Antibiotic Prophylaxis Prescribing Practices for Dental Implant Placement in Croatia: A Questionnaire-Based Cross-Sectional Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Questionnaire
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhurakivska, K.; Russo, L.L.; Muzio, L.L.; Caponio, V.C.A.; Laino, L.; Arena, C.; Cirillo, N.; Troiano, G. Antibiotic prophylaxis at the time of dental implant placement: A cost-effectiveness analysis. BMC Health Serv. Res. 2022, 22, 1073. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Muñoz-Cámara, D.; Sánchez-Siles, M. Attitudes of dental implantologists in Spain to prescribing antibiotics, analgesics and anti-inflammatories in healthy patients. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e752–e758. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Gewitz, M.; Lockhart, P.B.; Bolger, A.F.; DeSimone, D.C.; Kazi, D.S.; Couper, D.J.; Beaton, A.; Kilmartin, C.; Miro, J.M.; et al. Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e963–e978. [Google Scholar] [CrossRef] [PubMed]
- Kreč Potočki, M. Nove Smjernice u Antibiotskoj Profilaksi Kod Postave Dentalnih Implantata. Ph.D. Thesis, University of Zagreb, School of Dental Medicine, Zagreb, Croatia, 2024. Available online: https://urn.nsk.hr/urn:nbn:hr:127:132542 (accessed on 25 August 2024).
- Vidović Juras, D.; Škrinjar, I.; Križnik, T.; Andabak Rogulj, A.; Lončar Brzak, B.; Gabrić, D.; Granić, M.; Peroš, K.; Šutej, I.; Ivanišević, A. Antibiotic Prophylaxis Prior to Dental Procedures. Dent. J. 2024, 12, 364. [Google Scholar] [CrossRef]
- Rademacher, W.M.H.; Walenkamp, G.H.I.M.; Moojen, D.J.F.; Hendriks, J.G.E.; Goedendorp, T.A.; Rozema, F.R. Antibiotic prophylaxis is not indicated prior to dental procedures for prevention of periprosthetic joint infections: A systematic review and new guidelines from the Dutch Orthopaedic and Dental Societies. Acta Orthop. 2017, 88, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Watters, W.; Rethman, M.P.; Hanson, N.B.; Abt, E.; Anderson, P.A.; Carroll, K.C.; Futrell, H.C.; Garvin, K.; Glenn, S.O.; Hellstein, J.; et al. Prevention of orthopaedic implant infection in patients undergoing dental procedures. J. Am. Acad. Orthop. Surg. 2013, 21, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Sánchez, F.; Arteagoitia, I.; Rodríguez Andrés, C.; Caiazzo, A. Antibiotic prophylaxis habits in oral implant surgery among dentists in Italy: A cross-sectional survey. BMC Oral Health 2019, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Mira, J.C.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Prescription of Antibiotic Prophylaxis for Dental Implant Surgery in Healthy Patients: A Systematic Review of Survey-Based Studies. Front. Pharmacol. 2021, 11, 588333. [Google Scholar] [CrossRef] [PubMed]
- Borba, A.M.; Souza, D.F.M.; Brozoski, M.A.; Burim, R.A.; Naclério-Homem, M.d.G.; Deboni, M.C.Z. Can the use of antibiotics interfere with the success of dental osseointegrated implants in diabetic patients? J. Contemp. Dent. Pract. 2013, 14, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Guidelines for the Prevention of Surgical Site Infection Web Appendix 5 Summary of a Systematic Review on Optimal Timing for Preoperative Surgical Antibiotic Prophylaxis. 2024. Available online: https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/ssi/evidence/appendix5.pdf?sfvrsn=72e34df5_2 (accessed on 19 November 2024).
- Salgado-Peralvo, A.O.; Garcia-Sanchez, A.; Kewalramani, N.; Barone, A.; Martínez-González, J.M.; Velasco-Ortega, E.; López-López, J.; Kaiser-Cifuentes, R.; Guerra, F.; Matos-Garrido, N.; et al. Consensus Report on Preventive Antibiotic Therapy in Dental Implant Procedures: Summary of Recommendations from the Spanish Society of Implants. Antibiotics 2022, 11, 655. [Google Scholar] [CrossRef]
- Romandini, M.; Tullio IDe Congedi, F.; Kalemaj, Z.; D‘Ambrosio, M.; Laforí, A.; Quaranta, C.; Buti, J.; Perfetti, G. Antibiotic prophylaxis at dental implant placement: Which is the best protocol? A systematic review and network meta-analysis. J. Clin. Periodontol. 2019, 46, 382–395. [Google Scholar] [CrossRef]
- Dominiak, M.; Shuleva, S.; Silvestros, S.; Alcoforado, G. A prospective observational study on perioperative use of antibacterial agents in implant surgery. Adv. Clin. Exp. Med. 2020, 29, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Torof, E.; Morrissey, H.; Ball, P.A. Antibiotic Use in Dental Implant Procedures: A Systematic Review and Meta-Analysis. Medicina 2023, 59, 713. [Google Scholar] [CrossRef] [PubMed]
- Momand, P.; Naimi-Akbar, A.; Hultin, M.; Lund, B.; Götrick, B. Is routine antibiotic prophylaxis warranted in dental implant surgery to prevent early implant failure?—A systematic review. BMC Oral Health 2024, 24, 842. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Prophylactic antibiotic regimen and dental implant failure: A meta-analysis. J. Oral Rehabil. 2014, 41, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Abukaraky, A.; Sawair, F.; Alkaradsheh, O.; Eimar, H. Antibiotic prophylaxis and early dental implant failure: A quasi-random controlled clinical trial. Eur. J. Oral Implantol. 2011, 4, 31–38. Available online: https://www.researchgate.net/publication/51148189 (accessed on 25 August 2024).
- Al-Kattan, R.; Al-Shibani, N. Current trends in antibiotic prescription behavior among Saudi dentists performing implant surgery: A cross-sectional observational study. J. Investig. Clin. Dent. 2019, 10, e12383. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D. The Use of Antibiotic Prophylaxis in Implant Dentistry: A Microbiological and Clinical Perspective. Ph.D. Thesis, Karolinska Institutet, Stockholm, Sweden, 2017. Available online: https://hdl.handle.net/10616/46073 (accessed on 19 November 2024).
- Ireland, R.S.; Palmer, N.O.; Lindenmeyer, A.; Mills, N. An investigation of antibiotic prophylaxis in implant practice in the UK. Br. Dent. J. 2012, 213, E14. [Google Scholar] [CrossRef]
- Sánchez, F.R.; Arteagoitia, I.; Andrés, C.R.; Bruers, J. Antibiotic prophylaxis prescribing habits in oral implant surgery in the Netherlands: A cross-sectional survey. BMC Oral Health 2019, 19, 281. [Google Scholar]
- Sollecito, T.P.; Abt, E.; Lockhart, P.B.; Truelove, E.; Paumier, T.M.; Tracy, S.L.; Tampi, M.; Beltrán-Aguilar, E.D.; Frantsve-Hawley, J. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: Evidence-based clinical practice guideline for dental practitioners—A report of the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2015, 146, 11–16.e8. [Google Scholar] [CrossRef]
- Hickam, D.H.; Gordon, C.J.; Armstrong, C.E.; Paynter, R. Rapid Response Efficacy of Dental Services for Reducing Adverse Events in Those Undergoing Insertion of Implantable Cardiovascular Devices; 23-EHC020; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2023. [Google Scholar]
- American Heart Association. Dental Management of the Organ Transplant Patient 5. 2024. Available online: https://www.heart.org (accessed on 19 November 2024).
- Kwak, E.-J.; Kim, D.-J.; Choi, Y.; Joo, D.J.; Park, W. Importance of oral health and dental treatment in organ transplant recipients. Int. Dent. J. 2020, 70, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, H.; Kunzendorf, B.; Wiltfang, J. Dental implants and diabetes mellitus—A systematic review. Int. J. Implant. Dent. 2016, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Scottish Dental Clinical Effectiveness Programme. Management of Dental Patients Taking Anticoagulants or Antiplatelet Drugs; Scottish Dental Clinical Efectiveness Programme: Dundee, Scotland, 2022; Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.sdcep.org.uk/media/ypnl2cpz/sdcep-management-of-dental-patients-taking-anticoagulants-or-antiplatelet-drugs-2nd-edition.pdf&ved=2ahUKEwilyp7Ex-KKAxU5dPUHHe8cKPQQFnoECBQQAQ&usg=AOvVaw2cYYo0Plfyx5RiN9FBvH91 (accessed on 19 November 2024).
- Scottish Dental Clinical Effectiveness Programme. Is Dental Treatment Likely to Cause Bleeding? 2022. Available online: www.sdcep.org.uk (accessed on 19 November 2024).
- American Association of Endodontists. ENDODONTICS: Colleagues for Excellence. 2019. Available online: https://www.aae.org/colleagues (accessed on 19 November 2024).
- Blyleven, G.M.; Johnson, T.M.; Inouye, K.A.; Stancoven, B.W.; Lincicum, A.R. Factors influencing intraoperative and postoperative complication occurrence: A series of 1135 periodontal and implant-related surgeries. Clin. Exp. Dent. Res. 2024, 10, e849. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
| N (%) | p-Value | |
|---|---|---|
| Gender | 0.359 | |
| Male | 34 (44.7) | |
| Female | 42 (55.3) | |
| Age (in years) | <0.001 | |
| 25–30 | 10 (13.2) | |
| 31–40 | 25 (32.9) | |
| 41–50 | 22 (28.9) | |
| 51–60 | 16 (21.1) | |
| 61+ | 3 (3.9) | |
| Work Experience (in years) | 0.007 | |
| 0–5 | 11 (14.4) | |
| 6–10 | 13 (17.1) | |
| 11–20 | 30 (39.5) | |
| 21+ | 22 (28.9) | |
| Education Level | <0.001 | |
| DMD | 51 (67.1) | |
| PhD | 13 (17.1) | |
| Master’s Degree | 12 (15.8) | |
| Workplace | <0.001 | |
| Public Health Practice | 9 (11.8) 67 (88.15) | |
| Private Practice | ||
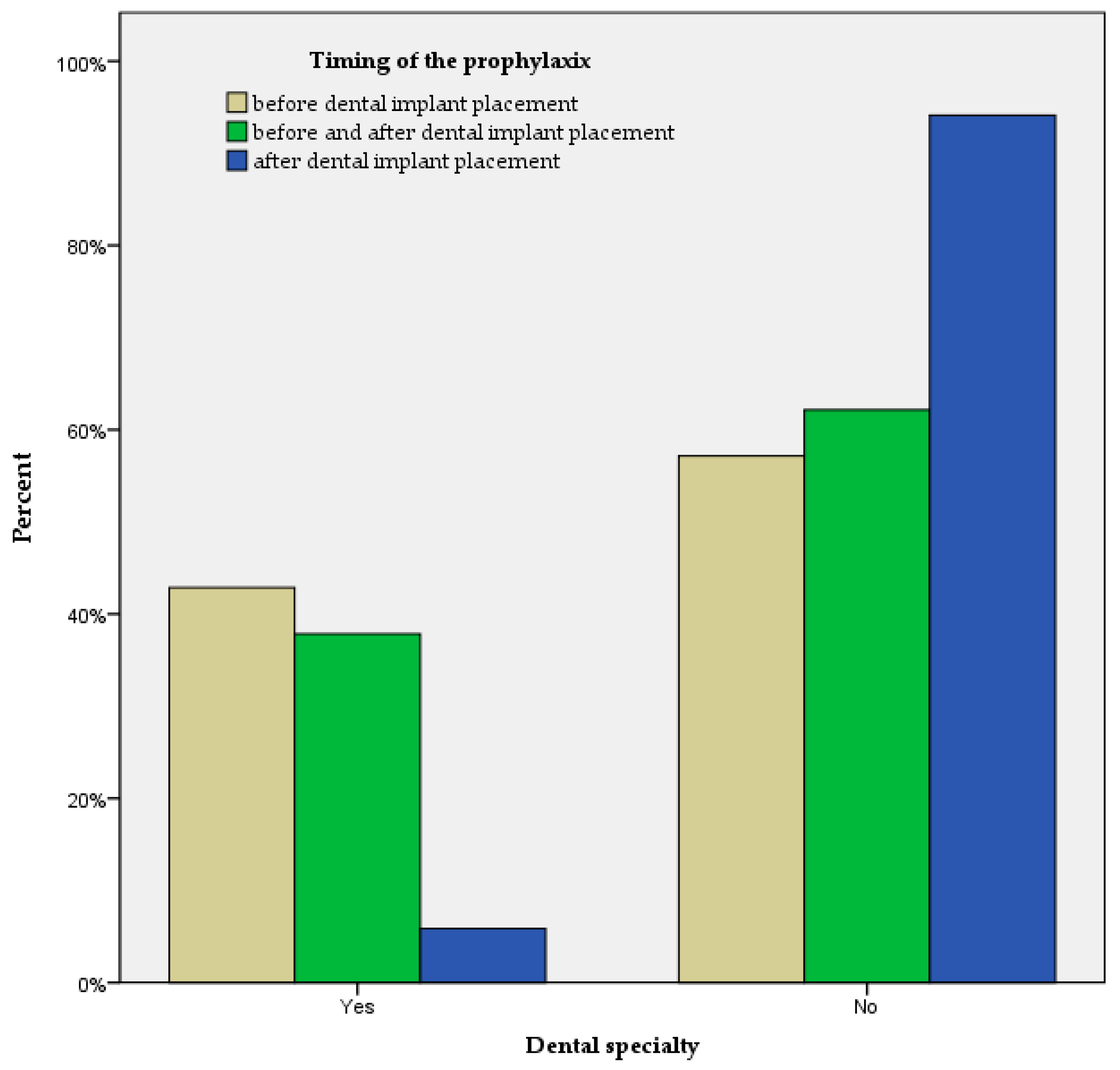
| Dental Specialty | 0.001 | |
| Yes | 24 (31.6) | |
| No | 52 (68.4) | |
| Specialty Field | <0.001 | |
| None | 52 (68.4) | |
| Oral Surgery Prosthodontics Periodonotology | 12 (15.8) 7 (9.2) 5 (6.6) |
| N (%) | p-Value | |
|---|---|---|
| Timing of the prophylaxis | <0.001 | |
| Before | 21 (27.6) | |
| After | 17 (22.4) | |
| Before and after | 37 (48.7) | |
| Never | 1 (1.3) | |
| Type of antibiotics | <0.001 | |
| Amoxicillin | 47 (61.8) | |
| Amoxicillin and Clavulanic acid | 22 (28.9) | |
| Clindamycin | 4 (5.3) | |
| Erythromycin | 1 (1.3) | |
| Amoxicillin and Clavulanic acid or Clindamycin | 1 (1.3) | |
| Does not use antibiotics | 1 (1.3) |
| Patient Condition | Yes N (%) | No N (%) | χ2 | p-Value |
|---|---|---|---|---|
| All patients | 17 (22.7) | 58 (77.3) | 22.41 | <0.001 |
| Artificial heart valves | 73 (97.3) | 2 (2.6) | 67.21 | <0.001 |
| History of infective endocarditis | 74 (98.7) | 1 (1.3) | 71.05 | <0.001 |
| Transplanted organs | 58 (77.3) | 17 (22.7) | 22.41 | <0.001 |
| Built-in pacemaker heart device | 50 (66.7) | 25 (33.3) | 8.33 | 0.003 |
| HIV | 51 (68.0) | 24 (32.0) | 9.72 | 0.001 |
| Dialysis | 50 (66.7) | 25 (33.3) | 8.33 | 0.003 |
| Artificial joints | 52 (69.3) | 23 (30.7) | 11.21 | 0.001 |
| Antiangiogenic therapy | 46 (61.3) | 29 (38.7) | 3.85 | 0.027 |
| Diabetes mellitus | 48 (64.0) | 27 (36.0) | 5.88 | 0.010 |
| Antiresorptive therapy | 46 (61.3) | 29 (38.7) | 3.85 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ković, M.; Pribisalić, A.; Viskić, J.; Martinić, J.; Grubišić, J.; Vardić, A.; Poklepović Peričić, T. Antibiotic Prophylaxis Prescribing Practices for Dental Implant Placement in Croatia: A Questionnaire-Based Cross-Sectional Study. Antibiotics 2025, 14, 47. https://doi.org/10.3390/antibiotics14010047
Ković M, Pribisalić A, Viskić J, Martinić J, Grubišić J, Vardić A, Poklepović Peričić T. Antibiotic Prophylaxis Prescribing Practices for Dental Implant Placement in Croatia: A Questionnaire-Based Cross-Sectional Study. Antibiotics. 2025; 14(1):47. https://doi.org/10.3390/antibiotics14010047
Chicago/Turabian StyleKović, Mare, Ajka Pribisalić, Joško Viskić, Jure Martinić, Josipa Grubišić, Ante Vardić, and Tina Poklepović Peričić. 2025. "Antibiotic Prophylaxis Prescribing Practices for Dental Implant Placement in Croatia: A Questionnaire-Based Cross-Sectional Study" Antibiotics 14, no. 1: 47. https://doi.org/10.3390/antibiotics14010047
APA StyleKović, M., Pribisalić, A., Viskić, J., Martinić, J., Grubišić, J., Vardić, A., & Poklepović Peričić, T. (2025). Antibiotic Prophylaxis Prescribing Practices for Dental Implant Placement in Croatia: A Questionnaire-Based Cross-Sectional Study. Antibiotics, 14(1), 47. https://doi.org/10.3390/antibiotics14010047







