Using Dalbavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) in Obese Patients: A Real-Life, Single-Center Observational Study
Abstract
1. Introduction
2. Results
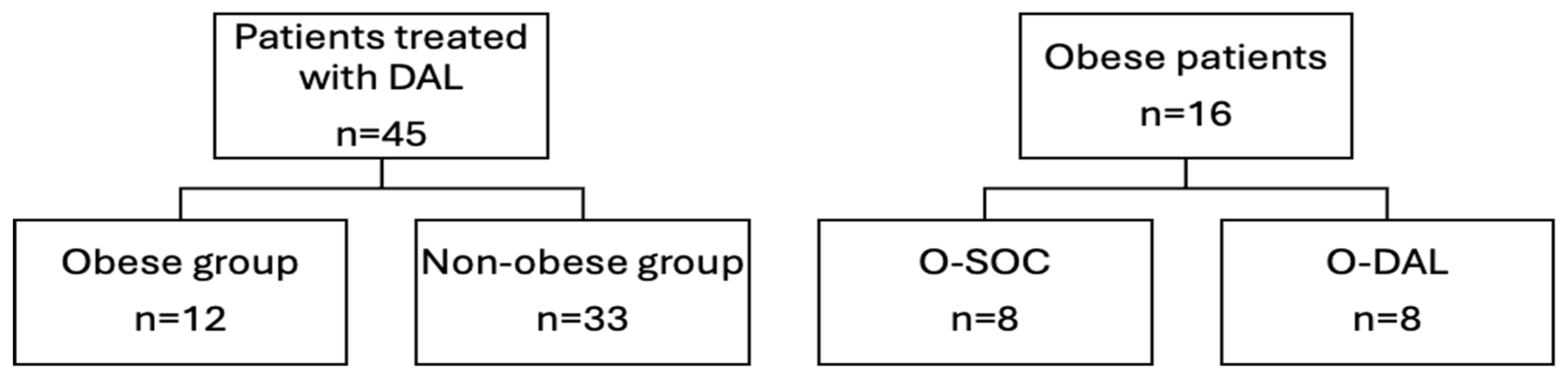
2.1. General Characteristics of Study Population
2.2. Comparison Between Obese and Non-Obese Patients
2.3. Comparison Between DAL and SOC in Obese Patients with ABSSSI Alone
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Definitions
4.3. Antimicrobial Regimens
4.4. Data Collection
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huttunen, R.; Syrjanen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Berghöfer, A.; Pischon, T.; Reinhold, T.; Apovian, C.M.; Sharma, A.M.; Willich, S.N. Obesity prevalence from a European perspective: A systematic review. BMC Public Health 2008, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Health Risks—Overweight or Obese Population—OECD Data, the OECD. Available online: http://data.oecd.org/healthrisk/overweight-or-obese-population.htm (accessed on 10 June 2024).
- Claeys, K.C.; Zasowski, E.J.; Lagnf, A.M.; Levine, D.P.; Davis, S.L.; Rybak, M.J. Novel application of published risk factors for methicillin-resistant S. aureus in acute bacterial skin and skin structure infections. Int. J. Antimicrob. Agents 2018, 51, 43–46. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, P.B.; Jenkins, T.C.; Stenehjem, E.; Doron, S.; Brown, J.; Goldwater, S.H.; Lopes, C.; Haynes, A.; Udeze, C.; Mo, Y.; et al. Impact of Outpatient vs Inpatient ABSSSI Treatment on Outcomes: A Retrospective Observational Analysis of Medical Charts Across US Emergency Departments. Open Forum Infect. Dis. 2018, 5, ofy109. [Google Scholar] [CrossRef] [PubMed]
- Carratalà, J.; Rosón, B.; Fernández-Sabé, N.; Shaw, E.; del Rio, O.; Rivera, A.; Gudiol, F. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2003, 22, 151–157. [Google Scholar] [CrossRef] [PubMed]
- FDA. Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment; Published Online; Food and Drug Administration: Silver Spring, MD, USA, 2013.
- Pollack, C.V.; Amin, A.; Ford, W.T.; Finley, R.; Kaye, K.S.; Nguyen, H.H.; Rybak, M.J.; Talan, D. Acute bacterial skin and skin structure infections (ABSSSI): Practice guidelines for management and care transitions in the emergency department and hospital. J. Emerg. Med. 2015, 48, 508–519. [Google Scholar] [CrossRef]
- Conway, E.L.; Sellick, J.A.; Kurtzhalts, K.; Mergenhagen, K.A. Obesity and Heart Failure as Predictors of Failure in Outpatient Skin and Soft Tissue Infections. Antimicrob. Agents Chemother. 2017, 61, e02389-16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Moore, E.; Bousfield, R. OPAT for cellulitis: Its benefits and the factors that predispose to longer treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Flegal, K.M. Criteria for definition of overweight in transition: Background and recommendations for the United States. Am. J. Clin. Nutr. 2000, 72, 1074–1081. [Google Scholar] [CrossRef]
- Riccobene, T.; Lock, J.; Lyles, R.D.; Georgiades, B.; Nowak, M.; Gonzalez, P.L.; Park, J.; Rappo, U. Dalbavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infection in Patients With Obesity or Diabetes: A Subgroup Analysis of Pooled Phase 3 Clinical Trials. Open Forum Infect. Dis. 2023, 10, ofad256. [Google Scholar] [CrossRef]
- Pai, M.P. Anti-infective Dosing for Obese Adult Patients: A Focus on Newer Drugs to Treat Methicillin-resistant Staphylococcus aureus Acute Bacterial Skin and Skin Structure Infections. Clin. Ther. 2016, 38, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection—From underweight to obesity. Clin. Microbiol. Infect. Clin. Microbiol. Infect. Dis. 2018, 24, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Grupper, M.; Nicolau, D.P. Obesity and skin and soft tissue infections: How to optimize antimicrobial usage for prevention and treatment? Curr. Opin. Infect. Dis. 2017, 30, 180–191. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/product-information/xydalba-epar-product-information_en.pdf (accessed on 11 June 2024).
- Oliva, A.; Carbonara, S.; Cianci, V.; Crapis, M.; Di Domenico, E.G.; Falcone, M.; Galardo, G.; Durante-Mangoni, E.; Venditti, M. Direct or early Discharge of Acute Bacterial Skin and Skin Structure Infection patients from the Emergency Department/Unit: Place in therapy of dalbavancin. Expert. Rev. Anti Infect. Ther. 2023, 21, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Almangour, T.A.; Alrasheed, M.A. Dalbavancin for the treatment of bone and joint infections: A meta-analysis. J. Infect. Chemother. 2024, in press. [Google Scholar] [CrossRef]
- Ritchie, H.; Aggarwal, A.; Schimmel, J.; Lorenzo, M.P. Clinical failure of dalbavancin for MRSA bacteremia in patient with severe obesity and history of IVDU. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2022, 28, 465–468. [Google Scholar] [CrossRef]
- Bai, F.; Aldieri, C.; Cattelan, A.; Raumer, F.; Di Meco, E.; Moioli, M.C.; Tordato, F.; Morelli, P.; Borghi, F.; Rizzi, M.; et al. Efficacy and safety of dalbavancin in the treatment of acute bacterial skin and skin structure infections (ABSSSIs) and other infections in a real-life setting: Data from an Italian observational multicentric study (DALBITA study). Expert. Rev. Anti Infect. Ther. 2020, 18, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.S.; Gallo, R.; Lugarà, M.; Gambardella, M.; Oliva, G.; Bertolino, L.; Andini, R.; Coppola, N.; Zampino, R.; Durante-Mangoni, E. Dalbavancin treatment for spondylodiscitis: Multi-center clinical experience and literature review. J. Chemother. Florence Italy 2022, 34, 360–366. [Google Scholar] [CrossRef]
- Charani, E.; Gharbi, M.; Frost, G.; Drumright, L.; Holmes, A. Antimicrobial therapy in obesity: A multicentre cross-sectional study. J. Antimicrob. Chemother. 2015, 70, 2906–2912. [Google Scholar] [CrossRef]
- Buckwalter, M.; Dowell, J.A. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J. Clin. Pharmacol. 2005, 45, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Oczkowski, S.; Alshamsi, F.; Belley-Cote, E.; Centofanti, J.E.; Møller, M.H.; Nunnaly, M.E.; Alhazzani, W. Surviving Sepsis Campaign Guidelines 2021: Highlights for the practicing clinician. Pol. Arch. Intern. Med. 2022, 132, 16290. [Google Scholar] [CrossRef] [PubMed]
| Total n = 45 | Obese n = 12 | Non-Obese n = 33 | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, median (IQR) | 64 (54–73) | 66.5 (50.7–75.2) | 64 (54–71) | 0.91 |
| Gender (male), n (%) | 28 (62.2) | 7 (58.3) | 21 (63.6) | 0.4526 |
| BMI, median (IQR) | 24 (23–30.5) | 35 (31.5–36.75) | 24 (22.5–25.5) | <0.0001 |
| Diabetes mellitus, n (%) | 8 (17.7) | 5 (41.7) | 3 (9.1) | 0.02 |
| CCI, median (IQR) | 3(1–5) | 4 (1.2–5) | 3 (1–5) | 0.51 |
| Clinical features | ||||
| ABSSSI without bone involvement, n (%) ABSSSI with bone involvement, n (%) | 17 (37) 28 (62.2) | 8 (66.7) 4 (33.3) | 9 (27.3) 24 (72.7) | 0.03 |
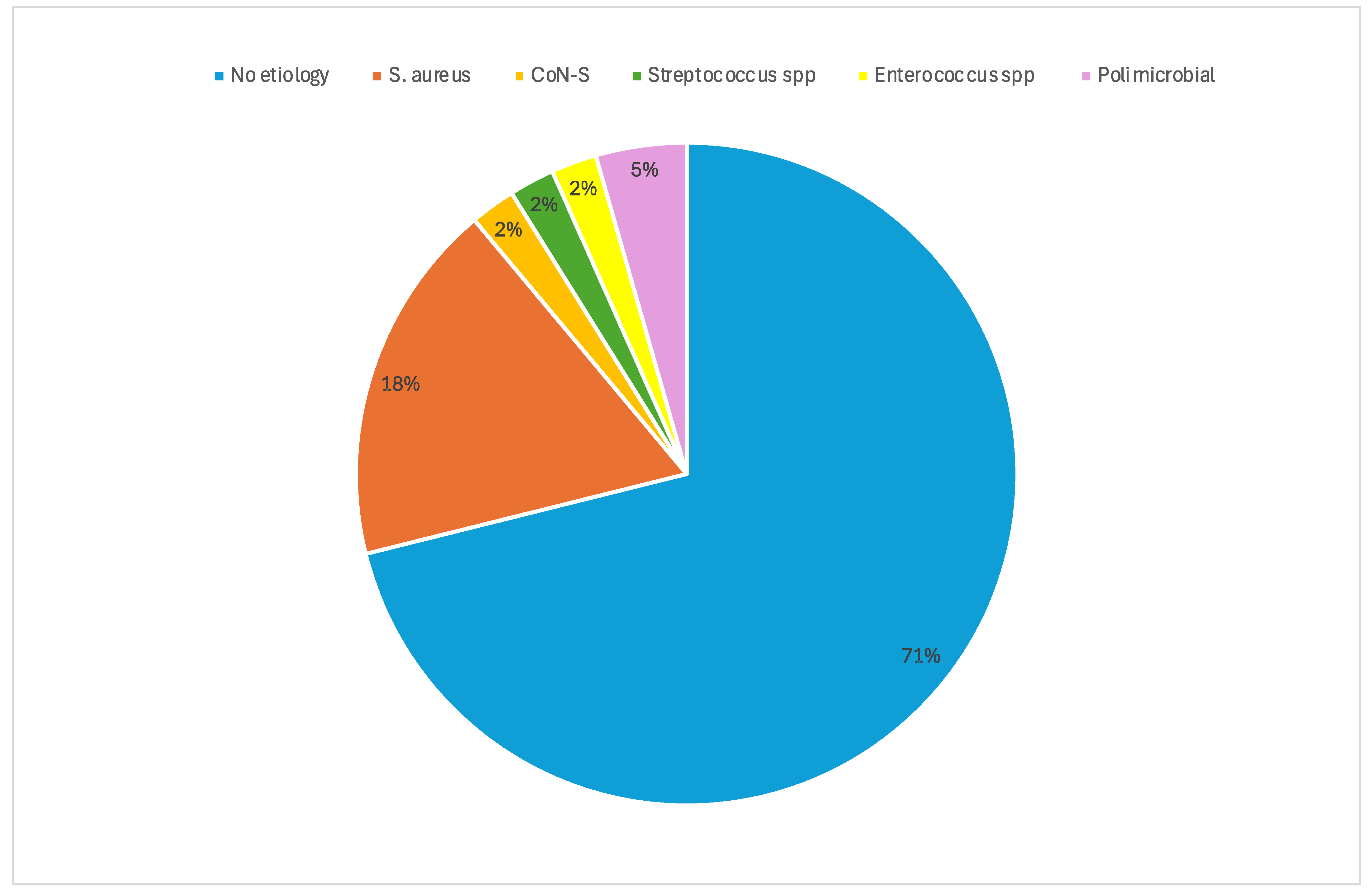
| Microbiological isolation, n (%) | 13 (28.9) | 4 (33.3) | 9 (27.3) | 0.72 |
| Fever °, n (%) | 22 (48.9) | 7 (58.3) | 15 (45.4) | 0.51 |
| Laboratory findings | ||||
| CRP mg/dl at infection onset, median (IQR) | 5.23 (0.8–17.2) | 2.5 (0.6–30.3) | 5.41 (0.8–14.1) | 0.82 |
| ESR at infection onset, median (IQR) | 60 (35–98) | 61.5 (24–114) | 60 (35–88) | 0.84 |
| WBC at infection onset, median (IQR) | 9940 (7300–14,105) | 8405 (5600–16,008) | 10170 (7820–14,030) | 0.52 |
| CRP mg/dL before DAL, median (IQR) | 1.25 (0.37–5.15) | 1.95 (0.37–5.7) | 1.2 (0.32–5) | 0.87 |
| ESR before DAL, median (IQR) | 43 (27–85) | 38 (20–51.5) | 57 (28–85) | 0.30 |
| WBC before DAL, median (IQR) | 6395 (5323–8260) | 6600 (5245–8025) | 6190 (5305–8320) | 0.87 |
| Antibiotic therapy prior to DAL | ||||
| Overall previous therapy duration, days median (IQR) | 20 (11.7–28) | 20 (11–28) | 20 (12–28) | 0.97 |
| Daptomycin, n (%) | 26 (57.7) | 6 (50) | 20 (60.6) | 0.734 |
| Glycopeptides, n (%) | 15 (33.3) | 3 (25) | 12 (36.4) | 0.72 |
| Linezolid, n (%) | 3 (6.7) | 0 (0) | 3 (9.1) | 0.55 |
| Fluoroquinolones, n (%) | 15 (33.3) | 3 (25%) | 12 (36.4) | 0.72 |
| β-lactam/β-lactamase inhibitors, n (%) | 15 (33.3) | 6 (50%) | 9 (27.3) | 0.17 |
| DAL therapy | ||||
| Number of DAL administration, mean (± SD) | 1.82 ± 0.98 | 1.41 ± 0.51 | 1.97 ± 1.07 | 0.16 |
| Reason for DAL use: favor hospital discharge, n (%) | 42 (93.3) | 10 (83.3) | 32 (96.9) | 0.16 |
| Reason for DAL use: failure of other regimens, n (%) | 3 (6.7) | 2 (16.6) | 1 (3.03) | 0.16 |
| Dalbavancin as 1st option, n (%) | 2 (4.4) | 1 (8.3) | 1 (3.1) | 0.46 |
| Dalbavancin as 2nd option, n (%) | 30 (66.7) | 7 (58.3) | 23 (69.7) | 0.72 |
| Dalbavancin as 3rd option, n (%) | 13 (28.9) | 5 (41.6) | 8 (24.2) | 0.28 |
| DAL 1500 mg single dose, n (%) | 31 (69.8) | 10 (83.3) | 21 (63.6) | 0.45 |
| DAL 1000 mg + 500 mg, n (%) | 7 (15.5) | 2 (16.7) | 5 (15.1) | >0.99 |
| DAL 1500 mg + 1000 mg + 500 mg, n (%) | 6 (13.3) | 0 (0) | 6 (18.2) | 0.16 |
| DAL 1000 mg, n (%) | 1 (2.2) | 0 (0) | 1 (3.0) | 0.16 |
| Outcomes | ||||
| 7 days clinical cure */improvement **, n (%) 7 days clinical cure, ABSSSI alone (total n = 17), n (%) 7 days clinical improvement ** with concomitant OAIs (total n = 28), n (%) | 23 (51.1) 10/17 (58.8) 13/28 (46.4) | 5 (41.6) 4/8 (50) 1/4 (25) | 18 (54.5) 6/9 (66.6) 12/24 (50) | 0.51 0.63 0.60 |
| 14 days clinical cure */improvement **, n (%) 14 days clinical cure, ABSSSI alone (total n = 17), n (%) 14 days clinical improvement ** with concomitant OAIs (total n = 28), n (%) | 41 (91.1) 16/17 (94.1) 25/28 (89.3) | 11 (91.6) 7/8 (87.5) 4/4 (100) | 30 (90.9) 9/9 (100) 21/24 (87.5) | >0.99 0.47 >0.99 |
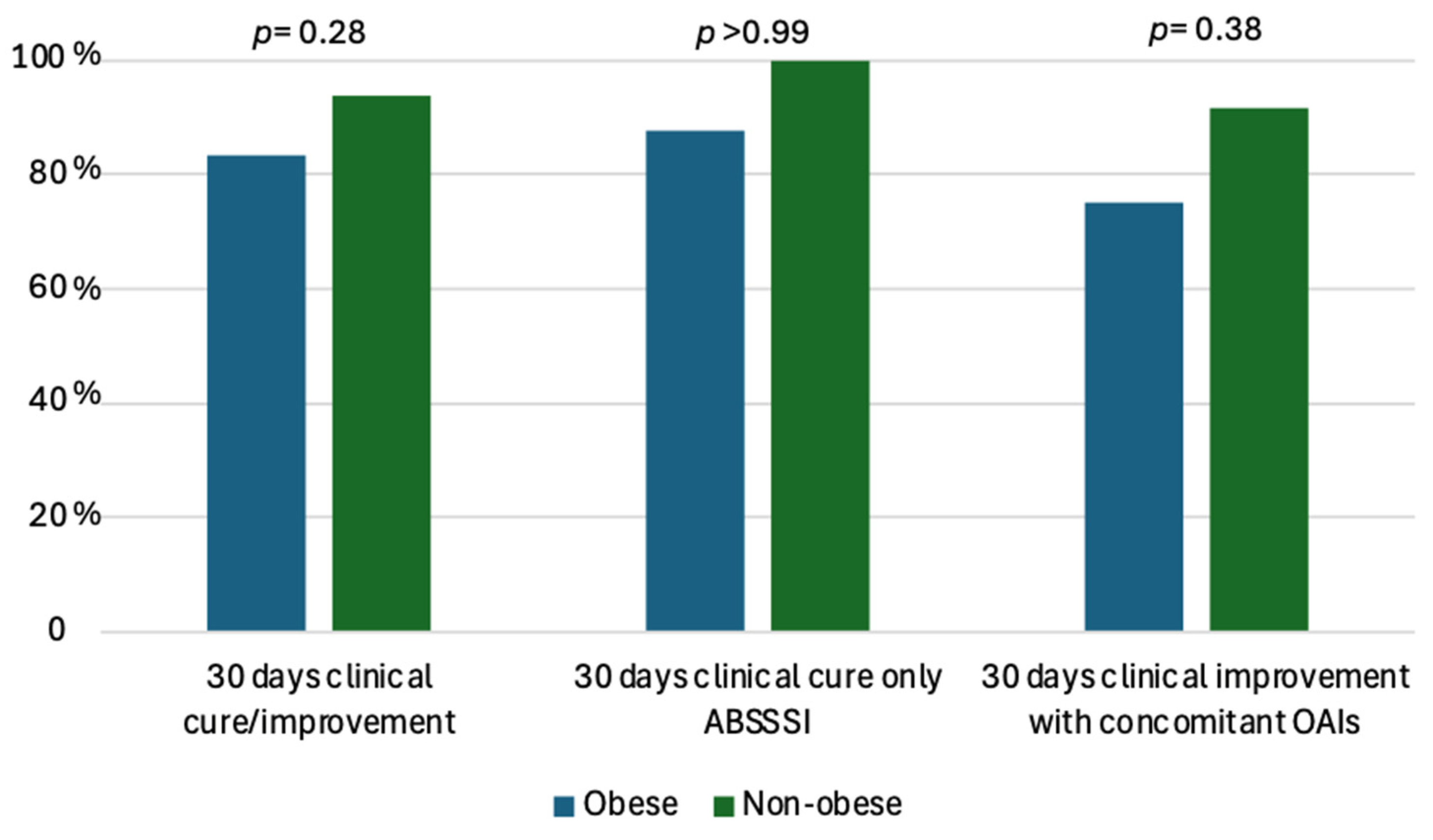
| 30 days clinical cure */improvement **, n (%) 30 days clinical cure *, ABSSSI alone (total n = 17), n (%) 30 days clinical improvement ** with concomitant OAIs (total n = 28), n (%) | 41 (91.1) 16/17 (94.1) 25/28 (89.3) | 10 (83.3) 7/8 (87.5) 3/4 (75) | 31 (93.9) 9/9 (100) 22/24 (91.7) | 0.28 >0.99 0.38 |
| 8 weeks clinical cure * with concomitant OAIs (total n = 28), n (%) | 24/28 (85.7) | 3/4 (75) | 21/24 (87.5) | 0.48 |
| 30 days outcome: relapse ***, n (%) | 4 (8.9) | 2 (16.7) | 2 (6) | 0.28 |
| Length of hospitalization (days, median IQR) | 21.7 ± 14.7 | 22.5 ± 16.7 | 24.8 ± 14.1 | 0.84 |
| Adverse effects, n (%) | 1 (2.2) | 1 (8.3) | 0 | NA |
| Total n = 12 | Obese with Diabetes n = 5 | Non-Obese with Diabetes n = 7 | p-Value | |
|---|---|---|---|---|
| 7 days clinical cure */improvement **, n (%) | 5 (41.6) | 2 (40) | 3 (42.8) | >0.999 |
| 14 days clinical cure */improvement **, n (%) | 11 (91.6) | 5 (100) | 6 (85.7) | >0.999 |
| 30 days clinical cure */improvement **, n (%) | 3 (25) | 1 (20) | 2 (28.6) | >0.999 |
| 30 days outcome: relapse ***, n (%) | 2 (16.7) | 0 (0) | 2 (28.6) | 0.469 |
| Total n = 16 | Dalbavancin § n = 8 | SOC ° n = 8 | p-Value | |
|---|---|---|---|---|
| Age, years median (IQR) | 57.6 ± 15.9 | 68.5 ± 9.6 | 50.7 ± 12 | 0.005 |
| BMI, median (IQR) | 36.7 ± 3.6 | 36.7 ± 4.8 | 36.9 ± 2.7 | 0.94 |
| Diabetes mellitus, n (%) | 7 (30) | 3 (37.5) | 4 (50) | >0.99 |
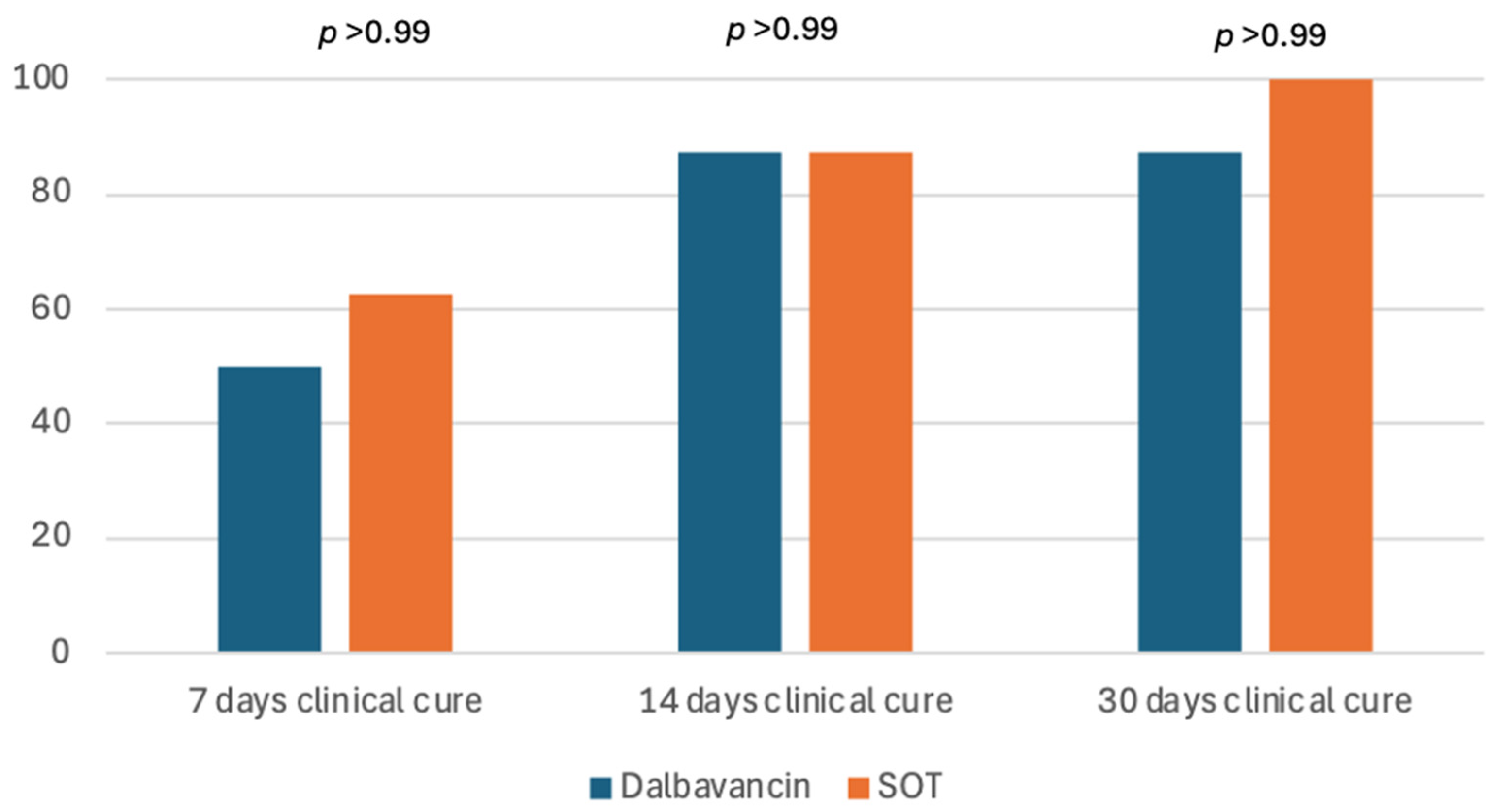
| 7 days clinical cure *, n (%) | 9 (56.2) | 4 (50) | 5 (62.5) | >0.99 |
| 14 days clinical cure *, n (%) | 14 (87.5) | 7 (87.5) | 7 (87.5) | >0.99 |
| 30 days clinical cure *, n (%) | 15 (93.7) | 7 (87.5) | 8 (100) | >0.99 |
| Length of hospitalization, days (±standard deviation) | 21.7 ± 12.4 | 22.5 ± 20 | 21 ± 7.9 | 0.845 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, A.; Petrucci, F.; Leanza, C.; Rivano Capparuccia, M.; Comi, M.; Mastroianni, C. Using Dalbavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) in Obese Patients: A Real-Life, Single-Center Observational Study. Antibiotics 2025, 14, 75. https://doi.org/10.3390/antibiotics14010075
Oliva A, Petrucci F, Leanza C, Rivano Capparuccia M, Comi M, Mastroianni C. Using Dalbavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) in Obese Patients: A Real-Life, Single-Center Observational Study. Antibiotics. 2025; 14(1):75. https://doi.org/10.3390/antibiotics14010075
Chicago/Turabian StyleOliva, Alessandra, Flavia Petrucci, Cristiana Leanza, Marco Rivano Capparuccia, Michela Comi, and Claudio Mastroianni. 2025. "Using Dalbavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) in Obese Patients: A Real-Life, Single-Center Observational Study" Antibiotics 14, no. 1: 75. https://doi.org/10.3390/antibiotics14010075
APA StyleOliva, A., Petrucci, F., Leanza, C., Rivano Capparuccia, M., Comi, M., & Mastroianni, C. (2025). Using Dalbavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) in Obese Patients: A Real-Life, Single-Center Observational Study. Antibiotics, 14(1), 75. https://doi.org/10.3390/antibiotics14010075






