
- McMullan, B.J.; Hall, L.; James, R.; Mostaghim, M.; Jones, C.A.; Konecny, P.; Blyth, C.C. Antibiotic appropriateness and guideline adherence in hospitalized children: Results of a nationwide study. J. Antimicrob. Chemother. 2020, 75, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Pulcini, C.; Binda, F.; Lamkang, A.S.; Trett, A.; Charani, E.; Goff, D.; Harbarth, S.; Hinrichsen, S.; Levy-Hara, G.; Mendelson, M.; et al. Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: A consensus approach. Clin. Microbiol. Infect. 2019, 25, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Newland, J.G.; Banerjee, R.; Gerber, J.S.; Hersh, A.L.; Steinke, L.; Weissman, S.J. Antimicrobial stewardship in pediatric care: Strategies and future directions. Pharmacotherapy 2012, 32, 735–743. [Google Scholar] [CrossRef]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 4, CD003543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okoli, C.; Pawlowski, S.D. The Delphi method as a research tool: An example, design considerations and applications. Inf. Manag. 2004, 42, 15–29. [Google Scholar] [CrossRef]
- Varndell, W.; Fry, M.; Elliott, D. Applying real-time Delphi methods: Development of a pain management survey in emergency nursing. BMC Nurs. 2021, 20, 149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoffelen, T.; Schouten, J.; Hoogerwerf, J.; Quirós, A.M.; May, L.S.; Oever, J.T.; Hulscher, M.E. Quality indicators for appropriate antimicrobial therapy in the emergency department: A pragmatic Delphi procedure. Clin. Microbiol. Infect. 2021, 27, 210–214. [Google Scholar] [CrossRef]
- Schwartz, K.L.; Xu, A.X.T.; Alderson, S.; Bjerrum, L.; Brehaut, J.; Brown, B.C.; Bucher, H.C.; De Sutter, A.; Francis, N.; Grimshaw, J.; et al. Best practice guidance for antibiotic audit and feedback interventions in primary care: A modified Delphi study from the Joint Programming Initiative on Antimicrobial resistance: Primary Care Antibiotic Audit and Feedback Network (JPIAMR-PAAN). Antimicrob. Resist. Infect. Control. 2023, 12, 72. [Google Scholar] [CrossRef]
- Dik, J.-W.H.; Hendrix, R.; Poelman, R.; Niesters, H.G.; Postma, M.J.; Sinha, B.; Friedrich, A.W. Measuring the impact of antimicrobial stewardship programs. Expert Rev. Anti Infect. Ther. 2016, 14, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Drekonja, D.M.; Filice, G.A.; Greer, N.; Olson, A.; MacDonald, R.; Rutks, I.; Wilt, T.J. Antimicrobial stewardship in outpatient settings: A systematic review. Infect. Control. Hosp. Epidemiol. 2015, 36, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.L.; Hall, M.; Shah, S.S.; Burns, A.; Goldman, J.L. Antibiotic Diversity Index: A novel metric to assess antibiotic variation among hospitalized children. J. Hosp. Med. 2025, 20, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Moehring, R.W.; Anderson, D.J.; Cochran, R.L.; Hicks, L.A.; Srinivasan, A.; Dodds Ashley, E.S. Structured Taskforce of Experts Working at Reliable Standards for Stewardship (STEWARDS) Panel. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin. Infect. Dis. 2017, 64, 377–383. [Google Scholar] [CrossRef]
- Brotherton, A.L. Metrics of Antimicrobial Stewardship Programs. Med. Clin. N. Am. 2018, 102, 965–976. [Google Scholar] [CrossRef]
- Versporten, A.; Gyssens, I.C.; Pulcini, C.; A Monnier, A.; Schouten, J.; Milanič, R.; Benić, M.S.; Tebano, G.; Le Maréchal, M.; Zanichelli, V.; et al. Metrics to assess the quantity of antibiotic use in the outpatient setting: A systematic review followed by an international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 2018, 73, vi59–vi66. [Google Scholar] [CrossRef] [PubMed]
- Coffin, S.E.; Abanyie, F.; Bryant, K.; Cantey, J.; Fiore, A.; Fritz, S.; Guzman-Cottrill, J.; Hersh, A.L.; Huskins, W.C.; Kociolek, L.K.; et al. Pediatric research priorities in healthcare-associated infections and antimicrobial stewardship. Infect. Control. Hosp. Epidemiol. 2021, 42, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Parker, S.K.; Todd, J.K.; Dominguez, S.R. Implementation of Rapid Molecular Infectious Disease Diagnostics: The Role of Diagnostic and Antimicrobial Stewardship. J. Clin. Microbiol. 2017, 55, 715. [Google Scholar] [CrossRef]
- Sick-Samuels, A.C.; Woods-Hill, C. Diagnostic Stewardship in the Pediatric Intensive Care Unit. Infect. Dis. Clin. N. Am. 2022, 36, 203–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claeys, K.C.; Johnson, M.D. Leveraging diagnostic stewardship within antimicrobial stewardship programmes. Drugs Context 2023, 12, 2022-9-5. [Google Scholar] [CrossRef]
- Williams, D.J.; Creech, C.B.; Walter, E.B.; Martin, J.M.; Gerber, J.S.; Newland, J.G.; Howard, L.; Hofto, M.E.; Staat, M.A.; Oler, R.E.; et al. Short- vs Standard-Course Outpatient Antibiotic Therapy for Community-Acquired Pneumonia in Children: The SCOUT-CAP Randomized Clinical Trial. JAMA Pediatr. 2022, 176, 253–261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.; Liu, M.; Yang, K.; Zhao, Y.; Tian, J.; Pernica, J.M.; Guyatt, G. Shorter Versus Longer-term Antibiotic Treatments for Community-Acquired Pneumonia in Children: A Meta-analysis. Pediatrics 2023, 151, e2022060097. [Google Scholar] [CrossRef]
- Same, R.G.; Amoah, J.; Hsu, A.J.; Hersh, A.L.; Sklansky, D.J.; E Cosgrove, S.; Tamma, P.D. The Association of Antibiotic Duration with Successful Treatment of Community-Acquired Pneumonia in Children. J. Pediatr. Infect. Dis. Soc. 2021, 10, 267–273. [Google Scholar] [CrossRef]
- Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H.; ARPEC Project Group; Calle, G.M.; Garrahan, J.P.; Clark, J.; Cooper, C.; et al. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: Developing hospital-quality indicators of antibiotic prescribing for children. J. Antimicrob. Chemother. 2016, 71, 1106–1117. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events with Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.S.; Jackson, M.A.; Tamma, P.D.; Zaoutis, T.E. Antibiotic Stewardship in Pediatrics. Pediatrics 2021, 147, e2020040295. [Google Scholar] [CrossRef]
- Gerber, J.S.; Prasad, P.A.; Fiks, A.G.; Localio, A.R.; Grundmeier, R.W.; Bell, L.M.; Wasserman, R.C.; Keren, R.; Zaoutis, T.E. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: A randomized trial. JAMA 2013, 309, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.; McKay, V.R.; Krucylak, C.; Powell, B.J.; Liu, J.; Terrill, C.; Saito, J.M.; Rangel, S.J.; Newland, J.G. A cluster randomized stepped-wedge trial to de-implement unnecessary post-operative antibiotics in children: The optimizing perioperative antibiotic in children (OPerAtiC) trial. Implement. Sci. 2021, 16, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diamond, I.R.; Grant, R.C.; Feldman, B.M.; Pencharz, P.B.; Ling, S.C.; Moore, A.M.; Wales, P.W. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J. Clin. Epidemiol. 2014, 67, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Sandford, B.A. The Delphi Technique: Making Sense of Consensus. Pract. Assess. Res. Eval. 2007, 12, 10. [Google Scholar] [CrossRef]

| Category | Participants (N/%) |
|---|---|
| Role | |
| Infectious Diseases Physician | 10 (50%) |
| Pharmacist | 8 (40%) |
| Other—Medication Safety Coordinator (Former ASP/ID) | 1 (5%) |
| Years of Experience | |
| 5–10 | 8 (40%) |
| 11–20 | 10 (50%) |
| More than 20 | 1 (5%) |
| Race | |
| White | 15 (75%) |
| Hispanic/Latin | 2 (10%) |
| Asian | 2 (10%) |
| Gender | |
| Male | 9 (45%) |
| Female | 10 (50%) |
| Total with demographic data | 19 (95%) |
| Missing demographic data | 1 (5%) |
| Themes Generated | Responses from Participants (Priority Items) |
|---|---|
| 1. Care Settings |
|
| 2. Prescriptions |
|
| 3a. Clinical Practice Strategies |
|
| 3b. Programmatic and System-Level Strategies |
|
| Themes | Priority Items | Votes (N) | Consensus (%) |
|---|---|---|---|
| 1. Care Settings | Outpatient ASP | 13 | 65 |
| Inpatient Care Unit ASP | 0 | 0 | |
| Urgent Care ASP | 2 | 10 | |
| ICU ASP | 6 | 30 | |
| 2. Prescriptions | Duration | 17 | 85 |
| Narrow/Broad Spectrum for community-acquired infections | 5 | 25 | |
| Discharge antibiotics (IV to PO transition) | 1 | 0.5 | |
| Dose | 0 | 0 | |
| Pathogen-drug mismatches | 0 | 0 | |
| 3a. Clinical Practice Strategies | Novel methods of stewardship other than prospective audit with feedback | 2 | 10 |
| Resource waste (environmental impact) | 2 | 10 | |
| Clinical pathway or practice guidelines development | 2 | 10 | |
| Improve consensus/goals of stewardship to the ID division as a whole | 2 | 10 | |
| Beta-lactam allergy de-labeling | 1 | 0.5 | |
| Antimicrobial stewardship in immunocompromised hosts | 1 | 0.5 | |
| Initiation of broad-spectrum antibiotics | 0 | 0 | |
| 3b. Programmatic and System-Level Strategies | Metrics | 10 | 50 |
| Executive leadership involvement | 5 | 25 | |
| Diagnostic stewardship | 4 | 20 | |
| Patient care outcomes following | 3 | 15 | |
| Burnout amongst antimicrobial stewardship clinicians, especially pharmacists | 3 | 15 | |
| More granular antibiotic measures that can help programs direct activities to outlier services, units, diagnoses, and antibiotics | 3 | 15 | |
| Relationship building | 2 | 10 | |
| Antibiotic harm index | 1 | 0.5 | |
| De-escalation | 0 | 0 | |
| Continued surgical prophylaxis | 0 | 0 |
| Theme | Priority Item | Votes (N) | Consensus (%) |
|---|---|---|---|
| Strategies | Metrics | 13 | 65 |
| Patient care outcome tracking | 2 | 10 | |
| Diagnostic Stewardship | 2 | 10 | |
| Executive Leadership involvement | 0 | 0 | |
| Burnout amongst antimicrobial stewardship clinicians, especially among pharmacists. | 0 | 0 |
| Theme | Priority Item/Subtheme | Number | Supporting Quote |
|---|---|---|---|
| Care Settings | ICU ASP | 1.1 | …so we have ICU stewardship up there, which I think is critically important due to the high level of clinical ambiguity in this setting. Patients are often complex, and stewardship decisions aren’t always straightforward. There’s rarely a single clear path. Yet the stakes are extremely high. For example, managing a multidrug-resistant organism in a patient on ECMO (extracorporeal membrane oxygenation) presents real challenges. This complexity makes the ICU a key area that deserves focused attention. |
| 1.2 | Going back to another’s comment about the invisibility of harm to patients, which I completely agree with, I’d tie that back to the ICU. Our ICU physicians are typically on for seven days, so when a patient develops C. difficile two weeks later, they never see it. And I’d go even further: a lot of that harm is invisible to us as well. We often only recognize it when ID is consulted later, or the patient is started on another antibiotic. Then a stewardship pharmacist goes back, reviews the case history, and realizes, ‘Oh, they were on antibiotics for (a viral infection)—and that’s what led down this path. | ||
| Outpatient ASP | 1.3 | Outpatient stewardship is critically important because it encompasses such a large and diverse portion of antibiotic prescribing in the U.S., from urgent care clinics to telehealth visits. It represents a substantial slice of overall antibiotic use and addressing it is essential. Many in this field are actively working on it, and it will remain a necessary area of focus. Outpatient stewardship requires approaches that account for the unique characteristics of prescribing in these settings | |
| Prescriptions | Duration | 1.4 | Mean duration really matters partly because we rarely agree on what it should be. We know that shorter is generally better, and we have evidence that many conditions don’t need 10 or 14 days; some need 7, 5, or even just 3 days. But the problem is especially noticeable in the outpatient setting, where there’s a lack of clear guidelines, and urgent care providers may not be up to date with the latest studies. We’re still seeing community-acquired pneumonia treated for 10 or 14 days, often with the wrong antibiotic. Another issue comes at hospital discharge where sometimes a patient gets four days of antibiotics in the hospital, only needs one more, but gets sent home with a five-day prescription. So, what was intended as a five-day course unintentionally becomes 9 or 10 days. It’s a common and preventable problem. |
| 1.5 | One of the appealing things about duration is that it’s a number and it’s concrete. It becomes the vessel that holds other antibiotic decisions, like whether to use a narrow- or broad-spectrum agent, or to go oral versus IV. Duration feels more measurable, more tangible. Thinking about it is feasible and desirable. It’s something I am motivated to work on. | ||
| Narrow/Broad-Spectrum Antibiotics for Community- Acquired Infections | 1.6 | One of the challenges we face is demonstrating that longer antibiotic durations are actually harmful. That’s why I often refer to (a) study on adverse effects. They took the extra step of calling patients directly. It showed that those who received broad-spectrum antibiotics experienced more adverse effects, but even among patients who received narrow-spectrum, first-line therapy, about 25% still reported side effects. These might not have resulted in hospital visits, but they were distressing to the patient. That matters. | |
| Strategies | Metrics | 1.7 | I think the biggest issue is actionable metrics. Working with DOT (days of therapy) per 1000 patient-days doesn’t tell us much, especially in aggregate. We need metrics that are more insightful and help guide program activities. I think about metrics in three categories: process measures, outcome measures, and - what we often overlook - program metrics. That third category is really about how our stewardship program is performing compared to others. We’ve worked on an impact scoring tool, and in trying to standardize it, we found that programs track their day-to-day interventions very differently, like whether they narrow, broaden, or stop therapy. So, I think standardizing how we track those interventions could really help us evaluate and improve stewardship across programs. |
| 1.8 | I think we need to restructure the way we think about antibiotics. Starting a patient on vancomycin without a clear need—that’s a near miss. They could develop an acute kidney injury, and we don’t treat it like a safety issue, but we should. I see a lot of parallels between stewardship and patient safety work. If we start framing unnecessary antibiotic use as a safety event, something that could cause real harm, I think we’d see more engagement. From a metrics standpoint, I really like the idea of an antibiotic harm index. It’s marketable, and it helps shift the conversation. We need to talk more about antibiotics in terms of safety, that’s probably what will get people to pay attention. | ||
| Patient Care Outcome Tracking | 1.9 | We have a lot of mixed data on the direct correlation between antibiotic exposure and resistance, or between exposure and adverse effects, especially when comparing five, seven, or ten-day courses. I think we need to dig deeper and focus on outcomes that truly matter to patients. For example, a study might show no difference in the incidence of diarrhea between five and ten days, but what about the number of days a patient had diarrhea? That often isn’t assessed. So, I would love to see just more of us do a little better job of trying to make a compelling argument that actually matters to the patients getting the antibiotics | |
| Diagnostic Stewardship | 2.0 | Before working in ID, I was in the ICU, and it’s interesting. While we often talk about ICU teams struggling with antibiotic use, they also have concerns about how we in ID order diagnostic tests. I think there’s a real opportunity for us to steward our own diagnostic practices, being more thoughtful about the cost of care and asking what we’ll actually do with the results. This is an area where we might have more control compared to others. There are certain tests, like some syndromic panels, that are being overused, and I’ve seen firsthand how quickly things can spiral. We need to set an example, because when we’re not careful with our own diagnostic decisions, it can influence and even amplify misuse by others. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obeng, H.; Tetteh, E.; Malone, S.; Walsh, L.; Walsh, T.; Bula-Rudas, F.J.; Banerjee, R.; Brothers, A.W.; Herigon, J.C.; Namtu, K.; et al. Establishing Priority Pediatric Antimicrobial Stewardship Interventions in the US: Findings from a Delphi Consensus Study. Antibiotics 2025, 14, 1011. https://doi.org/10.3390/antibiotics14101011
Obeng H, Tetteh E, Malone S, Walsh L, Walsh T, Bula-Rudas FJ, Banerjee R, Brothers AW, Herigon JC, Namtu K, et al. Establishing Priority Pediatric Antimicrobial Stewardship Interventions in the US: Findings from a Delphi Consensus Study. Antibiotics. 2025; 14(10):1011. https://doi.org/10.3390/antibiotics14101011
Chicago/Turabian StyleObeng, Harry, Emmanuel Tetteh, Sara Malone, Lauren Walsh, Tyler Walsh, Fernando J. Bula-Rudas, Ritu Banerjee, Adam W. Brothers, Joshua C. Herigon, Katie Namtu, and et al. 2025. "Establishing Priority Pediatric Antimicrobial Stewardship Interventions in the US: Findings from a Delphi Consensus Study" Antibiotics 14, no. 10: 1011. https://doi.org/10.3390/antibiotics14101011
APA StyleObeng, H., Tetteh, E., Malone, S., Walsh, L., Walsh, T., Bula-Rudas, F. J., Banerjee, R., Brothers, A. W., Herigon, J. C., Namtu, K., Weissman, S., Riggsbee, D., Olson, J., Palazzi, D. L., Wirtz, A., Sattler, M., Tansmore, J., Rodriguez, B. A., Abdelnour, M., ... McKay, V. R. (2025). Establishing Priority Pediatric Antimicrobial Stewardship Interventions in the US: Findings from a Delphi Consensus Study. Antibiotics, 14(10), 1011. https://doi.org/10.3390/antibiotics14101011







