Bacteriophage Treatment Induces Phenotype Switching and Alters Antibiotic Resistance of ESBL Escherichia coli
Abstract
1. Introduction
2. Results
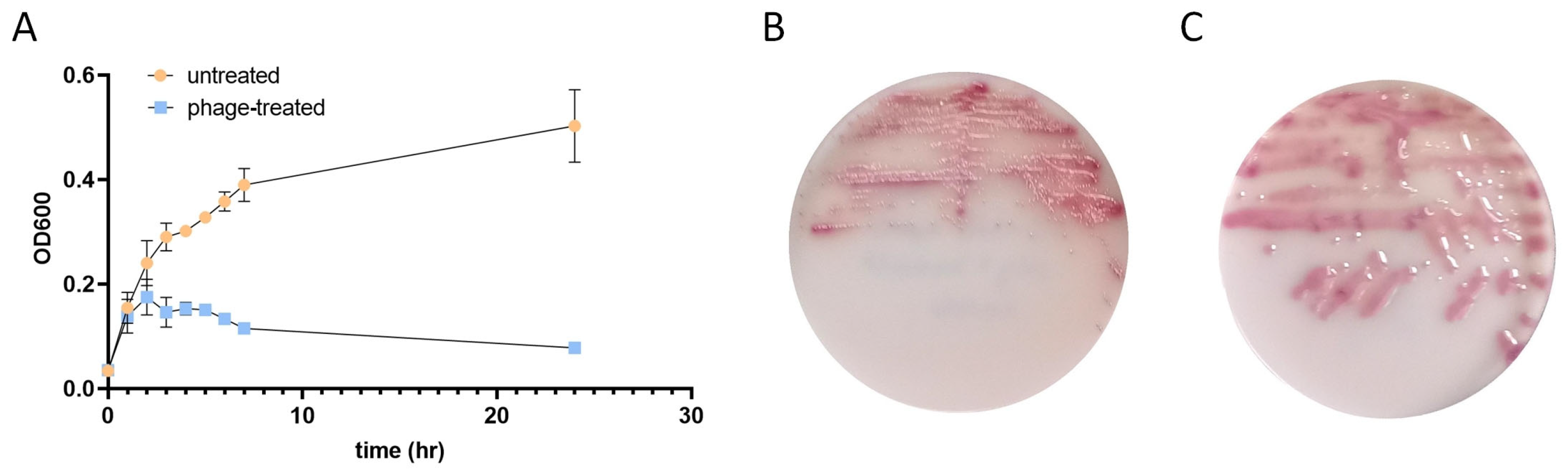
2.1. Bacteriophage Infection Alters the Growth Kinetics and Colony Morphology of E. coli
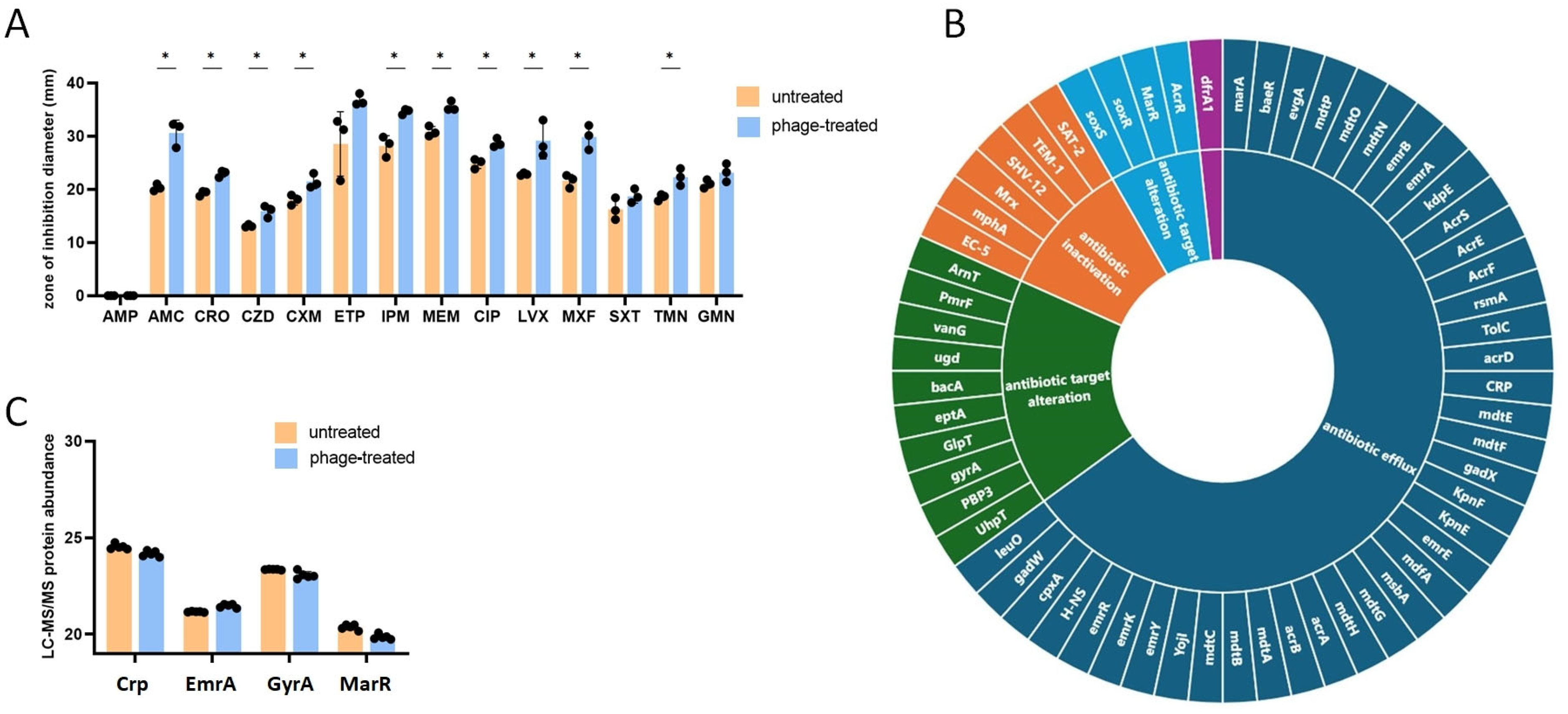
2.2. Impact of Bacteriophage Infection on the Antimicrobial Resistance of E. coli
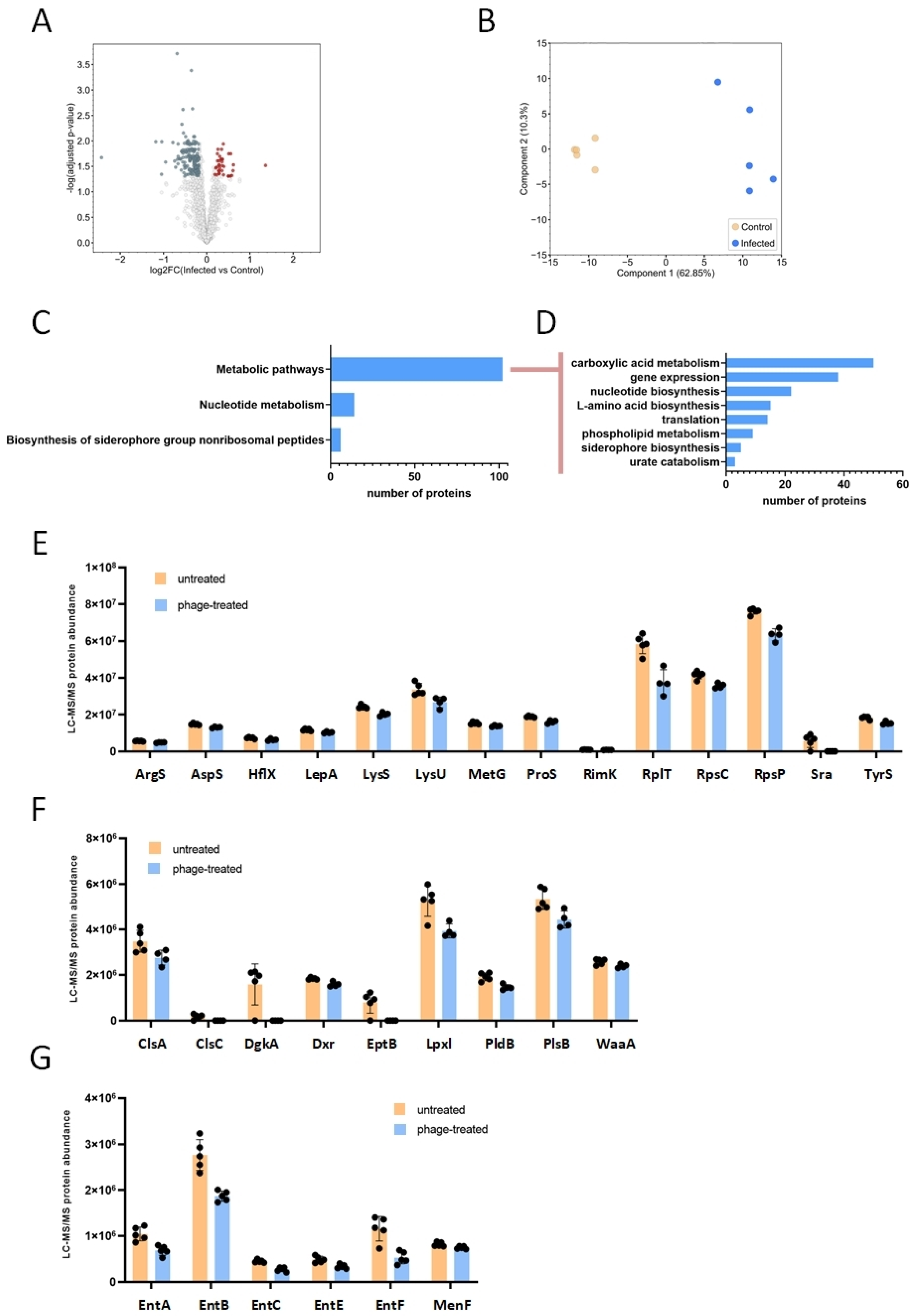
2.3. Proteomic Analysis of Bacteriophage-Treated and Untreated E. coli
2.4. Impact of Bacteriophage Treatment on the Membrane Permeability of E. coli
3. Discussion
4. Materials and Methods
4.1. Bacterium Isolate and Reagents
4.2. Antibiotic Susceptibility and Growth Kinetic Tests
4.3. Genome Sequencing of Bacteriophage-Treated and Untreated E. coli
4.4. Proteome Analysis of Bacteriophage-Treated and Untreated E. coli by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS)
4.5. Real-Time Ethidium Bromide Accumulation Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinson, J.N.V.; Walk, S.T. Escherichia Coli Residency in the Gut of Healthy Human Adults. EcoSal Plus 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia Coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia Coli. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. Coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- WHO. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage Therapy: An Alternative to Antibiotics in the Age of Multi-Drug Resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162. [Google Scholar] [CrossRef]
- Bolocan, A.S.; Callanan, J.; Forde, A.; Ross, P.; Hill, C. Phage Therapy Targeting Escherichia Coli-a Story with No End? FEMS Microbiol. Lett. 2016, 363, fnw256. [Google Scholar] [CrossRef]
- Johri, A.V.; Johri, P.; Hoyle, N.; Nadareishvili, L.; Pipia, L.; Nizharadze, D. Case Report: Successful Treatment of Recurrent E. Coli Infection with Bacteriophage Therapy for Patient Suffering from Chronic Bacterial Prostatitis. Front. Pharmacol. 2023, 14, 1243824. [Google Scholar] [CrossRef]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef]
- Green, S.I.; Clark, J.R.; Santos, H.H.; Weesner, K.E.; Salazar, K.C.; Aslam, S.; Campbell, J.W.; Doernberg, S.B.; Blodget, E.; Morris, M.I.; et al. A Retrospective, Observational Study of 12 Cases of Expanded-Access Customized Phage Therapy: Production, Characteristics, and Clinical Outcomes. Clin. Infect. Dis. 2023, 77, 1079–1091. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef]
- Valério, N.; Oliveira, C.; Jesus, V.; Branco, T.; Pereira, C.; Moreirinha, C.; Almeida, A. Effects of Single and Combined Use of Bacteriophages and Antibiotics to Inactivate Escherichia Coli. Virus Res. 2017, 240, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, M.; De Zoysa, M.; Shin, H.-J. Application of Phage Therapy: Synergistic Effect of Phage EcSw (ΦEcSw) and Antibiotic Combination towards Antibiotic-Resistant Escherichia Coli. Transbound. Emerg. Dis. 2020, 67, 2809–2817. [Google Scholar] [CrossRef] [PubMed]
- Shigella Phage SHFML-11. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=1863008 (accessed on 7 January 2025).
- Shigella Phage SHFML-26. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=1863009 (accessed on 7 January 2025).
- Wang, R.H.; Yang, S.; Liu, Z.; Zhang, Y.; Wang, X.; Xu, Z.; Wang, J.; Li, S.C. PhageScope: A Well-Annotated Bacteriophage Database with Automatic Analyses and Visualizations. Nucleic Acids Res. 2024, 52, D756–D761. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Disk Diffusion Test Methodology. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology (accessed on 7 January 2025).
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded Curation, Support for Machine Learning, and Resistome Prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Tanji, Y.; Hattori, K.; Suzuki, K.; Miyanaga, K. Spontaneous Deletion of a 209-Kilobase-Pair Fragment from the Escherichia Coli Genome Occurs with Acquisition of Resistance to an Assortment of Infectious Phages. Appl. Environ. Microbiol. 2008, 74, 4256–4263. [Google Scholar] [CrossRef]
- Sørensen, P.E.; Baig, S.; Stegger, M.; Ingmer, H.; Garmyn, A.; Butaye, P. Spontaneous Phage Resistance in Avian Pathogenic Escherichia Coli. Front. Microbiol. 2021, 12, 782757. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Chang, H.-W.; Kim, K.-H. Comparative Genomic Analysis of Bacteriophage EP23 Infecting Shigella Sonnei and Escherichia Coli. J. Microbiol. 2011, 49, 927–934. [Google Scholar] [CrossRef]
- Villarroel, J.; Larsen, M.V.; Kilstrup, M.; Nielsen, M. Metagenomic Analysis of Therapeutic PYO Phage Cocktails from 1997 to 2014. Viruses 2017, 9, 328. [Google Scholar] [CrossRef]
- Wolfram-Schauerte, M.; Pozhydaieva, N.; Viering, M.; Glatter, T.; Höfer, K. Integrated Omics Reveal Time-Resolved Insights into T4 Phage Infection of E. Coli on Proteome and Transcriptome Levels. Viruses 2022, 14, 2502. [Google Scholar] [CrossRef]
- Berger, P.; Kouzel, I.U.; Berger, M.; Haarmann, N.; Dobrindt, U.; Koudelka, G.B.; Mellmann, A. Carriage of Shiga Toxin Phage Profoundly Affects Escherichia Coli Gene Expression and Carbon Source Utilization. BMC Genom. 2019, 20, 504. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.W.; Logel, D.Y.; Mirzai, M.; Pascovici, D.; Molloy, M.P.; Jaschke, P.R. Proteomic and Transcriptomic Analysis of Microviridae φX174 Infection Reveals Broad Upregulation of Host Escherichia Coli Membrane Damage and Heat Shock Responses. mSystems 2021, 6, e00046-21. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, C.; van Heerden, J.H.; Nordholt, N.; Schmidt, P.; Bruggeman, F.J. Taking Chances and Making Mistakes: Non-Genetic Phenotypic Heterogeneity and Its Consequences for Surviving in Dynamic Environments. J. R. Soc. Interface 2017, 14, 20170141. [Google Scholar] [CrossRef]
- Proctor, R.A.; von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small Colony Variants: A Pathogenic Form of Bacteria That Facilitates Persistent and Recurrent Infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef]
- Chiarelli, A.; Cabanel, N.; Rosinski-Chupin, I.; Zongo, P.D.; Naas, T.; Bonnin, R.A.; Glaser, P. Diversity of Mucoid to Non-Mucoid Switch among Carbapenemase-Producing Klebsiella Pneumoniae. BMC Microbiol. 2020, 20, 325. [Google Scholar] [CrossRef]
- Mégroz, M.; Kleifeld, O.; Wright, A.; Powell, D.; Harrison, P.; Adler, B.; Harper, M.; Boyce, J.D. The RNA-Binding Chaperone Hfq Is an Important Global Regulator of Gene Expression in Pasteurella Multocida and Plays a Crucial Role in Production of a Number of Virulence Factors, Including Hyaluronic Acid Capsule. Infect. Immun. 2016, 84, 1361–1370. [Google Scholar] [CrossRef]
- Jia, J.; King, J.E.; Goldrick, M.C.; Aldawood, E.; Roberts, I.S. Three Tandem Promoters, Together with IHF, Regulate Growth Phase Dependent Expression of the Escherichia Coli Kps Capsule Gene Cluster. Sci. Rep. 2017, 7, 17924. [Google Scholar] [CrossRef]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An Archetype for Microbial Iron Transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef]
- Lisiecki, P. Antibiotic resistance and siderophore production in enterococci. Med. Dosw. Mikrobiol. 2014, 66, 1–10. [Google Scholar]
- Khazaal, M.T.; El-Hendawy, H.H.; Mabrouk, M.I.; Faraag, A.H.I.; Bakkar, M.R. Antibiotic Resistance and Siderophores Production by Clinical Escherichia Coli Strains. Biotechnologia 2022, 103, 169–184. [Google Scholar] [CrossRef]
- Cacace, E.; Kim, V.; Varik, V.; Knopp, M.; Tietgen, M.; Brauer-Nikonow, A.; Inecik, K.; Mateus, A.; Milanese, A.; Mårli, M.T.; et al. Systematic Analysis of Drug Combinations against Gram-Positive Bacteria. Nat. Microbiol. 2023, 8, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Yilancioglu, K. Antimicrobial Drug Interactions: Systematic Evaluation of Protein and Nucleic Acid Synthesis Inhibitors. Antibiotics 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.C.; Pfrunder-Cardozo, K.R.; Meinel, D.; Egli, A.; Hall, A.R. Associations among Antibiotic and Phage Resistance Phenotypes in Natural and Clinical Escherichia Coli Isolates. mBio 2017, 8, e01341-17. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Ahn, J. Associations between Antibiotic Resistance and Bacteriophage Resistance Phenotypes in Laboratory and Clinical Strains of Salmonella Enterica Subsp. Enterica Serovar Typhimurium. Microb. Pathog. 2020, 143, 104159. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, F.; Li, K.W.; Klaassen, R.V.; Smit, A.B. MS-DAP Platform for Downstream Data Analysis of Label-Free Proteomics Uncovers Optimal Workflows in Benchmark Data Sets and Increased Sensitivity in Analysis of Alzheimer’s Biomarker Data. J. Proteome Res. 2023, 22, 374–386. [Google Scholar] [CrossRef]
- Paixão, L.; Rodrigues, L.; Couto, I.; Martins, M.; Fernandes, P.; de Carvalho, C.C.C.R.; Monteiro, G.A.; Sansonetty, F.; Amaral, L.; Viveiros, M. Fluorometric Determination of Ethidium Bromide Efflux Kinetics in Escherichia Coli. J. Biol. Eng. 2009, 3, 18. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rózsa, Á.; Orosz, L.; Szemerédi, N.; Spengler, G.; Kecskeméti, G.; Vágó, O.; Sárvári, K.P.; Szabó, D.; Szabó, Z.; Burián, K.; et al. Bacteriophage Treatment Induces Phenotype Switching and Alters Antibiotic Resistance of ESBL Escherichia coli. Antibiotics 2025, 14, 76. https://doi.org/10.3390/antibiotics14010076
Rózsa Á, Orosz L, Szemerédi N, Spengler G, Kecskeméti G, Vágó O, Sárvári KP, Szabó D, Szabó Z, Burián K, et al. Bacteriophage Treatment Induces Phenotype Switching and Alters Antibiotic Resistance of ESBL Escherichia coli. Antibiotics. 2025; 14(1):76. https://doi.org/10.3390/antibiotics14010076
Chicago/Turabian StyleRózsa, Árpád, László Orosz, Nikoletta Szemerédi, Gabriella Spengler, Gábor Kecskeméti, Otília Vágó, Károly Péter Sárvári, Diana Szabó, Zoltán Szabó, Katalin Burián, and et al. 2025. "Bacteriophage Treatment Induces Phenotype Switching and Alters Antibiotic Resistance of ESBL Escherichia coli" Antibiotics 14, no. 1: 76. https://doi.org/10.3390/antibiotics14010076
APA StyleRózsa, Á., Orosz, L., Szemerédi, N., Spengler, G., Kecskeméti, G., Vágó, O., Sárvári, K. P., Szabó, D., Szabó, Z., Burián, K., & Virok, D. P. (2025). Bacteriophage Treatment Induces Phenotype Switching and Alters Antibiotic Resistance of ESBL Escherichia coli. Antibiotics, 14(1), 76. https://doi.org/10.3390/antibiotics14010076








