A Quantitative Examination and Comparison of the Ability of Australian Gentamicin Dosing Guidelines to Achieve Target Therapeutic Concentrations in Neonates
Abstract
1. Introduction
2. Results
2.1. Comparison of Neonatal Gentamicin Dosing Guidelines
2.2. Population Pharmacokinetic Model and Simulation Dataset
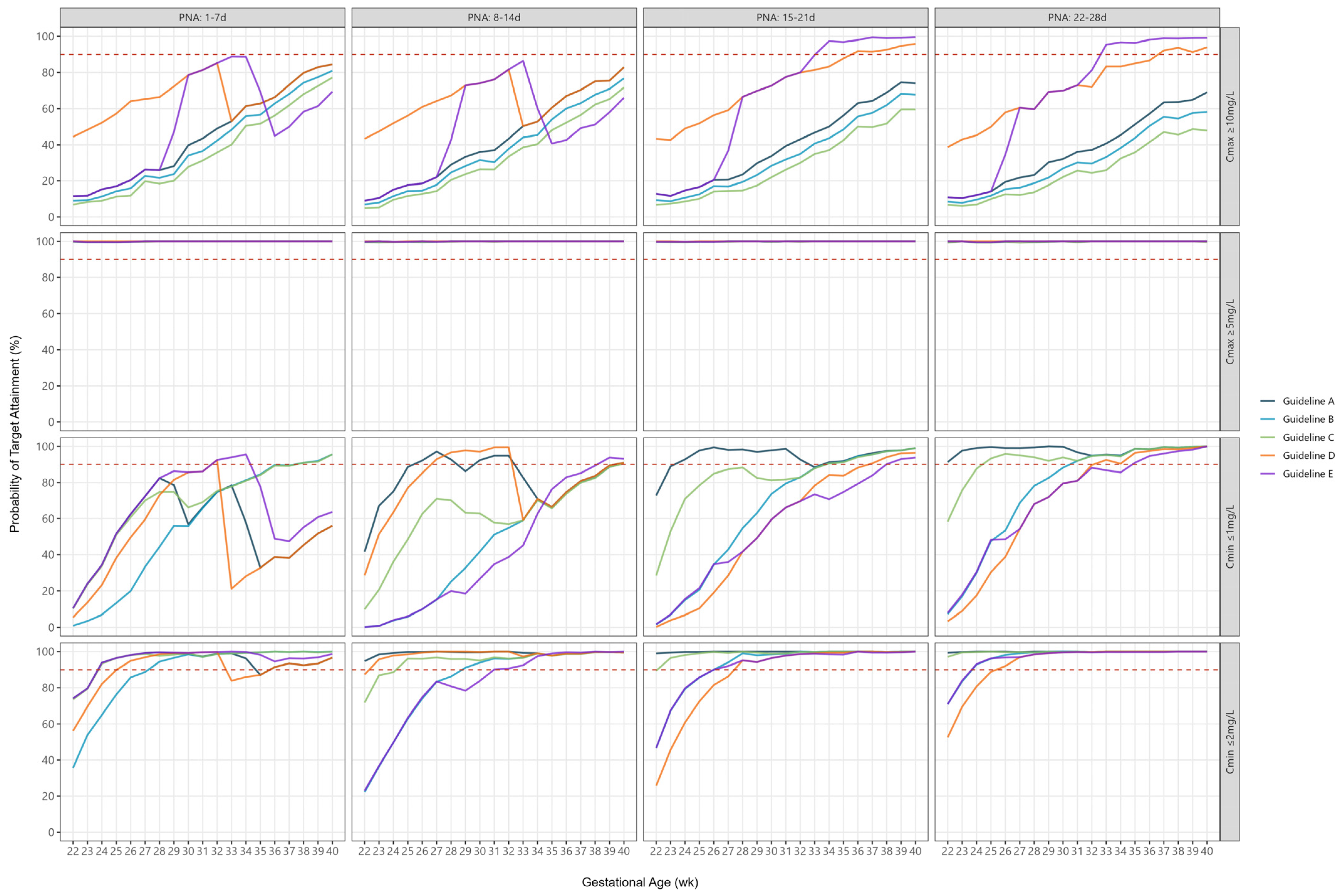
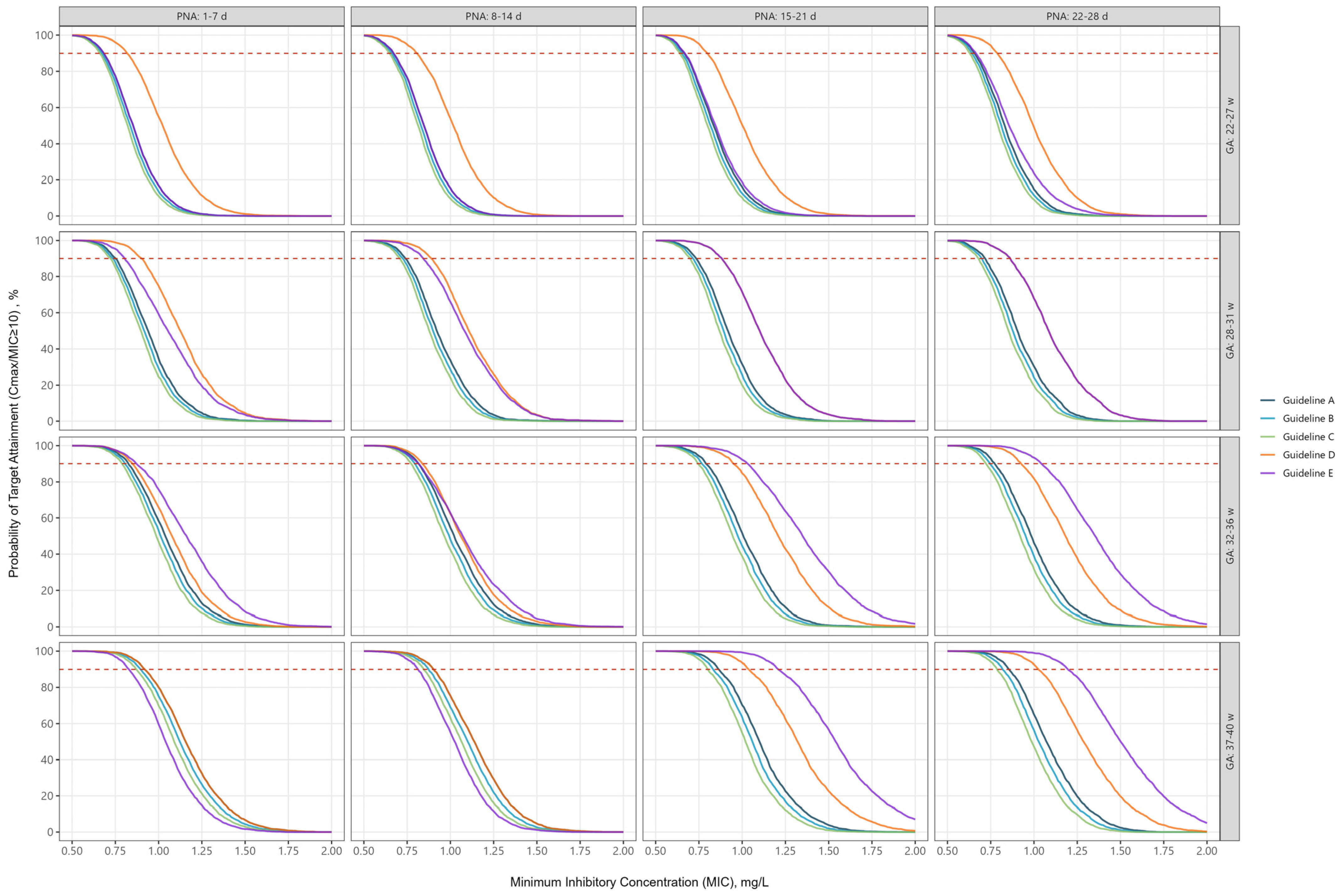
2.3. Peak Concentrations
2.4. Trough Concentrations
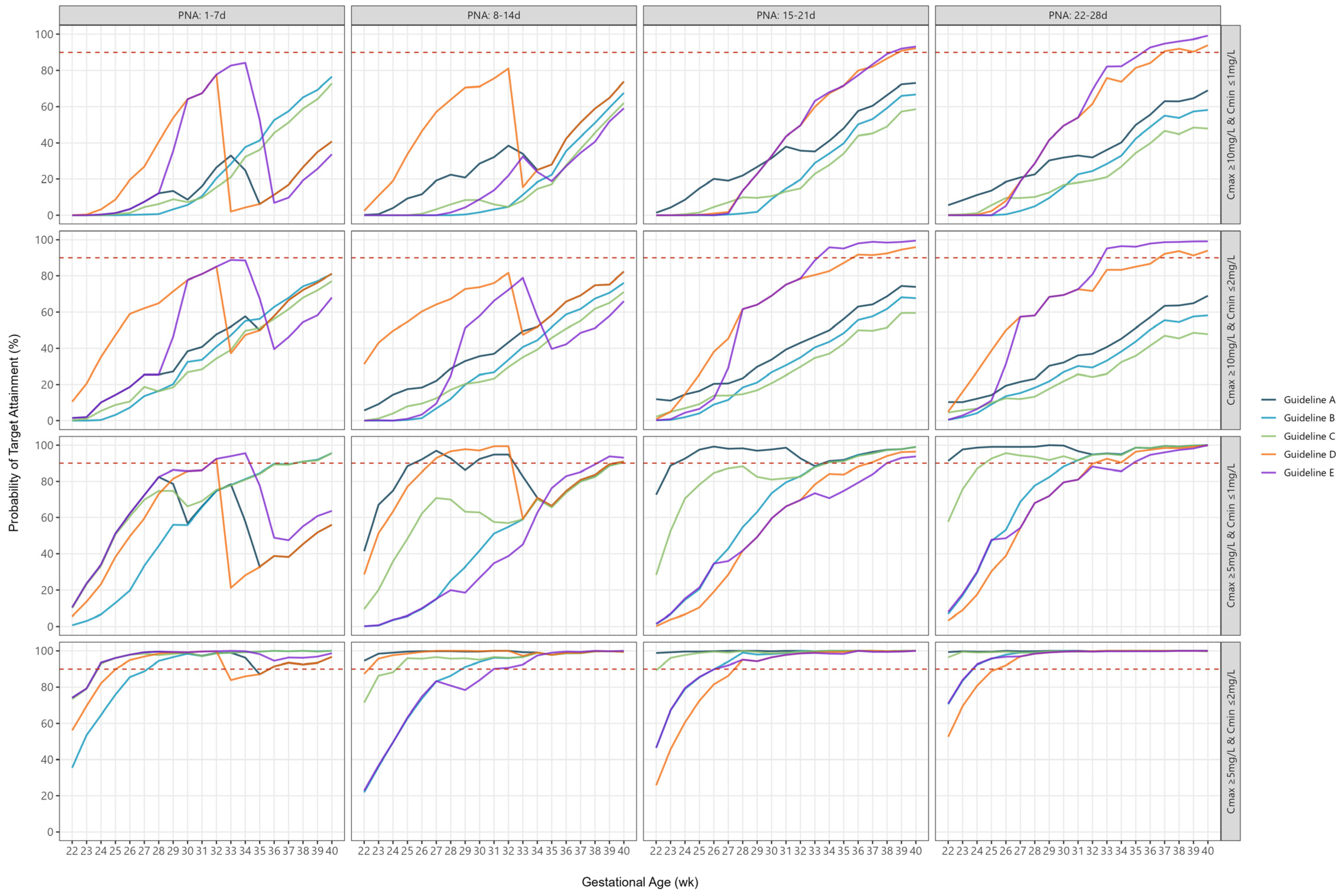
2.5. Combined Peak and Trough Concentrations
3. Discussion
4. Materials and Methods
4.1. Identification of Neonatal Gentamicin Dosing Guidelines
4.2. Population Pharmacokinetic Modelling
4.3. Probability of Target Attainment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleischmann, C.; Reichert, F.; Cassini, A.; Horner, R.; Harder, T.; Markwart, R.; Tröndle, M.; Savova, Y.; Kissoon, N.; Schlattmann, P.; et al. Global incidence and mortality of neonatal sepsis: A systematic review and meta-analysis. Arch. Dis. Child. 2021, 106, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.L.; Stortz, J.A.; Mira, J.C.; Larson, S.D.; Wynn, J.L.; Moldawer, L.L. Immunological defects in neonatal sepsis and potential therapeutic approaches. Front. Pediatr. 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, P.; Dickinson, P.; Smith, C.L. Early life response to infection. Curr. Opin. Infect. Dis. 2013, 26, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.; Barday, M.; Okomo, U.; Dramowski, A.; Sharland, M.; Bekker, A. Early-versus late-onset sepsis in neonates–time to shift the paradigm? Clin. Microbiol. Infect. 2024, 30, 38–43. [Google Scholar] [CrossRef]
- Kim, F.; Polin, R.A.; Hooven, T.A. Neonatal sepsis. BMJ 2020, 371, m3672. [Google Scholar] [CrossRef]
- Kent, A.; Kortsalioudaki, C.; Monahan, I.M.; Bielicki, J.; Planche, T.D.; Heath, P.T.; Sharland, M.; Neonatal Gram Negative MIC Group. Neonatal gram-negative infections, antibiotic susceptibility and clinical outcome: An observational study. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F507–F512. [Google Scholar] [CrossRef]
- Singh, T.; Barnes, E.H.; Isaacs, D. Early-onset neonatal infections in Australia and New Zealand, 2002–2012. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F248–F252. [Google Scholar] [CrossRef]
- Duggan, H.L.; Chow, S.S.; Austin, N.C.; Shah, P.S.; Lui, K.; Tan, K. Early-onset sepsis in very preterm neonates in Australia and New Zealand, 2007–2018. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 31–37. [Google Scholar] [CrossRef]
- McMullan, B.; Cooper, C.; Spotswood, N.; James, R.; Jones, C.; Konecny, P.; Blyth, C.; Karen, T. Antibiotic prescribing in neonatal sepsis: An Australian nationwide survey. BMJ Paediatr. Open. 2020, 4, e000643. [Google Scholar] [CrossRef]
- Bastone, E.B.; Li, S.C.; Ioannides-Demos, L.L.; Spicer, W.; McLean, A.J. Kill kinetics and regrowth patterns of Escherichia coli exposed to gentamicin concentration-time profiles simulating in vivo bolus and infusion dosing. Antimicrob. Agents Chemother. 1993, 37, 914–917. [Google Scholar] [CrossRef]
- Moore, R.D.; Lietman, P.S.; Smith, C.R. Clinical response to aminoglycoside therapy: Importance of the ratio of peak concentration to minimal inhibitory concentration. J. Infect. Dis. 1987, 155, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Turnidge, J. Pharmacodynamics and dosing of aminoglycosides. Infect. Dis. Clin. N. Am. 2003, 17, 503–528. [Google Scholar] [CrossRef] [PubMed]
- Germovsek, E.; Kent, A.; Metsvaht, T.; Lutsar, I.; Klein, N.; Turner, M.A.; Sharland, M.; Nielsen, E.I.; Heath, P.T.; Standing, J.F. Development and evaluation of a gentamicin pharmacokinetic model that facilitates opportunistic gentamicin therapeutic drug monitoring in neonates and infants. Antimicrob. Agents Chemother. 2016, 60, 4869–4877. [Google Scholar] [CrossRef] [PubMed]
- Black, M.J.; Sutherland, M.R.; Gubhaju, L.; Kent, A.L.; Dahlstrom, J.E.; Moore, L. When birth comes early: Effects on nephrogenesis. Nephrology 2013, 18, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Friis-Hansen, B. Body water compartments in children: Changes during growth and related changes in body composition: Kenneth D. Blackfan memorial lecture. Pediatrics 1961, 28, 169–181. [Google Scholar] [CrossRef]
- Fuchs, A.; Guidi, M.; Giannoni, E.; Werner, D.; Buclin, T.; Widmer, N.; Csajka, C. Population pharmacokinetic study of gentamicin in a large cohort of premature and term neonates. Br. J. Clin. Pharmacol. 2014, 78, 1090–1101. [Google Scholar] [CrossRef]
- Lingvall, M.; Reith, D.; Broadbent, R. The effect of sepsis upon gentamicin pharmacokinetics in neonates. Br. J. Clin. Pharmacol. 2005, 59, 54–61. [Google Scholar] [CrossRef]
- De Cock, R.F.; Allegaert, K.; Brussee, J.M.; Sherwin, C.M.; Mulla, H.; de Hoog, M.; van den Anker, J.N.; Danhof, M.; Knibbe, C.A. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: Towards a semi-physiological function for maturation in glomerular filtration. Pharm. Res. 2014, 31, 2643–2654. [Google Scholar] [CrossRef]
- García, B.; Barcia, E.; Pérez, F.; Molina, I.T. Population pharmacokinetics of gentamicin in premature newborns. J. Antimicrob. Chemother. 2006, 58, 372–379. [Google Scholar] [CrossRef]
- Bijleveld, Y.A.; van den Heuvel, M.E.; Hodiamont, C.J.; Mathôt, R.A.; de Haan, T.R. Population pharmacokinetics and dosing considerations for gentamicin in newborns with suspected or proven sepsis caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2017, 61, e01304-16. [Google Scholar] [CrossRef]
- Botha, J.; Du Preez, M.; Adhikari, M. Population pharmacokinetics of gentamicin in South African newborns. Eur. J. Clin. Pharmacol. 2003, 59, 755–759. [Google Scholar] [CrossRef] [PubMed]
- van Donge, T.; Pfister, M.; Bielicki, J.; Csajka, C.; Rodieux, F.; van den Anker, J.; Fuchs, A. Quantitative analysis of gentamicin exposure in neonates and infants calls into question its current dosing recommendations. Antimicrob. Agents Chemother. 2018, 62, e02004-17. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-N.R.; Mosel, C.; Grzeskowiak, L.E. Interventions to reduce medication errors in neonatal care: A systematic review. Ther. Adv. Drug Saf. 2018, 9, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Achten, N.B.; Klingenberg, C.; Benitz, W.E.; Stocker, M.; Schlapbach, L.J.; Giannoni, E.; Bokelaar, R.; Driessen, G.J.A.; Brodin, P.; Uthaya, S.; et al. Association of use of the neonatal early-onset sepsis calculator with reduction in antibiotic therapy and safety: A systematic review and meta-analysis. JAMA Pediatr. 2019, 173, 1032–1040. [Google Scholar] [CrossRef]
- Devi, R.; Priyadarshi, M.; Singh, P.; Chaurasia, S.; Basu, S. Efficacy and safety of short- vs. standard-course antibiotics for culture-negative neonatal sepsis: A systematic review and meta-analysis. J. Trop. Pediatr. 2024, 70, fmae002. [Google Scholar] [CrossRef]
- Gathwala, G.; Sindwani, A.; Singh, J.; Choudhry, O.; Chaudhary, U. Ten days vs. 14 days antibiotic therapy in culture-proven neonatal sepsis. J. Trop. Pediatr. 2010, 56, 433–435. [Google Scholar] [CrossRef]
- Rohatgi, S.; Dewan, P.; Faridi, M.M.A.; Kumar, A.; Malhotra, R.K.; Batra, P. Seven versus 10 days antibiotic therapy for cultureproven neonatal sepsis: A randomised controlled trial. J. Paediatr. Child Health 2017, 53, 556–562. [Google Scholar] [CrossRef]
- Chowdhary, G.; Dutta, S.; Narang, A. Randomized controlled trial of 7-day vs. 14-day antibiotics for neonatal sepsis. J. Trop. Pediatr. 2006, 52, 427–432. [Google Scholar] [CrossRef]
- Kumar, A. An alternate pathophysiologic paradigm of sepsis and septic shock: Implications for optimizing antimicrobial therapy. Virulence 2014, 5, 80–97. [Google Scholar] [CrossRef]
- Sumpter, A.L.; Holford, N.H. Predicting weight using postmenstrual age–neonates to adults. Paediatr. Anaesth. 2011, 21, 309–315. [Google Scholar] [CrossRef]
- Touw, D.J.; Westerman, E.M.; Sprij, A.J. Therapeutic drug monitoring of aminoglycosides in neonates. Clin. Pharmacokinet. 2009, 48, 71–88. [Google Scholar] [CrossRef]
| Guideline | Dose and Frequency | Infusion Time | Target Peak | Target Trough |
|---|---|---|---|---|
| A | PMA < 30 w: 5 mg/kg every 48 h PMA 30–35 w: 5 mg/kg every 36 h PMA ≥ 35 w: 5 mg/kg every 24 h | 5 min | 5–12 mg/L | <2 mg/L |
| B | PNA ≤ 7 d: 5 mg/kg every 36 h PNA ≥ 8 d: 5 mg/kg every 24 h | 20 min | Not Stated | <1 mg/L |
| C | Birthweight < 1200 g: PNA ≤ 7 d: 5 mg/kg every 48 h PNA 8–30 d: 5 mg/kg every 36 h Birthweight ≥ 1200 g: PNA ≤ 7 d: 5 mg/kg every 36 h PNA > 7 d: 5 mg/kg every 24 h | 30 min | Not Stated | <1 mg/L |
| D | GA < 33 w: PNA 0–14 d: 6 mg/kg every 48 h PNA > 14 d: 6 mg/kg every 24 h GA ≥ 33 w: PNA 0–14 d: 5 mg/kg every 24 h PNA > 14 d: 6 mg/kg every 24 h | 5 min | Not Stated | <1 mg/L |
| E | PMA < 30 w: PNA 0–7 d: 5 mg/kg every 48 h PNA > 7 d: 5 mg/kg every 24 h PMA 30–35 w: PNA 0–7 d: 6 mg/kg every 48 h PNA > 7 d: 6 mg/kg every 24 h PMA > 35 w: PNA 0–14 d: 4.5 mg/kg every 24 h PNA > 14 d: 7 mg/kg every 24 h | 10 min | >10 mg/L | <1 mg/L |
| Therapeutic Target | PNA Group (Days) | Guideline | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| Median (Min–Max) | ||||||
| Peak ≥ 10 mg/L | 1–7 | 44 (12–84) | 37 (9–81) | 31 (7–77) | 66 (44–85) | 50 (12–89) |
| 8–14 | 37 (9–83) | 31 (7–77) | 26 (5–72) | 67 (43–83) | 49 (9–86) | |
| 15–21 | 39 (12–74) | 32 (9–68) | 26 (7–60) | 77 (43–96) | 77 (12–100) | |
| 22–28 | 36 (11–69) | 30 (8–58) | 24 (6–49) | 72 (39–94) | 73 (11–99) | |
| Peak ≥ 5 mg/L | 1–7 | 100 (99–100) | 100 (99–100) | 100 (99–100) | 100 (100–100) | 100 (99–100) |
| 8–14 | 100 (99–100) | 100 (99–100) | 100 (99–100) | 100 (99–100) | 100 (99–100) | |
| 15–21 | 100 (99–100) | 100 (99–100) | 100 (99–100) | 100 (99–100) | 100 (99–100) | |
| 22–28 | 100 (99–100) | 100 (99–100) | 100 (99–100) | 100 (100–100) | 100 (99–100) | |
| Trough ≤ 1 mg/L | 1–7 | 56 (10–82) | 66 (1–96) | 75 (10–96) | 45 (5–92) | 64 (10–96) |
| 8–14 | 86 (42–97) | 51 (0–91) | 63 (10–90) | 84 (29–99) | 35 (0–94) | |
| 15–21 | 97 (73–99) | 80 (2–99) | 87 (29–99) | 66 (0–96) | 66 (2–94) | |
| 22–28 | 99 (91–100) | 92 (7–100) | 95 (58–100) | 81 (3–100) | 81 (8–100) | |
| Trough ≤ 2 mg/L | 1–7 | 96 (74–99) | 98 (36–100) | 99 (74–100) | 93 (56–99) | 98 (74–100) |
| 8–14 | 99 (95–100) | 96 (22–99) | 97 (72–99) | 99 (87–100) | 90 (23–100) | |
| 15–21 | 100 (99–100) | 99 (47–100) | 99 (89–100) | 98 (26–100) | 98 (47–100) | |
| 22–28 | 100 (99–100) | 100 (71–100) | 100 (97–100) | 99 (53–100) | 99 (71–100) | |
| Peak ≥ 10 mg/L and Trough ≤ 1 mg/L | 1–7 | 12 (0–41) | 11 (0–76) | 10 (0–73) | 20 (0–78) | 19 (0–84) |
| 8–14 | 28 (0–74) | 3 (0–68) | 8 (0–62) | 51 (2–81) | 14 (0–59) | |
| 15–21 | 35 (1–73) | 15 (0–67) | 13 (0–59) | 44 (0–92) | 44 (0–93) | |
| 22–28 | 32 (6–69) | 23 (0–58) | 18 (0–48) | 54 (0–94) | 54 (0–99) | |
| Peak ≥ 10 mg/L and Trough ≤ 2 mg/L | 1–7 | 41 (1–81) | 34 (0–81) | 28 (0–77) | 62 (11–85) | 46 (1–89) |
| 8–14 | 37 (6–82) | 27 (0–76) | 23 (0–71) | 66 (31–82) | 49 (0–79) | |
| 15–21 | 39 (11–74) | 31 (0–68) | 25 (2–60) | 75 (1–96) | 75 (0–99) | |
| 22–28 | 36 (10–69) | 29 (0–58) | 24 (4–49) | 72 (5–94) | 73 (0–99) | |
| Peak ≥ 5 mg/L and Trough ≤ 1 mg/L | 1–7 | 56 (10–82) | 66 (1–96) | 75 (10–96) | 45 (5–92) | 64 (10–96) |
| 8–14 | 86 (41–97) | 51 (0–91) | 63 (9–90) | 84 (29–99) | 35 (0–94) | |
| 15–21 | 97 (73–99) | 80 (1–99) | 87 (28–99) | 66 (0–96) | 66 (2–94) | |
| 22–28 | 99 (91–100) | 92 (7–100) | 94 (58–99) | 81 (3–100) | 81 (8–100) | |
| Peak ≥ 5 mg/L and Trough ≤ 2 mg/L | 1–7 | 96 (74–99) | 98 (36–100) | 99 (73–100) | 93 (56–99) | 98 (74–100) |
| 8–14 | 99 (95–100) | 96 (22–99) | 97 (71–99) | 99 (87–100) | 90 (23–100) | |
| 15–21 | 100 (99–100) | 99 (46–100) | 99 (89–100) | 98 (82–100) | 98 (46–99) | |
| 22–28 | 100 (99–100) | 99 (70–100) | 99 (96–100) | 99 (53–100) | 99 (71–100) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzeskowiak, L.E.; Wynne, S.; Stark, M.J. A Quantitative Examination and Comparison of the Ability of Australian Gentamicin Dosing Guidelines to Achieve Target Therapeutic Concentrations in Neonates. Antibiotics 2025, 14, 48. https://doi.org/10.3390/antibiotics14010048
Grzeskowiak LE, Wynne S, Stark MJ. A Quantitative Examination and Comparison of the Ability of Australian Gentamicin Dosing Guidelines to Achieve Target Therapeutic Concentrations in Neonates. Antibiotics. 2025; 14(1):48. https://doi.org/10.3390/antibiotics14010048
Chicago/Turabian StyleGrzeskowiak, Luke E., Sheree Wynne, and Michael J. Stark. 2025. "A Quantitative Examination and Comparison of the Ability of Australian Gentamicin Dosing Guidelines to Achieve Target Therapeutic Concentrations in Neonates" Antibiotics 14, no. 1: 48. https://doi.org/10.3390/antibiotics14010048
APA StyleGrzeskowiak, L. E., Wynne, S., & Stark, M. J. (2025). A Quantitative Examination and Comparison of the Ability of Australian Gentamicin Dosing Guidelines to Achieve Target Therapeutic Concentrations in Neonates. Antibiotics, 14(1), 48. https://doi.org/10.3390/antibiotics14010048





