Antimicrobial Effects of Metal Coatings or Physical, Chemical Modifications of Titanium Dental Implant Surfaces for Prevention of Peri-Implantitis: A Systematic Review of In Vivo Studies
Abstract
1. Introduction
2. Results
2.1. Animal and Human Models and Follow-Up
2.2. Implant Surface Modification
2.3. Implant Characteristics
2.4. Infection Set-Up Procedure and Antibacterial Efficacy Test
2.5. In Vivo Antibacterial Results
3. Discussion
Limitations to Consider
4. Materials and Methods
4.1. Protocol Development
- PICO question:
- P (Participants/Test group): Titanium dental implants in human/animal models.
- I (Intervention): Ti surfaces after physical/morphological modifications and/or modifications using various metal elements.
- C (Control group): Ti surfaces without modifications or coatings.
- O (Outcome): in vivo antimicrobial effect.
4.2. Search Strategy
- PubMed:
- Embase:
- Scopus:
- Web of Science:
4.3. Study Inclusion/Exclusion Criteria
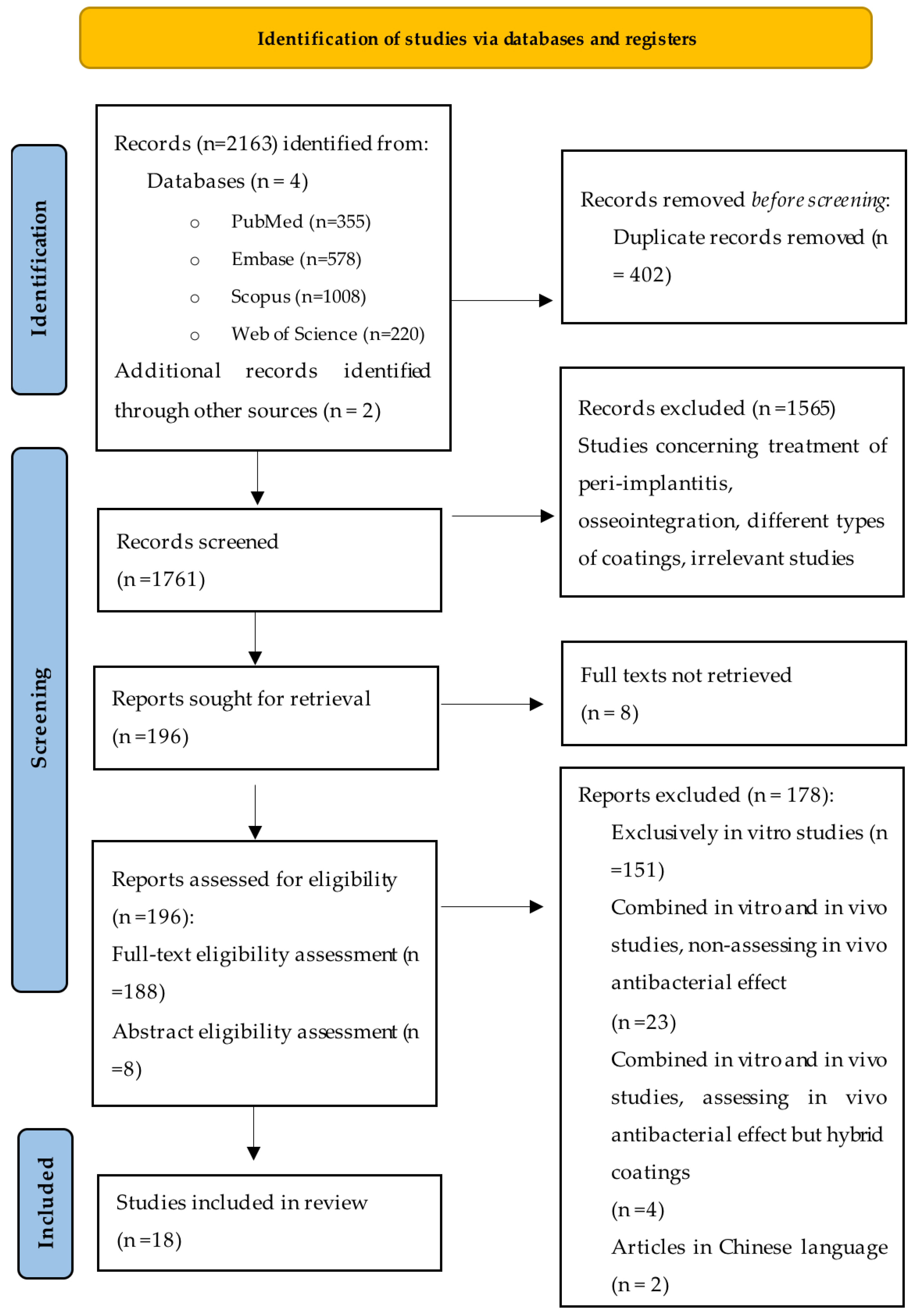
4.4. Identification and Study Selection
4.5. Data Extraction
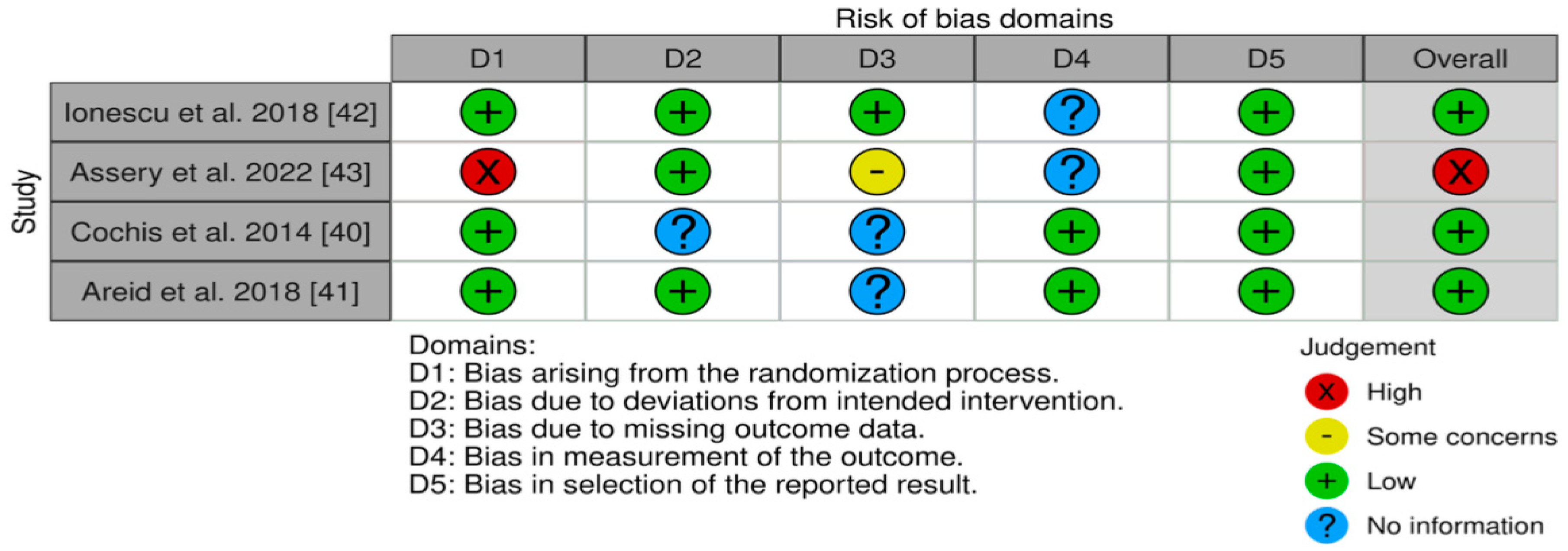
4.6. Risk of Bias and Assessment of Quality for the Selected Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennes, M.E.; Naumann, M.; Peroz, S.; Beuer, F.; Schmidt, F. Antibacterial effects of modified implant abutment surfaces for the prevention of peri-implantitis—A systematic review. Antibiotics 2021, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Brunetto, P.S.; Woischnig, A.-K.; Varisco, M.; Rajacic, Z.; Vosbeck, J.; Terracciano, L.; Fromm, K.M.; Khanna, N. Preventing Implant-Associated infections by silver coating. Antimicrob. Agents Chemother. 2016, 60, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Quirynen, M. Risk indicators for peri-implantitis. A narrative review. Clin. Oral Implant. Res. 2015, 26, 15–44. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N. Microbiological and immuno-pathological aspects of peri-implant diseases. Arch. Oral Biol. 2014, 59, 66–72. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Aaboe, M.; Araujo, M.; Carrión, J.B.; Cavalcanti, R.; Cionca, N.; Cochran, D.; Darby, I.; Funakoshi, E.; Gierthmuehlen, P.C.; et al. Group 4 ITI Consensus Report: Risks and biologic complications associated with implant dentistry. Clin. Oral Implant. Res. 2018, 29, 351–358. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-implant Diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and treatment of peri-implant diseases—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50, 4–76. [Google Scholar] [CrossRef]
- Roccuzzo, A.; De Ry, S.P.; Sculean, A.; Roccuzzo, M.; Salvi, G.E. Current Approaches for the Non-surgical Management of Peri-implant Diseases. Curr. Oral Health Rep. 2020, 7, 274–282. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Stähli, A.; Monje, A.; Sculean, A.; Salvi, G.E. Peri-implantitis: A clinical update on prevalence and surgical treatment outcomes. J. Clin. Med. 2021, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, H.; Bakitian, F.; Neilands, J.; Andersen, O.Z.; Stavropoulos, A. Antimicrobial Properties of Strontium Functionalized Titanium Surfaces for Oral Applications, A Systematic Review. Coatings 2021, 11, 810. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Qiu, W.; Fang, F. Overview of Antibacterial Strategies of Dental Implant Materials for the Prevention of Peri-Implantitis. Bioconjugate Chem. 2021, 32, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomaterialia. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface modified techniques and emerging functional coating of dental implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. Biomed. Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Alipal, J.; Lee, T.C.; Koshy, P.; Abdullah, H.Z.; Idris, M.I. Evolution of anodised titanium for implant applications. Heliyon 2021, 7, e07408. [Google Scholar] [CrossRef]
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings 2020, 10, 568. [Google Scholar] [CrossRef]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018, 9, 492–495. [Google Scholar] [CrossRef]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 220. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Inchingolo, A.D.; Mancini, A.; Annicchiarico, C.; Inchingolo, F.; Dipalma, G.; et al. Surface Coatings of Dental Implants: A Review. J. Funct. Biomater. 2023, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef]
- Sun, H.; Chan, Y.; Li, X.; Xu, R.; Zhang, Z.; Hu, X.; Wu, F.; Deng, F.; Yu, X. Multi-omics analysis of oral bacterial biofilm on titanium oxide nanostructure modified implant surface: In vivo sequencing-based pilot study in beagle dogs. Mater. Today Bio 2022, 15, 100275. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Luo, H.; Zhang, D.; Lei, S.; Zhou, K. Dual-Purpose Magnesium-Incorporated Titanium Nanotubes for Combating Bacterial Infection and Ameliorating Osteolysis to Realize Better Osseointegration. ACS Biomater. Sci. Eng. 2019, 5, 5368–5383. [Google Scholar] [CrossRef]
- Wang, X.; Fan, H.; Zhang, F.; Zhao, S.; Liu, Y.; Xu, Y.; Wu, R.; Li, D.; Yang, Y.; Liao, L.; et al. Antibacterial Properties of Bilayer Biomimetic Nano-ZnO for Dental Implants. ACS Biomater. Sci. Eng. 2020, 6, 1880–1886. [Google Scholar] [CrossRef]
- Lu, S.; Li, R.; Chai, M.; Wang, J.; Duan, W.; Yao, X.; Zhang, X.; Tang, B. Nanostructured Cu-doped TiO2 with photothermal effect for prevention of implant-associated infection. Colloids Surf. B Biointerfaces 2022, 217, 112695. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Palmer, J.A.; Bock, N.; Reynolds, E.C.; Webster, T.J.; Deva, A.; Morrison, W.A.; O’Connor, A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019, 14, 4613–4624. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Abraham, S.; Stephen, S.; Babu, A.S.; Gowd, S.G.; Vinod, V.; Biswas, R.; Nair, M.B.; Unni, A.K.K.; Menon, D. Superhydrophilic multifunctional nanotextured titanium dental implants: In vivo short and long-term response in a porcine model. Biomater. Sci. 2022, 10, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Shi, X.; Li, X.; Liu, W.; Liu, Y.; Zhang, R.; Yu, Y.; Su, J. Mesoporous TiO2 Coatings Regulate ZnO Nanoparticle Loading and Zn2+ Release on Titanium Dental Implants for Sustained Osteogenic and Antibacterial Activity. ACS Appl. Mater. Interfaces 2023, 15, 15235–15249. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Sun, N.; Jiang, F.; Lu, Y.; Yang, G.; Wu, X.; Lin, S.; Zhang, W.; Jiang, X. The Translation from In Vitro Bioactive Ion Concentration Screening to In Vivo Application for Preventing Peri-implantitis. ACS Appl. Mater. Interfaces 2021, 13, 5782–5794. [Google Scholar] [CrossRef]
- Liu, H.; Tang, Y.; Zhang, S.; Liu, H.; Wang, Z.; Li, Y.; Wang, X.; Ren, L.; Yang, K.; Qin, L. Anti-infection mechanism of a novel dental implant made of titanium-copper (TiCu) alloy and its mechanism associated with oral microbiology. Bioact. Mater. 2021, 8, 381–395. [Google Scholar] [CrossRef]
- Zhao, F.; Gao, A.; Liao, Q.; Li, Y.; Ullah, I.; Zhao, Y.; Ren, X.; Tong, L.; Li, X.; Zheng, Y.; et al. Balancing the Anti-Bacterial and Pro-Osteogenic Properties of Ti-Based Implants by Partial Conversion of ZnO Nanorods into Hybrid Zinc Phosphate Nanostructures. Adv. Funct. Mater. 2024, 34, 2311812. [Google Scholar] [CrossRef]
- Pan, Q.; Zheng, Y.; Zhou, Y.; Zhang, X.; Yuan, M.; Guo, J.; Xu, C.; Cheng, Z.; Kheraif AA, A.; Liu, M.; et al. Doping Engineering of Piezo-Sonocatalytic Nanocoating Confer Dental Implants with Enhanced Antibacterial Performances and Osteogenic Activity. Adv. Funct. Mater. 2024, 34, 2313553. [Google Scholar] [CrossRef]
- Wang, X.; Dong, H.; Liu, J.; Qin, G.; Chen, D.; Zhang, E. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater. Sci. Eng. C 2019, 100, 38–47. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; Chiesa, R.; Arciola, C.R.; Rimondini, L. Biofilm formation on titanium implants counteracted by grafting gallium and silver ions. J. Biomed. Mater. Res. Part A 2014, 103, 1176–1187. [Google Scholar] [CrossRef]
- Areid, N.; Söderling, E.; Tanner, J.; Kangasniemi, I.; Närhi, T.O. Early Biofilm Formation on UV Light Activated Nanoporous TiO2 Surfaces In Vivo. Int. J. Biomater. 2018, 2018, 7275617. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Brambilla, E.; Azzola, F.; Ottobelli, M.; Pellegrini, G.; Francetti, L.A.C. Laser microtextured titanium implant surfaces reduce in vitro and in situ oral biofilm formation. PLoS ONE 2018, 13, e0202262. [Google Scholar] [CrossRef]
- Assery, N.; Alomeir, N.; Zeng, Y.; Xiao, J.; Tsigarida, A. The effect of Er:YAG laser treatment on biofilm formation on titanium and zirconia disc surfaces. J. Periodontol. 2023, 94, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, X.; Liang, Z.; Wei, Z.; Chen, Z.; Wang, Y.; Li, W.; Zhang, W.; Yang, R.; Qiu, H.; et al. A programmed surface on dental implants sequentially initiates bacteriostasis and osseointegration. Colloids Surf. B Biointerfaces 2023, 230, 113477. [Google Scholar] [CrossRef]
- Duarte, P.M.; Reis, A.F.; de Freitas, P.M.; Ota-Tsuzuki, C. Bacterial Adhesion on Smooth and Rough Titanium Surfaces after Treatment with Different Instruments. J. Periodontol. 2009, 80, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, H.; Qiao, S.; Gu, Y.; Luo, H.; Liu, X.; Lai, H.; Wang, M.; Meng, F. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomed. 2015, 10, 6659–6674. [Google Scholar] [CrossRef]
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial dental implant functionalization strategies—A systematic review. Dent. Mater. J. 2016, 35, 545–558. [Google Scholar] [CrossRef]
- Shi, A.; Zhu, C.; Fu, S.; Wang, R.; Qin, G.; Chen, D.; Zhang, E. What controls the antibacterial activity of Ti-Ag alloy, Ag ion or Ti2Ag particles? Mater. Sci. Eng. C 2019, 109, 110548. [Google Scholar] [CrossRef]
- Soma, T.; Iwasaki, R.; Sato, Y.; Kobayashi, T.; Ito, E.; Matsumoto, T.; Kimura, A.; Homma, F.; Saiki, K.; Takahashi, Y.; et al. An ionic silver coating prevents implant-associated infection by anaerobic bacteria in vitro and in vivo in mice. Sci. Rep. 2022, 12, 18387. [Google Scholar] [CrossRef]
- Pruchova, E.; Kosova, M.; Fojt, J.; Jarolimova, P.; Jablonska, E.; Hybasek, V.; Joska, L. A two-phase gradual silver release mechanism from a nanostructured TiAlV surface as a possible antibacterial modification in implants. Bioelectrochemistry 2019, 127, 26–34. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Fernández-Arias, M.; del Val, J.; Buxadera-Palomero, J.; Rodríguez, D.; Lusquiños, F.; Gil, F.; Pou, J. Synthesis and deposition of silver nanoparticles on cp Ti by laser ablation in open air for antibacterial effect in dental implants. Mater. Lett. 2018, 231, 126–129. [Google Scholar] [CrossRef]
- Chen, C.J.; Ding, S.J.; Chen, C.C. Effects of surface conditions of titanium dental implants on bacterial adhesion. Photomed. Laser Surg. 2016, 34, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Esteves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and Antibiofilm Coating of Dental Implants—Past and New Perspectives. Antibiotics 2022, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Cao, H.; Zhao, Y.; Jin, G.; Cheng, M.; Wang, J.; Jiang, Y.; An, Z.; Zhang, X.; Liu, X. Antimicrobial and Osteogenic Properties of Silver-Ion-Implanted Stainless Steel. ACS Appl. Mater. Interfaces. 2015, 7, 10785–10794. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Qiao, Y.; Zhu, H.; Ding, C. Antimicrobial activity and cytocompatibility of Ag plasma-modified hierarchical TiO2 film on titanium surface. Colloids Surf. B Biointerfaces 2014, 113, 134–145. [Google Scholar] [CrossRef]
- Chen, M.; Yang, L.; Zhang, L.; Han, Y.; Lu, Z.; Qin, G.; Zhang, E. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater. Sci. Eng. C 2017, 75, 906–917. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Silva Lima Mendes, D.T.; Leite Matos, G.R.; Stwart de Araújo Souza, S.A.; Souza Silva Macedo, M.C.; Tavares, D.D.S.; Resende, C.X. Does the incorporation of zinc into TiO2 on titanium surfaces increase bactericidal activity? A systematic review and meta-analysis. J. Prosthet. Dent. 2024, 13, 510–519. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Gupta, A.; Makabenta, J.M.V.; Park, J.; Amante, J.J.; Chattopadhyay, A.N.; Matuwana, D.; Kearney, C.J.; Rotello, V.M. Ultrasound-Enhanced Antibacterial Activity of Polymeric Nanoparticles for Eradicating Bacterial Biofilms. Adv. Healthc. Mater. 2022, 11, 2201060. [Google Scholar] [CrossRef]
- Wang, R.; He, X.; Gao, Y.; Zhang, X.; Yao, X.; Tang, B. Antimicrobial property, cytocompatibility and corrosion resistance of Zn-doped ZrO2/TiO2 coatings on Ti6Al4V implants. Mater. Sci. Eng. C 2017, 75, 7–15. [Google Scholar] [CrossRef]
- Pan, C.; Liu, T.; Yang, Y.; Liu, T.; Gong, Z.; Wei, Y.; Quan, L.; Yang, Z.; Liu, S. Incorporation of Sr2+ and Ag nanoparticles into TiO2 nanotubes to synergistically enhance osteogenic and antibacterial activities for bone repair. Mater. Des. 2020, 196, 109086. [Google Scholar] [CrossRef]
- Hameed, H.A.; Hasan, H.A.; Luddin, N.; Husein, A.; Ariffin, A.; Alam, M.K. Osteoblastic Cell Responses of Copper Nanoparticle Coatings on Ti-6Al-7Nb Alloy Using Electrophoretic Deposition Method. Biomed. Res. Int. 2022, 2022, 3675703. [Google Scholar] [CrossRef] [PubMed]
- Hang, R.; Gao, A.; Huang, X.; Wang, X.; Zhang, X.; Qin, L.; Tang, B. Antibacterial activity and cytocompatibility of Cu–Ti–O nanotubes. J. Biomed. Mater. Res. Part A 2013, 102, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, S.; Zhang, Y.; Jin, D.; Lin, Z.; Tao, X.; Chen, T.; Zheng, L.; Zhang, Z.; Wu, Q. Titanium surfaces with biomimetic topography and copper incorporation to modulate behaviors of stem cells and oral bacteria. Front. Bioeng. Biotechnol. 2023, 11, 1223339. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Eifler, R.; Rais, B.; Mueller, P.P. Alkalization is responsible for antibacterial effects of corroding magnesium. J. Biomed. Mater. Res. A 2015, 103, 3526–3532. [Google Scholar] [CrossRef]
- Rahim, M.I.; Rohde, M.; Rais, B.; Seitz, J.M.; Mueller, P.P. Susceptibility of metallic magnesium implants to bacterial biofilm infections. J. Biomed. Mater. Res. A 2016, 104, 1489–1499. [Google Scholar] [CrossRef]
- Liu, Z.; Schade, R.; Luthringer, B.; Hort, N.; Rothe, H.; Müller, S.; Liefeith, K.; Willumeit-Römer, R.; Feyerabend, F. Influence of the Microstructure and Silver Content on Degradation, Cytocompatibility, and Antibacterial Properties of Magnesium-Silver Alloys in Vitro. Oxidative Med. Cell. Longev. 2017, 2017, 8091265. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Jiang, W.; Bai, H.; Liu, H.; Wang, J. In Vivo Antibacterial Efficacy of Nanopatterns on Titanium Implant Surface: A Systematic Review of the Literature. Antibiotics 2021, 10, 1524. [Google Scholar] [CrossRef]
- Gulati, K.; Ivanovski, S. Dental implants modified with drug releasing titania nanotubes: Therapeutic potential and developmental challenges. Expert Opin. Drug Deliv. 2016, 14, 1009–1024. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Nath, S.; Sugawara, Y.; Divakarla, K.; Das, T.; Manos, J.; Chrzanowski, W.; Matsushita, T.; Kokubo, T. Two-in-One Biointerfaces-Antimicrobial and Bioactive Nanoporous Gallium Titanate Layers for Titanium Implants. Nanomaterials 2017, 7, 229. [Google Scholar] [CrossRef]
- Narendrakumar, K.; Kulkarni, M.; Addison, O.; Mazare, A.; Junkar, I.; Schmuki, P.; Sammons, R.; Iglič, A. Adherence of oral streptococci to nanostructured titanium surfaces. Dent. Mater. 2015, 31, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, J.; Alcudia, A.; Begines, B.; Beltrán, A.M.; Rodríguez-Ortiz, J.A.; Trueba, P.; Villarraga, J.; Torres, Y. Biofunctionalization of Porous Ti Substrates Coated with Ag Nanoparticles for Potential Antibacterial Behavior. Metals 2021, 11, 692. [Google Scholar] [CrossRef]
- Parnia, F.; Yazdani, J.; Javaherzadeh, V.; Maleki Dizaj, S. Overview of Nanoparticle Coating of Dental Implants for Enhanced Osseointegration and Antimicrobial Purposes. J. Pharm. Pharm. Sci. 2017, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Golshan, N.H.; Deng, X.; Hickey, D.J.; Zeimer, K.; Li, H.; Webster, T.J. Selenium nanoparticles incorporated into titania nanotubes inhibit bacterial growth and macrophage proliferation. Nanoscale 2016, 8, 15783–15794. [Google Scholar] [CrossRef]
- Park, J.H.; Heo, S.J.; Koak, J.Y.; Kim, S.K.; Han, C.H.; Lee, J.H. Effects of Laser Irradiation on Machined and Anodized Titanium Disks. Int. J. Oral Maxillofac. Implant. 2012, 27, 265–272. [Google Scholar]
- Galli, C.; Macaluso, G.M.; Elezi, E.; Ravanetti, F.; Cacchioli, A.; Gualini, G.; Passeri, G. The Effects of Er:YAG Laser Treatment on Titanium Surface Profile and Osteoblastic Cell Activity: An In Vitro Study. J. Periodontol. 2011, 82, 1169–1177. [Google Scholar] [CrossRef]
- Romanos, G.E.; Everts, H.; Nentwig, G.H. Effects of Diode and Nd:YAG Laser Irradiation on Titanium Discs: A Scanning Electron Microscope Examination. J. Periodontol. 2000, 71, 810–815. [Google Scholar] [CrossRef]
- Schwarz, F.; Rothamel, D.; Sculean, A.; Georg, T.; Scherbaum, W.; Becker, J. Effects of an Er: YAG laser and the Vector® ultrasonic system on the biocompatibility of titanium implants in cultures of human osteoblast-like cells. Clin. Oral Implant. Res. 2003, 14, 784–792. [Google Scholar] [CrossRef]
- Kreisler, M.; Kohnen, W.; Christoffers, A.; Götz, H.; Jansen, B.; Duschner, H.; D’Hoedt, B. In vitro evaluation of the biocompatibility of contaminated implant surfaces treated with an Er: YAG laser and an air powder system. Clin. Oral Implant. Res. 2004, 16, 36–43. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, H.; Zhang, L.; Yan, X.; Zhu, B.; Meng, H. Peri-implant mucositis sites with suppuration have higher microbial risk than sites without suppuration. J. Periodontol. 2020, 91, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Chun Giok, K.; Menon, R.K. The Microbiome of Peri-Implantitis: A Systematic Review of Next-Generation Sequencing Studies. Antibiotics 2023, 12, 1610. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef]
| Studies | In Vivo Model | Location of Implant Placement | Surface Modification | Procedure of Infection Set-Up | Follow-Up | Antibacterial Efficacy Tests | Conclusions |
|---|---|---|---|---|---|---|---|
| Animals | |||||||
| Rats | |||||||
| Pan et al., 2024 [38] | 30 eight-week-old male SD rats (Sprague–Dawley) | In the alveolar bone in front of the maxillary first molar. | Anodization (5 h, 50 V), annealing treatment (450 °C, 2 h) and hydrothermal treatment to construct SrTiO3/TiO2 nanotubes (STNT) and and Al-STNT. Ultrasound treatment every 2 days with a power of 1.5 W cm−2 for 5 min, 7 treatments over 2 weeks. | After one month of implantation, the peri-implantitis model was constructed by a silk 4-0 ligature around the neck of the implant and bacteria injection (5 × 107 CFU/mL P. gingivalis and 5 × 107 CFU/mL F. nucleatum) every other day around the thread, for 2 weeks. | 2 weeks | Hematoxylin and eosin (H&E) staining Giemsa staining method Bacterial culture. | “After ultrasound treatment, bacteria stained in dark purple were abundantly distributed in the epithelial and submucosal layers in the Ti group. The bacteria in the mucosa of the other experimental groups gradually decreased, and almost no bacteria stained by Giemsa were observed in the last Al-STNT group.” “In vivo experiments proved that the ultrasound-treated Al-STNT implant could effectively resist bacteria-induced peri-implantitis.” |
| Wen et al., 2023 [34] | Eight male SD rats | The buccal, mesial root alveolar fossa of the first maxillary molar after tooth extraction. | ZnO nanoparticle loaded mesoporous TiO2 coatings (nZnO/MTC-Ti) via the evaporation-induced self-assembly method (EISA) and one-step spin coating. | Porphyromonas gingivalis (Pg) at a density of 104/mL. | 14 days | Two groups of bacterial culture under aerobic and anaerobic conditions, respectively, for 24 h. Finally, the culture solution was photographed, and its optical density value was read at 600 nm. | “The synergistic effects of MTC and ZnO NPs on nZnO/MTC-Ti could control the Zn2+ release at long-term, steady rates while avoiding overdose cytotoxicity without triggering excessive ROS or inducing cell apoptosis.” “Compared to Ti, MTC-Ti also exhibited mild bacterial resistance, suggesting the enhanced inhibitory effects of the mesoporous structure on bacterial adhesion.” |
| Lu et al., 2022 [30] | Nine rats | The dorsal area of each rat. | Cu doped TiO2 (TiO2-Cu) films were prepared on Ti by magnetron sputtering and subsequent annealing. Irradiation with 808 nm (0.8 W cm− 2) NIR light for 20 min. | 50 μL of S. mutans (1 × 105 CFU/mL) was dropped in for 5 min. | 2 days | Spread plate technique Photothermal images of Ti, TiO2-0Cu and TiO2-1Cu after 10 min and 20 min in vivo irradiation of NIR light. | “Upon the irradiation of 808 nm NIR light, TiO2-1Cu film with low Cu content also showed favorable in vivo antibacterial activity due to the synergistic effect of photothermal effect and Cu.” “Large number of bacteria still remained on the agar plates of Ti and TiO2-0Cu groups, while only a few bacteria were found on the agar plates of TiO2-1Cu group, and the antibacterial efficiency reached 96%, showing the excellent in vivo antibacterial performance.” |
| Wang et al., 2020 [29] | 10 male SD rats (Sprague–Dawley) | The dorsal area of each rat. | Ti ZnO nanospheres Ti ZnO nanorods Ti ZnO nanorods−nanospheres hierarchical structure (NRS) Ti-Zr ZnO nanospheres Ti-Zr ZnO nanorods Ti-Zr ZnO nanorods−nanospheres hierarchical structure (NRS) | A 100 μL injection of the bacterial suspension of S. aureus was injected to the implant position once a day for 2 weeks. | 2 weeks | Bacterial culture/ plate colony counting method | “ZnO nanorods and ZnO nanospheres both had certain levels of antibacterial property, while ZnO NRS had the best antibacterial effect.” “The experimental results exhibited that these doubled-layered structures had great antibacterial activity, good stability, and low toxicity.” |
| Yang et al., 2019 [28] | 45 male SD rats | The femoral medullary cavity in the left knee. | Magnesium (Mg)-incorporated nanotube-modified titanium implants (NT-Mg) by electrochemical anodization and hydrothermal treatment NT-Mg3/3 h. | 50 μL of PBS containing methicillin-resistant Staphylococcus aureus, 5378 [MRSA], ATCC43300 at a concentration of 1 × 106 CFUs/mL was injected into the medullary cavity using a micropipette. | 3, 4, and 35 days | X-ray Micro-CT Histopathological analysis Giemsa staining was used to assess bacterial burden in decalcified histological transverse sections. | “The NT group exhibited slightly reduced radiographic scores compared with the Ti group at day 35 post-surgery (p < 0.05), indicating that the nanotubular structure itself also exhibited slight anti-infection potential in vivo.” “Histopathological scores in the Ti and NT groups were all significantly increased compared with the NT-Mg group (p < 0.01). The total scores in the NT group were also slightly reduced compared with the Ti group (p < 0.05), indicating alleviated bone infection in the NT group.” |
| Tran et al., 2019 [31] | SD rats, number? | Two femurs for each rat, femoral medullary cavity. | Two-hole 1.5 mm titanium plates and screws, titanium substrates coated at a density of approximately 6 × 106 particles/mm2 Se NP through surface-induced nucleation-deposition. | 105 CFU for MRSE and 102 CFU for MRSA were injected in 100 μL of sterile 0.9% saline on top of the plate. | 4 weeks | Scanning electron microscopy SEM imaging Immunostaining for bacteria with rabbit anti-MRSA and mouse anti-Staph epidermidis. Colony counting of biofilm bacteria of extracted screws and of surrounding tissue after swabbing wound pockets. | “Confocal microscopy imaging of the bacteria within the biofilms showed thick and dense layers on the uncoated plates compared to more individual, separated bacteria and bacterial aggregates on the coated plates.” |
| Jin et al., 2014 [32] | 20 male SD rats (four groups of five) | The left femoral medullary cavity. | Plasma immersion ion implantation of Ag and Zn at 30 kV for 90 min. | 20 mL of the bacterial suspension with a concentration of 104 CFUs/mL S. aureus. | 6 weeks | Bacterial culture Radiographic examination (osseous destruction) Histological evaluation (Giemsa staining) | “The bacterial growth on the Ag-PIII and Zn/Ag-PIII groups on agar plates is significantly reduced and the corresponding TSB cultures are negative (clear appearance) disclosing that both Ag-PIII and Zn/Ag-PIII have excellent antibacterial ability in vivo.” |
| Mice | |||||||
| Kuehl et al., 2016 [2] | Female C57BL/6 mice, at least 39? Nineteen mice for S. epidermis eight mice for S. aureus, at least twelve for combination with DAP/VAN | The dorsal area of each mouse. | Ag-coated titanium-aluminum-niobium (TiAlNb) alloy | 5 × 102 to 1 × 108 CFU of bacteria S. epidermidis 1457, which were injected directly into the cage percutaneously either immediately after implantation (i.e., perioperative infection) or 2 weeks later (i.e., postoperative infection). S. aureus SA113 in a perioperative infection using the minimal infective dose of 1 × 103 CFU per cage. | 2, 6, 9, and 14 days for S. epidermidis | Samples of TCF (tissue cage fluid) on MHA (Mueller Hinton agar) plates. | “The Ag coating prevents an infection with S. epidermidis in an inoculum- and time-dependent manner.” “As single agents, neither preoperative DAP nor VAN nor Ag-coated cages were sufficient to prevent a persistent infection with MRSA. Remarkably, in combination with preoperatively applied DAP, Ag coating prevented the growth of planktonic as well as adherent MRSA cells, resulting in a 100% prevention rate.” |
| Dogs | |||||||
| Sun et al., 2023 [27] | Three adult male beagle dogs | The third and fourth premolar and the first molar teeth in both mandibular quadrants 3 months after extraction (six implants—two implants of each group—in No. 1 and 2 beagle dogs and three implants—one implant of each group—(Q3) in No. 3). | Nanophase calcium phosphate embedded to TiO2 nanotubes after anodic oxidation, annealing process, and electrochemical deposition on selective laser melting (SLM) titanium substrates. | Oral microbioflora without infection set-up. | 8 weeks | Next-generation sequencing (NGS) technology, 16S rRNA gene/RNA sequencing. | “The modified nanostructured titanium surfaces affected the genes associated with microbial metabolism, protein synthesis, bacterial locomotion, localization and the integrity of organism cellular membranes.” “The destruction of bacterial cellular membrane on the NTN surface could be one of the vital mechanisms of antibacterial activities of nanostructured titanium surfaces.” |
| Liu et al., 2022 [36] | Six male beagle dogs | At mandibular premolar extraction site where implants were placed 3 months after teeth extractions. | TiCu alloy with microstructure of α-Ti + Ti2Cu. | Peri-implant infection model by native oral microbiota (ligature and sucrose-rich diet model). | Oral samples (plaque) collected monthly. | Micro CT Histopathological analysis (hematoxylin and eosin) 16S rRNA gene and metagenomic sequence technology | “Histological score of TiCu group was significantly lower than that of Ti group, which indicated the excellent anti-infection ability of TiCu implant.” “Similarity between the saliva microbial compositions of animals with Ti and TiCu implants in the normal implantation model.” “TiCu implant can still maintain the oral microbiota balance in the infection model.” |
| Yin et al., 2021 [35] | Labrador dogs | At mandibular premolar extraction site after establishment of the experimental canine periodontitis model, 3 months after extraction. | Micro-arc oxidation (MAO) technology to create Zn/Sr experimental implants. The concentrations of the two ions in the electrolyte were 6 times that of 40 μM Zn2+ and 6 mM Sr2+. | 2-0 sutures were tied around the cervical area of the implant to induce peri-implantitis 4 weeks after implant insertion. | 8 weeks | H&E staining Gram staining Immunofluorescence staining of CD3 | “Large number of bacteria existed in the soft tissue of the control group, while the experimental group had a good antibacterial ability, which could delay the next stage of inflammation.” |
| Rabbits | |||||||
| Zhao et al., 2024 [37] | ?male New Zealand white rabbits | Femur defects of rabbits. | Ti-ZnO, and Ti-ZnP2 The ZnO nanorod arrays were synthesized on the pristine Ti by a hydrothermal method. | S. aureus suspension (105 CFU mL−1) for 30 min. | 2 weeks | Hematoxylin and eosin (H&E) staining Giemsa staining Colony counting method | “Numerous bacteria are found in the Ti group, but fewer bacteria are observed from Ti-ZnO.” |
| Wang et al., 2019 [39] | 24 healthy New Zealand big white rabbits (two groups of twelve) | Back muscle of the rabbit. | Ti-Cu sintered alloy with 10wt% Cu (Ti-10Cu). | 20 μL of the bacterial suspension with a concentration of 105 CFUs/mL S. aureus strain ATCC 6538. | 1, 4, 7, and 14 days | Bacterial culture Hematoxylin and eosin (H&E) staining. | “The colonies number in the Ti-10Cu group was significantly less than the number in the cp-Ti group at all investigated intervals although same amount of S. aureus was added in the two groups during the surgical procedure (p < 0.01).” |
| Pigs | |||||||
| Mathew et al., 2021 [33] | 15 adult White Yorkshire Desi female pigs (50% crossbred) | Titanium plates mounted on acrylic were attached to the pigs’ teeth. | Microtexturing and hydrothermal treatment of the commercially available sand blasted acid etched Ti COM implant to create micro-nano textured Ti implant SAN and nanotextured Ti implant TNL. | Oral microbioflora without infection set-up. | 2 days | Gram staining, colony counting. | “Microbial attachment was above 5 × 105 CFU cm−2 for the commercial implant, while SAN and TNL implants revealed a significantly low microbial count of 1 × 105 CFU cm−2 and 0.5 × 105 CFU cm−20, respectively.” |
| Humans | |||||||
| Assery et al., 2022 [43] | Four systemically healthy, ≥18-year-old, nonsmoking subjects. | Custom-made acrylic stents on maxillary arch. | Extraoral surface decontamination with Er: YAG 2940 nm. | Oral microbioflora without infection set-up. | 12 h overnight (9 p.m. to 9 a.m.) | Multiphoton confocal laser scanning microscopy and Flu-oView software FV1000were used to evaluate and capture the biofilm 3D structure and the live/dead bacteria ratio. Computational analyses of confocal biofilm images. | “Er:YAG laser treatment of titanium implant surfaces does not significantly affect early biofilm formation in the oral cavity.” |
| Ionescu et al., 2018 [42] | 10 subjects (seven women, three men; aged 20–32 years old with a mean age of 22.5 years). | Individual mandibular thermoformed acrylic customized tray. Three half-implants, one for each experimental surface, were fixed horizontally on each buccal side (three on the right and three on the left). | Laser-microtextured in 136 s, Nd: YAG source diode pumped solid state (DPSS) laser (355 nm wavelength). | Oral microbioflora without infection set-up. | 2 days | MTT assay, confocal laser scanning microscopy (CLSM), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) | “Machined and laser-treated surfaces were less colonized than grit-blasted ones, while no significant differences were identified between machined and laser-treated surfaces” “Laser-created microtopography can reduce biofilm formation, with a maximum effect when the surface is blasted orthogonally by the laser beam.” |
| Areid et al., 2018 [41] | 10 healthy, nonsmoking adult volunteers (six males, four females, mean age 39.7 years, from 25 to 56 years) | Titanium discs were attached on subjects’ buccal surfaces of their maxillary molars with flowable composite resin. | Nanoporous titanium dioxide surfaces (TiO2) obtained by the hydrothermal HT coating method. Half of the discs: UV light for 60 min under ambient conditions using a 36 W puritec HNS germicidal ultraviolet lamp (Osram GmbH; Germany), with a dominant wavelength of 254 nm. | Oral microbioflora without infection set-up. | 24 h | Colony counting of plaque and bacterial samples. | “The plaque samples of noncoated groups (NC and UVNC) showed more often S. mutans in the biofilms than the coated hydrothermal groups (HT and UVHT) with the number of colonized surfaces equal to seven and three, respectively.” “Hydrothermally induced nanoporous TiO2 surfaces inhibited S. mutans adhesion and decreased biofilm formation when compared with noncoated titanium alloy.” |
| Cochis et al., 2015 [40] | Seven subjects (four males and three females; aged 20– 27 years, mean age 24 years) | Oral appliances containing six specimens (1 mm diameter) | Addition of silver (Ag) and gallium (Ga) by electrochemical surface modification using the anodic spark deposition (ASD) method. AgNPs, AgNO3, Ga(NO3)3 appropriately mixed with L-cysteine and oxalic acid dehydrate as chelating agents. | Oral microbioflora without infection set-up. | 24 h | Colony counting method Colorimetric assay 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-((phenyl amino) carbonyl)-2H-tetrazolium hydroxide (XTT; Sigma-Aldrich) | “Gallium-based samples showed the best bactericidal activity among the antibacterial treatments developed. In fact, the values of CFU/mm2 were reduced by about 48% with GaCis (Ga(NO3)3 with chelating agent l-Cysteine) and by 40% with GaOss (Ga(NO3)3 with chelating agent oxalic acid), when compared with the control SiB-Na (alkali treatment), while the samples containing silver displayed a bacterial inhibition of about 30% (AgCis—AgNO3 with chelating agent l-Cysteine) to 34% (AgNPs—Ag nanoparticles).” “Bacteria viability on gallium-treated samples as measured by the inhibition ratio was in a range between 27% (GaOss) and 35% (GaCis) compared to controls. Silver samples confirmed CFU results, but with a slightly lower inhibition ratio than gallium.” |
| Study | Implant Number | Implant Dimensions | Implant Shape | Surface Structure of Tested Implants | Comparison | In Vivo Antibacterial Result |
|---|---|---|---|---|---|---|
| Ag modified | ||||||
| Kuehl et al., 2016 [2] | At least 39 implants? | 8.5 × 1 × 30 mm | Cylindrical cages (so-called “tissue cages”; 8.5 by 1 by 30 mm | Ag-coated titanium-aluminum-niobium (TiAlNb) alloy | Uncoated TiAlNb alloys | “Ag coating prevents an infection with S. epidermidis in an inoculum- and time-dependent manner.” “As single agents, neither preoperative DAP nor VAN nor Ag-coated cages were sufficient to prevent a persistent infection with MRSA. Remarkably, in combination with preoperatively applied DAP, Ag coating prevented the growth of planktonic as well as adherent MRSA cells, resulting in a 100% prevention rate.” |
| Zn modified | ||||||
| Zhao et al., 2024 [37] | ? implants (Three groups: Ti, Ti-ZnO, and Ti-ZnP2 rods) | Ø 3 mm L: 5 mm | Rods | Ti-ZnO, and Ti-ZnP2 The ZnO nanorod arrays were synthesized on thepristine Ti by a hydrothermal method. | Pure Ti implants | “Numerous bacteria are found in the Ti group, but fewer bacteria are observed from Ti-ZnO.” |
| Wen et al. 2023 [34] | 16 implants | Ø 1.6 mm L: 3.5 mm | Oral implants | ZnO nanoparticle-loaded mesoporous TiO2 coating-titanium (nZnO/MTC-Ti) | Pure titanium (Ti), mesoporous TiO2 coating-titanium (MTC-Ti), ZnO nanoparticle-loaded-titanium (nZnO-Ti). | “Compared to Ti, MTC-Ti also exhibited mild bacterial resistance, suggesting the enhanced inhibitory effects of the mesoporous structure on bacterial adhesion. Thus, nZnO/MTC-Ti achieved long-term antibiosis success in vivo through the MTC structure regulating Zn2+ release. Sustained Zn2+ release created lasting antibacterial environment and desirable inhibitory effects.” |
| Wang et al., 2020 [29] | 20 (n in each group?) | 8 × 8 × 1 mm | Foils/slices | Ti-ZnO nanorods, Ti-ZnO nanospheres, Ti-ZnO NRS Ti-Zr-ZnO nanorods, Ti-Zr-ZnO nanospheres, Ti-Zr-ZnO NRS | Ti, Ti-Zr | “The bacteria on the surfaces of samples with ZnO nanorods and ZnO nanospheres modification were less than that on naked Ti or Ti−Zr implants, and the samples with ZnO NRS modification had the least number of bacteria.” |
| Jin et al., 2014 [32] | 20 implants | Ø 2 mm L: 7 mm | Cylinder | Zn-PIII, Ag-PIII, and Zn/Ag-PIII | Pure Ti | “Both Ag-PIII and Zn/Ag-PIII have excellent antibacterial ability in vivo.” The small degree of bacterial growth on the roll-over cultures, absence of neutrophils (and presence of spindle-shaped fibroblast cells indicate the excellent antibacterial ability of the Ag-PIII group in vivo.” |
| Cu modified | ||||||
| Lu et al., 2022 [30] | Nine implants (n = 3, 3 groups) | Ø 5 mm L: 10 mm | Oblong | TiO2-1Cu | Ti, TiO2-0Cu | “A large number of bacteria still remained on the agar plates of Ti and TiO2-0Cu groups, while only a few bacteria were found on the agar plates of TiO2-1Cu group, and the antibacterial efficiency reached 96%, showing the excellent in vivo antibacterial performance.” |
| Liu et al., 2022 [36] | 24 implants (12 Ti and 12 TiCu) | Ø 3.6 mm L: 8 mm | Oral implants | TiCu alloy with microstructure of α-Ti + Ti2Cu, sandblasted with large grift and acid etching (SLA) treatment. | Pure Ti implants | “Histological score of TiCu group was significantly lower than that of Ti group, which indicated the excellent anti-infection ability of TiCu implant” “Similarity between the saliva microbial compositions of animals with Ti and TiCu implants in the normal implantation model.” “TiCu implant can still maintain the oral microbiota balance in the infection model.” |
| Wang et al., 2019 [39] | 24 implants (Two groups of twelve) | 10 mm in length, 3 mm in width and 1 mm in thickness. | Oral implants | Ti-10Cu | Commercial pure cp-Ti | “Ti-10Cu alloy could kill the bacteria shortly after 1 day implantation and nearly kill all bacteria after 4 days while no such function could be found in the case of the cp-Ti.” “Many colonies were found in the cp-Ti group at all intervals, but only several bacteria colonies could be found in the Ti-Cu group after 1–4 days postimplantation and no bacteria colonies after 7 days postimplantation.” |
| Mg modified | ||||||
| Yang et al., 2019 [28] | 45 implants (n = 15, 3 groups) | Ø 2 mm L: 15 mm | Rods | Titanium substrates coated with Mg incorporated TNTs (Electrochemical anodization and hydrothermal treatment to create NT-Mg3). | (1) Pure titanium implant group (Ti) n = 15; (2) TNT-coated titanium implant group (NT) n = 15. | “The radiographic scores recorded in the NT-Mg group were significantly reduced compared with that in the Ti and NT groups from days 14 to 35 after implantation (p < 0.01). The NT group exhibited slightly reduced radiographic scores compared with the Ti group at day 35 post-surgery (p < 0.05).” “The histopathological scores in the Ti and NT groups were all significantly increased compared with the NT-Mg group (p < 0.01). The total scores in the NT group were also slightly reduced compared with the Ti group (p < 0.05).” |
| Se modified | ||||||
| Tran et al., 2019 [31] | ? | Width 1.5 mm plate | Plates and screws | Selenium NP coated titanium substrates | Non-coated titanium substrates | “Se NP coatings strongly inhibited biofilm formation on the implants and reduced the number of viable bacteria in the surrounding tissue.” |
| Ga modified | ||||||
| Cochis et al., 2015 [40] | 42 titanium discs (six groups: Ti, SiB-Na, AgCis, GaCis, GaOss, AgNPs) | Ø 12 mm 0.5 mm thickness | Discs | AgCis, GaCis, GaOss, AgNPs | Ti control, SiB-Na (alkali treatment). | “CFU counts showed a reduction in bacterial colonies on the treated samples compared with the controls. Silver-coated samples resulted in 30–34% decrease in bacterial colonies compared to the controls.” A reduction in the bacterial metabolic activity was observed in gallium- and silver-treated specimens, with the best inhibition ratio in the gallium specimens (27–35%), as confirmed by XTT analysis.” |
| Combined metals | ||||||
| Pan et al., 2024 [38] | 30 implants | Ø 1.8 mm L: 3.5 mm | Oral implants | TNT STNT Al-STNT | Ti | “Introducing Al3+ ions doping in SrTiO3 created crystal distortion, which leads to enhanced sonocatalytic efficiency by oxygen vacancies and piezoelectric properties. This increases the generation of ROS induced by ultrasound, making Al-STNT a promising candidate for antibacterial applications.” |
| Yin et al., 2021 [35] | 12 implants | Ø 2.0 mm L: 10 mm | Rods | Micro-arc oxidation (MAO) technology to create Zn/Sr experimental implants MAO-6. | MAO | “A large number of bacteria existed in the soft tissue of the control group, while the experimental group had a good antibacterial ability, which could delay the next stage of inflammation.” |
| Physical/Chemical modifications | ||||||
| Sun et al., 2023 [27] | 15 (Five titanium implants of each group). 12 implants (MP = 3, NT = 5, NTN = 4) were recruited in this study. | Ø 4.5 mm L: 13 mm | - | Nanophase calcium phosphate embedded to TiO2 nanotubes (NTN). | (i) Mechanical polishing (MP), (ii) TiO2 nanotubes (NT). | “The nanostructured titanium had little effect on the community composition of the sub-mucosal and supra-mucosal microbiota established on implant surfaces. No significant difference of diversity and species richness were found among all groups.” |
| Assery et al. 2022 [43], | 24 titanium discs (two groups of twelve) Three titanium discs were discarded during processing. | Ø 5 mm 1 mm in thickness | Discs | Titanium discs that underwent extraoral surface decontamination with Er: YAG 2940 nm. | Untreated titanium discs (12) | “Er: YAG laser treatment of titanium implant surfaces does not significantly affect the early biofilm formation in the oral cavity.” |
| Mathew et al., 2021 [33] | 45 implants (n = 15, 3 groups) | Ø 4.2 mm L: 12 mm | Cylinder | Micro-nano textured Ti (SAN) and nanotextured Ti (TNL). | Commercially available sand blasted and acid etched Ti (Nano polished) (COM). | “SAN and TNL surfaces with their superhydrophilic character and high surface energy could reduce bacterial attachment by nearly 90% in vivo, as compared to the microscale surface of COM.” “The micro-nano and nanotextured Ti dental implants in our study demonstrate anti-adhesive properties, resulting in reduced bacterial activity, which in turn can inhibit bacterial film formation.” |
| Ionescu et al., 2018 [42] | 30 half-implants | Implant model “Milano” Ø 4.0 mm, L: 9.0 mm | Oral implants | Laser-treated laser-microtextured in 136 s | Machined, grit-blasted | “Machined and laser-treated surfaces were less colonized than grit-blasted ones, while no significant differences were identified between machined and laser-treated surfaces. When the beam blasts the titanium surface at a different angle, as on the inclined portion of the threads, this effect is lost. “ |
| Areid at al., 2018 [41] | 40 Titanium (Ti-6Al-4V) alloy discs (4 groups) | Ø 4 mm 1 mm thickness | Discs | UV-treated noncoated titanium alloy (UVNC), hydrothermally induced TiO2 coating (HT), UV-treated titanium alloy with hydrothermally induced TiO2 coating (UVHT). | Noncoated titanium alloy (NC) | “Noncoated Ti-6Al-4V (NC) surfaces showed over 2 times more S. mutans in the early biofilm when compared with the hydrothermally (HT) induced nanoporous TiO2 surface. The numbers of colonized surfaces on NC and HT surfaces were equal to 7 and 3, respectively.” |
| # | Signalling Question | Wang et al., 2020 [29] | Sun et al., 2023 [27] | Lu et al., 2022 [30] | Mathew et al., 2021 [33] | Yang et al., 2019 [28] | Jin et al., 2014 [32] | Wen et al., 2023 [34] | Kuehl et al., 2016 [2] | Tran et al., 2019 [31] | Yin et al., 2021 [35] | Liu et al., 2021 [36] | Zhao et al., 2024 [37] | Wang et al., 2019 [39] | Pan et al., 2024 [38] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Was the allocation sequence adequately generated and applied? (Selection) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 2 | Were the groups similar at baseline or were they adjusted for confounders in the analysis? (Selection) | Low | Low | Unclear | Low | Low | Low | Low | Low | Low | Unclear | Low | Low | Low | Low |
| 3 | Was the allocation adequately concealed? (Selection) | High | High | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Unclear |
| 4 | Were the animals randomly housed during the experiment? (Performance) | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Unclear |
| 5 | Were the caregivers and/or investigators blinded from knowledge which intervention each animal received during the experiment? (Performance) | High | High | High | Unclear | Low | Unclear | Unclear | High | Unclear | Unclear | Low | Unclear | Unclear | Unclear |
| 6 | Were animals selected at random for outcome assessment? (Detection) | High | High | High | Unclear | Unclear | Low | Unclear | Unclear | Unclear | Unclear | High | Unclear | Unclear | Unclear |
| 7 | Was the outcome assessor blinded? (Detection) | Unclear | High | High | Unclear | High | Low | Unclear | High | High | Unclear | Low | Unclear | Unclear | Unclear |
| 8 | Were incomplete outcome data adequately addressed? (Attrition) | Unclear | Unclear | Unclear | Unclear | High | Unclear | High | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 9 | Are reports of the study free of selective outcome reporting? (Reporting) | High | High | High | High | High | High | High | High | High | High | High | High | High | High |
| 10 | Was the study apparently free of other problems that could result in high risk of bias? (Other) | High | High | High | High | High | High | High | High | High | High | High | High | High | High |
| Items | Wang et al., 2020 [29] | Sun et al., 2023 [27] | Lu et al., 2022 [30] | Mathew et al., 2021 [33] | Yang et al., 2019 [28] | Jin et al., 2014 [32] | Wen et al., 2023 [34] | Kuehl et al., 2016 [2] | Tran et al., 2019 [31] | Yin et al., 2021 [35] | Liu et al., 2022 [36] | Zhao et al., 2024 [37] | Wang et al., 2019 [39] | Pan et al., 2024 [38] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Title | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Abstract | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| INTRODUCTION | ||||||||||||||
| 3. Background | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4. Objectives | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| METHODS | ||||||||||||||
| 5. Ethical statement | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6. Study design | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7. Experimental procedures | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8. Experimental animals | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9. Housing and husbandry | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| 10. Sample size | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| 11. Allocating animals to experimental groups | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12. Experimental outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 13. Statistical methods | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| RESULTS | ||||||||||||||
| 14. Baseline data | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15. Numbers analysed | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| 16. Outcomes and estimation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 17. Adverse events | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DISCUSSION | ||||||||||||||
| 18. Interpretation/scientific implication | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 19. Generalisability/translation | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20.Funding | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| TOTAL | 16 | 16 | 16 | 19 | 17 | 17 | 16 | 17 | 15 | 14 | 15 | 14 | 15 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkioka, M.; Rausch-Fan, X. Antimicrobial Effects of Metal Coatings or Physical, Chemical Modifications of Titanium Dental Implant Surfaces for Prevention of Peri-Implantitis: A Systematic Review of In Vivo Studies. Antibiotics 2024, 13, 908. https://doi.org/10.3390/antibiotics13090908
Gkioka M, Rausch-Fan X. Antimicrobial Effects of Metal Coatings or Physical, Chemical Modifications of Titanium Dental Implant Surfaces for Prevention of Peri-Implantitis: A Systematic Review of In Vivo Studies. Antibiotics. 2024; 13(9):908. https://doi.org/10.3390/antibiotics13090908
Chicago/Turabian StyleGkioka, Maria, and Xiaohui Rausch-Fan. 2024. "Antimicrobial Effects of Metal Coatings or Physical, Chemical Modifications of Titanium Dental Implant Surfaces for Prevention of Peri-Implantitis: A Systematic Review of In Vivo Studies" Antibiotics 13, no. 9: 908. https://doi.org/10.3390/antibiotics13090908
APA StyleGkioka, M., & Rausch-Fan, X. (2024). Antimicrobial Effects of Metal Coatings or Physical, Chemical Modifications of Titanium Dental Implant Surfaces for Prevention of Peri-Implantitis: A Systematic Review of In Vivo Studies. Antibiotics, 13(9), 908. https://doi.org/10.3390/antibiotics13090908