Abstract
The rise in antibiotic-resistant bacteria highlights the need for novel antimicrobial agents. This study presents the design and synthesis of a series of rhein (RH)-derived compounds with improved antimicrobial properties. The lead compound, RH17, exhibited a potent antibacterial activity against Staphylococcus aureus (S. aureus) isolates, with minimum inhibitory concentrations (MICs) ranging from 8 to 16 μg/mL. RH17 disrupted bacterial membrane stability, hindered metabolic processes, and led to an increase in reactive oxygen species (ROS) production. These mechanisms were confirmed through bacterial growth inhibition assays, membrane function assessments, and ROS detection. Notably, RH17 outperformed the parent compound RH and demonstrated bactericidal effects in S. aureus. The findings suggest that RH17 is a promising candidate for further development as an antimicrobial agent against Gram-positive pathogens, addressing the urgent need for new therapies.
1. Introduction
The growing issue of antimicrobial resistance in bacteria poses a significant global threat, as it diminishes the effectiveness of current treatments and limits options for managing certain persistent infections [1,2,3]. Methicillin-resistant Staphylococcus aureus (MRSA) significantly contributes to mortality among antibiotic-resistant pathogens [4,5,6,7]. MRSA infections are complicated to treat due to their ability to form biofilms and exist as metabolically inactive persister cells, which are both highly tolerant to antibiotics [8,9,10]. Therefore, the development of new therapeutic strategies that effectively target both biofilm-forming bacteria and persisters is crucial. Ideally, new antibacterial agents should disrupt biofilm integrity and demonstrate potent activity against actively growing bacteria and persisters [11,12].
With their structural diversity and biological activity, natural products are a valuable source for drug discovery [13,14,15]. Rhein (RH), an anthraquinone derived from rhubarb (Rheum palmatum) [16], has attracted attention due to its wide range of biological activities, including anti-inflammatory [17,18], anticancer [19,20], antiviral [21,22], and antimicrobial properties [23,24,25]. Although RH has demonstrated some antimicrobial activity, with a minimum inhibitory concentration (MIC) of 64 μg/mL against S. aureus ATCC 25923, its therapeutic potential is limited due to its relatively low potency [26]. In recent years, various studies have modified the chemical structure of RH to enhance its antibacterial efficacy, achieving some success [27,28].
Cationic antimicrobial peptides (CAMPs) are another promising class of antimicrobial agents known for their potent broad-spectrum activity [29,30]. CAMPs function by interacting with bacterial membranes, thereby causing cell lysis and bacterial death due to their amphiphilic structure, which consists of positively charged hydrophilic regions and hydrophobic tails [31,32]. This unique mechanism of action makes CAMPs particularly effective against drug-resistant pathogens. However, the clinical application of CAMPs has been limited due to challenges with toxicity and stability [33,34,35]. To overcome these limitations, researchers have developed small-molecule mimics of CAMPs, which retain their membrane-disrupting capabilities while minimizing toxicity [32]. Some CAMP mimetics have shown promise in clinical trials, such as LTX-109 (phase Ⅱ) [36] and PMX30063 (phase Ⅱ) [37], demonstrating solid antibacterial activity while reducing the likelihood of bacterial resistance. These small-molecule mimics effectively disrupt bacterial membranes, providing a potent antimicrobial option with improved safety profiles.
In this study, we aimed to develop novel rhein derivatives by incorporating features of CAMPs, such as amphiphilicity and a membrane-targeting ability, to enhance their antibacterial activity. When compared to the parent RH compound, our lead compound, RH17, exhibited a significantly improved activity against S. aureus, with an MIC of 8 μg/mL. Additionally, RH17 demonstrated a dual mechanism of action, disrupting bacterial membrane homeostasis and collapsing bacterial metabolism. This study suggests that RH17 and its analogs represent a new class of membrane-targeting antibacterial agents with the potential for further development.
2. Results
2.1. Rational Design and Synthesis of RH Analogs
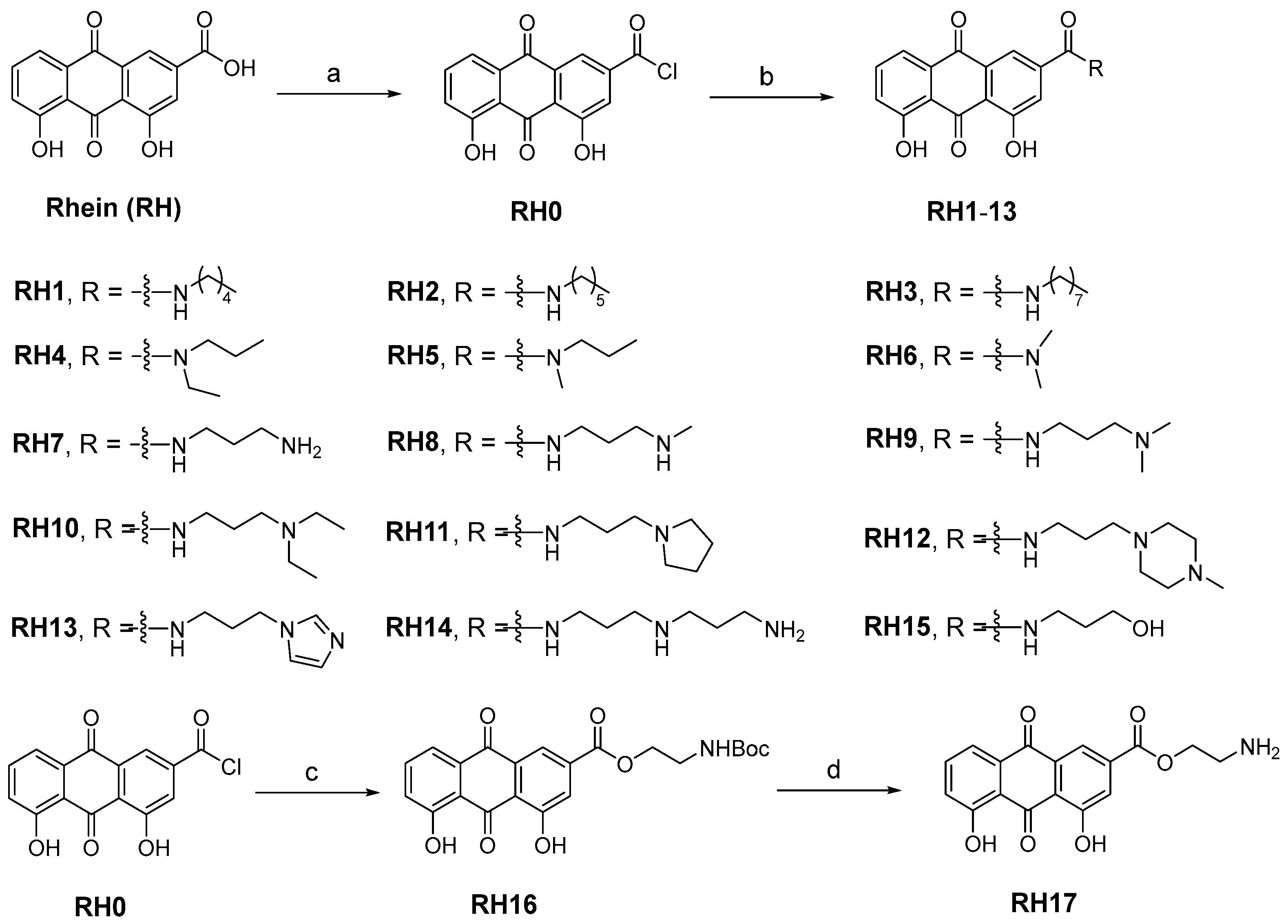
The research on the structural modification of RH mainly focuses on the 1,8-dihydroxy and 3-carboxyl positions of RH [14]. According to the analysis of RH, emodin methyl ether, and other homologues, the key component that exerts antimicrobial activity are 1,8-dihydroxy moieties. Therefore, we mainly modify RH by modifying its carboxyl group. The synthetic route of all target compounds, RH1–17, is described in Figure 1. Rhein was treated with oxalyl chloride at reflux temperature in dichloromethane (DCM) solution to yield intermediate RH0. The acetamide derivatives of RH were prepared by adding the corresponding amines into intermediate RH0, and the ammonolysis reaction was completed at room temperature. The structures of derivatives (RH1–17) were characterized by nuclear magnetic resonance and mass spectrometry.
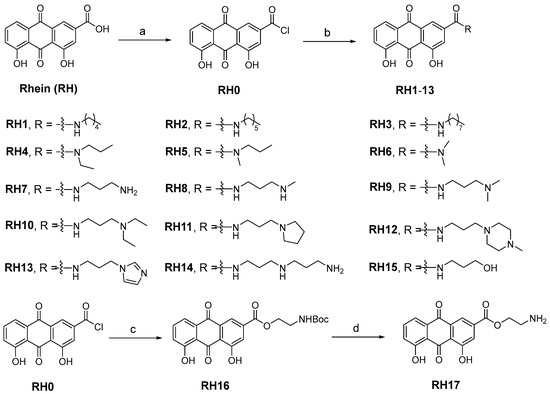
Figure 1.
The synthesis of RH derivatives (RH1–17). Reagents and conditions: (a) DCM (0.01 mM DMF), oxalyl chloride, N2, reflux, overnight; (b) anhydrous THF, corresponding amines, r.t., 12–24 h; (c) DCM, TEA, Boc-NH-PEG1-OH, r.t., 3 h; (d) Dioxane, HCl, r.t., 5 h.
2.2. Structure–Activity Relationships of Derivatives
Initially, we assessed the in vitro antibacterial activity of synthetic RH analogs against various S. aureus and E. faecalis strains by determining their MICs [38]. Gentamicin, a commercially available antimicrobial agent, was used as a reference in this evaluation (Table 1).

Table 1.
In vitro antimicrobial activities against S. aureus and E. faecalis and the physicochemical properties of RH derivatives (RH1–17).
To examine the impact of alkyl chains of amines on antibacterial activity, we synthesized the compounds RH1, RH2, RH3, RH4, RH5, and RH6. The clogD7.4 values of these compounds were reflective of their lipophilicity, which is also attributed to their relatively weak antibacterial potency. We speculated that the introduced amine groups and the carbonyl group of the backbone would form an acetamide bond, which may improve lipophilicity, inhibit ionization, and exhibit weak antibacterial activity. Based on this, we introduced diamino substituents to explore the influence of ionizable amine on antibacterial activity, and compounds (RH7, RH8, RH9, RH10, RH11, RH12, and RH13) were prepared. All compounds showed antibacterial activity equivalent to RH (32 μg/mL), and the MIC values of RH8 (16 μg/mL) and RH11 (16 μg/mL) were reduced by one time. By analyzing their physicochemical properties, it was found that the lipophilicity and ionization ability of these compounds were relatively balanced, and finally, their antibacterial effects were improved. The compound RH14 exhibited a weak antibacterial activity, presumably because its side chain is too long, which leads to too much flexibility to bind to the target. Overall, we used the aminoethanol group to replace the hydroxyl group to obtain compound RH17, which has low lipophilicity (1.37) and a high ionization ability (7.65). As a result, compound RH17 exhibited good antibacterial activity with an MIC of 8 μg/mL. To sum up, we found that lipophilicity and ionization ability are the main properties affecting antibacterial activity.
2.3. RH17 Exhibits Antibacterial Activity against Gram-Positive Bacteria
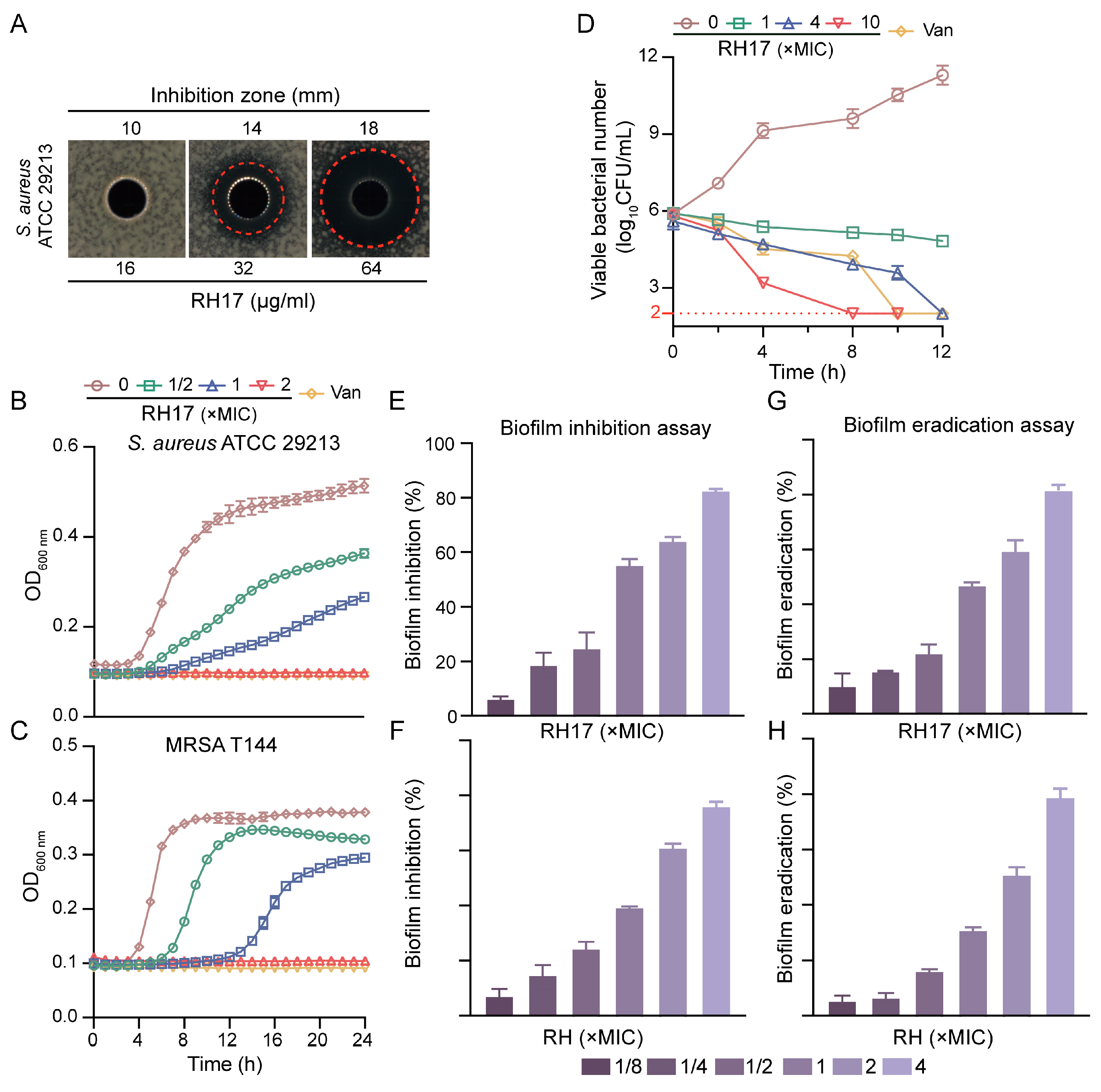
In our quest to identify potential antibacterial mechanisms of RH analogs against S. aureus, we selected RH17 as a model compound. RH17 exhibited inhibitory activity against the standard strain S. aureus ATCC 29213, with an MIC of 8 μg/mL, which is four times more potent than RH. Additionally, the antimicrobial activity of RH17 was assessed using the well diffusion method against S. aureus ATCC 29213. The results indicated that RH17 displayed a significant dose-dependent antibacterial activity against S. aureus, with inhibition zones of 10, 14, and 18 mm at concentrations of 8, 16, and 32 μg/mL, respectively (Figure 2A).
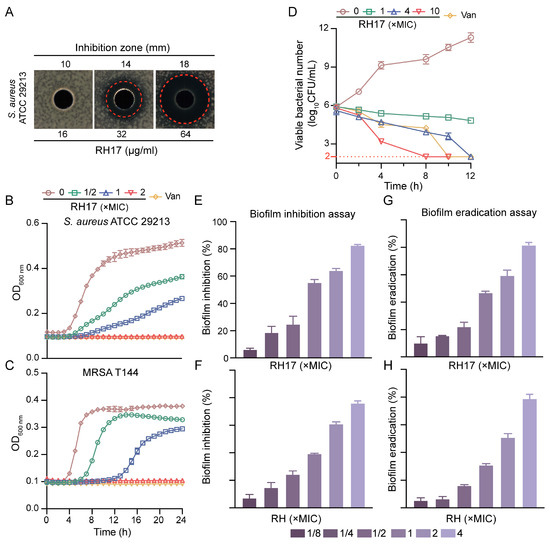
Figure 2.
The antibacterial potency of RH17. (A) Antimicrobial activity of RH17 against S. aureus ATCC 29213. (B,C) Effect of varying concentrations of RH17 on growth curves of S. aureus isolates. The presented data are depicted as means ± standard deviation (SD). (B) S. aureus ATCC 29213; (C) MRSA T144. (D) Time-kill curve of S. aureus ATCC 29213 at different concentrations of RH17. (E–H) The antibiofilm activity of RH17 and RH. (E,F) RH17 and RH treatment inhibited biofilm formation on S. aureus ATCC 29213 after 24 h. (E) RH17; (F) RH. (G,H) RH17 and RH eradicated 24 h pre-formed S. aureus ATCC 29213 biofilms. (G) RH17; (H) RH.
To further explore the antibacterial potential, we determined the MICs of both RH and RH17 against 40 clinical S. aureus isolates, which included 20 methicillin-susceptible S. aureus (MSSA) and 20 methicillin-resistant S. aureus (MRSA). RH17 exhibited strong antimicrobial activity against MSSA and MRSA isolates, with MIC values ranging from 8 to 16 μg/mL. Specifically, the MIC50 and MIC90 values for MSSA were both 8 μg/mL, while for MRSA, the MIC50 was 8 μg/mL, and MIC90 was 16 μg/mL. In comparison, RH showed significantly higher MIC values, ranging from 32 to 128 μg/mL for the same MSSA and MRSA isolates, emphasizing the enhanced potency of RH17. Regarding bactericidal activity, the minimum bactericidal concentrations (MBCs) of RH17 against S. aureus isolates ranged from 16 to 64 μg/mL, while RH exhibited higher MBC values, ranging from 64 to >128 μg/mL. These findings indicate that RH17 exhibits a more muscular bactericidal activity than RH across MSSA and MRSA strains (Table 2 and Table S1). Additionally, RH17’s activity was evaluated against E. faecalis and vancomycin-resistant Enterococcus (VRE). For E. faecalis, the MIC50 and MIC90 values were 16 and 32 μg/mL, respectively. For VRE, the MIC50 and MIC90 values were both 32 μg/mL (Table 2).

Table 2.
Antimicrobial activity of RH17 against S. aureus and E. faecalis isolates.
To understand its inhibitory effects on bacterial growth, we conducted growth curve experiments. The data revealed that RH17 inhibited the growth of planktonic cells of MSSA and MRSA in a dose-dependent manner. While complete inhibition was observed at 2 × MIC, lower concentrations (ranging from 1/2 × MIC to 1 × MIC) showed reduced growth compared to the control but did not achieve full inhibition (Figure 2B,C). These findings underscore RH17’s extensive potential against Gram-positive bacteria, encompassing not only S. aureus but also E. faecalis, another clinically significant pathogen associated with healthcare-associated infections and antibiotic resistance.
2.4. Time-Killing Curves of RH17 against S. aureus
The determination of MBCs provided a clearer insight into the bactericidal efficiency of RH17 against S. aureus isolates. To further analyze the time-dependent killing effect of RH17, time-kill experiments were conducted on S. aureus ATCC 29213, as depicted in Figure 2D. The results visually demonstrate the bactericidal activity of RH17. At 1 × MIC, RH17 exhibited weak bactericidal activity against S. aureus ATCC 29213. However, at a concentration of 4 × MIC, no bacterial colonies were observed on the plate after a 24 h incubation at 37 °C. When the RH17 concentration reached 10 × MIC, the remaining CFU fell to the detection limit within eight hours, indicating that RH17 kills S. aureus in a concentration-dependent manner.
2.5. Antibiofilm Activity of RH17
To assess the antibiofilm potential of RH17, crystal violet (CV) staining was employed in biofilm formation and eradication assays. S. aureus ATCC 29213 was initially examined for its biofilm-forming ability at various concentrations (1/8 × to 4 × MIC) of RH17 and RH over 24 h. The findings revealed that biofilm formation was inhibited in a concentration-dependent manner (Figure 2E). For RH17, significant inhibition was observed at 1/8 × MIC (6.78%), while RH exhibited a slightly lower inhibition rate of 5.34% at the same concentration. At the highest concentration (4 × MIC), RH17 showed an 82% reduction in biofilm formation, whereas RH demonstrated a comparable inhibition of 78%. Additionally, a biofilm eradication experiment was conducted to evaluate the capacity of RH17 and RH to disrupt pre-formed 24 h-old biofilms of the same strain. A similar concentration-dependent pattern of biofilm eradication was observed for both compounds (Figure 2F). At 1/8 × MIC, RH17 eradicated 10.23% of the biofilm, while RH showed an eradication rate of 6.14%. At 4 × MIC, RH17 demonstrated a biofilm eradication rate of 83%, whereas RH exhibited a slightly lower eradication rate of 81.2%. These results collectively highlight the significant antibiofilm activity of both RH17 and RH against S. aureus, with RH17 showing marginally better performance in biofilm formation inhibition and eradication at higher concentrations.
2.6. Preliminary Antibacterial Mechanism of RH17
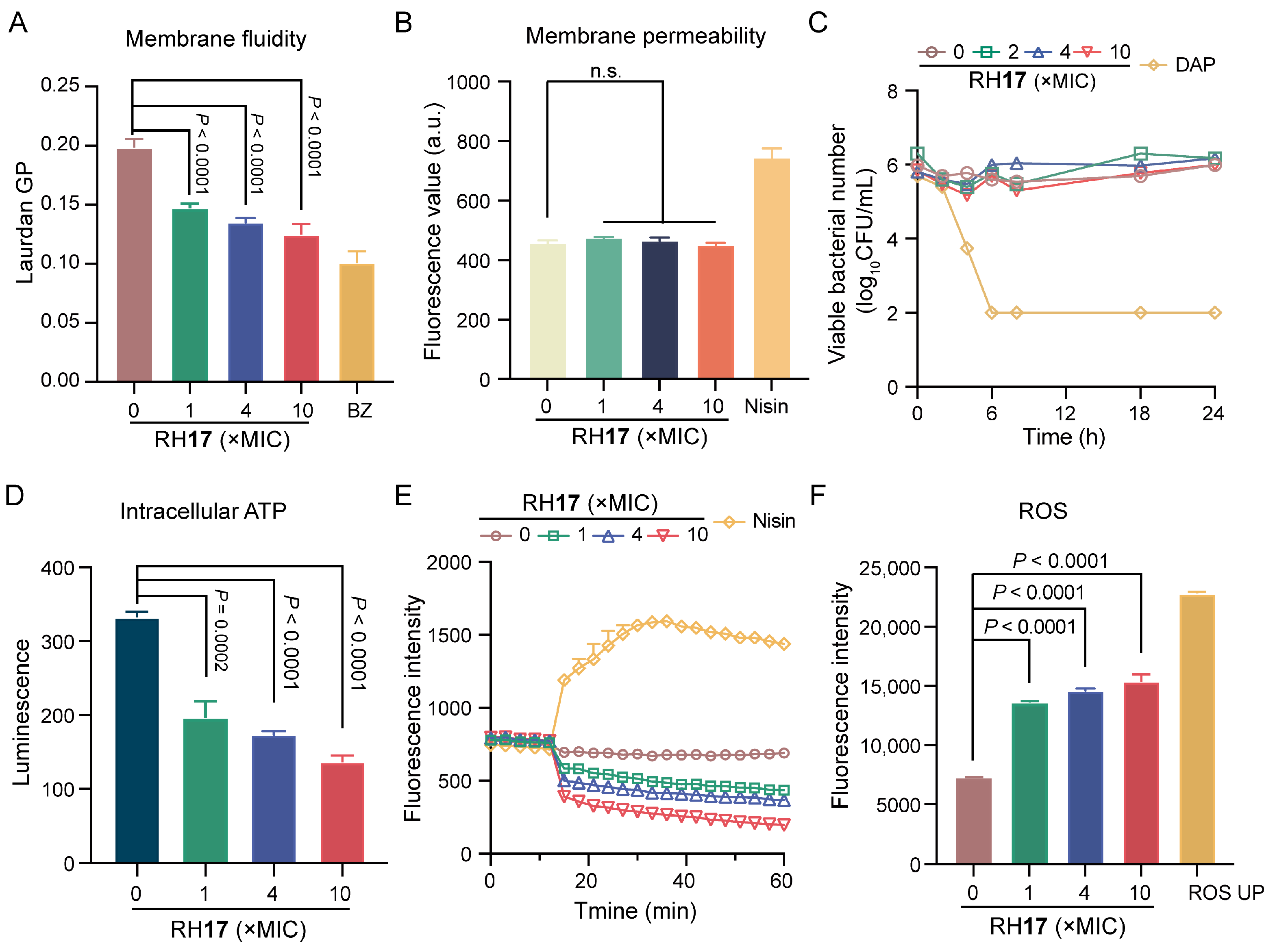
To investigate if the potent bactericidal effect of RH17 on S. aureus is linked to cell membrane damage, a Laurdan fluorescent dye was used to assess its impact on membrane fluidity. Upon treatment with RH17 at concentrations ranging from 1 × MIC to 10 × MIC, a significant decrease in fluorescence intensity was observed (Figure 3A), indicating reduced membrane fluidity. This reduction can impair the barrier function of bacterial membranes [39]. Subsequently, we assessed cytoplasmic membrane permeability using a propidium iodide (PI) fluorescent dye and detected no significant change in fluorescence intensity (Figure 3B). This suggests that RH17’s antibacterial action may operate through mechanisms other than direct bacterial membrane disruption.
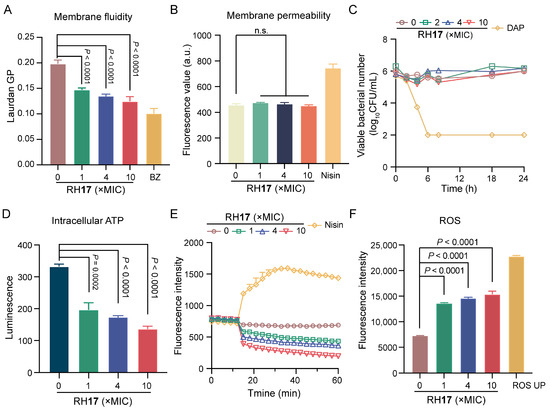
Figure 3.
The antibacterial mechanism of RH17. (A) The fluidity of the membrane was decreased for S. aureus ATCC 29213 after treatment with RH17 for 1 h. Benzyl alcohol (BZ) at 50 mM was used as the positive control. (B) There were no effects on the membrane permeability for S. aureus ATCC 29213 after treatment with RH17 for 1 h. Nisin at 200 μg/mL was used as the positive control. (C) The effect of RH17 on the time-kill curves of S. aureus ATCC 29213 arrested by cold temperature (0 °C in an ice-water bath) showed no significant impact. Daptomycin (DAP) was used as the positive control, which demonstrated strong bactericidal activity under the same conditions. (D) The effect of RH17 on S. aureus ATCC 29213 after 1 h treatment showed a reduction in intracellular ATP levels. (E) The effect of RH17 on S. aureus ATCC 29213 showed a decrease in fluorescence intensity. Nisin at 200 μg/mL was used as the positive control. (F) The effect of RH17 on S. aureus ATCC 29213 after 1 h treatment resulted in increased ROS production. ROS UP at 500 μg/mL was used as the positive control. A p-value of “n.s.” means not significant, suggesting no meaningful difference between groups.
In the literature, it has been observed that in a model where bacterial metabolism was inhibited by cold temperature, daptomycin, through its direct action on the bacterial membrane, consistently exhibited bactericidal activity, whereas vancomycin, targeting cell wall synthesis, does not [40]. Subsequently, RH17 was examined for its effects on the growth of S. aureus cultures that were arrested by cold temperature. Time-kill curves demonstrated that daptomycin remained bactericidal against cold-arrested S. aureus, whereas RH17 was not effective (Figure 3C). Those results demonstrated that RH17 may exert its antibacterial activity by disturbing normal metabolism.
Adenosine triphosphate (ATP), produced by the electronic respiratory chain, is necessary for bacterial metabolism [41]. Therefore, we measured the ATP levels within cells exposed to RH17 and found that RH17 significantly reduced intracellular ATP levels (Figure 3D). Previous studies have shown that reduced ATP levels can decrease the proton motive force (PMF) via the F1F0-ATPase [42,43]. We hypothesized that RH17 might disrupt PMF, which includes the membrane potential (ΔΨ) and the transmembrane proton gradient (ΔpH) [44]. To examine this, we used the fluorescence probe 3,3′-dipropylthiadicarbocyanine iodide (DiSC3(5)). When ΔΨ is compromised, DiSC3(5) is released from the inner membrane, increasing fluorescence [45,46,47]. Conversely, dissipating ΔpH enhances DiSC3(5) uptake, decreasing fluorescence. Our results showed a dose-dependent decrease in fluorescence units, indicating that RH17 primarily dissipated the ΔpH component of bacterial PMF. Notably, RH17, especially at 10 × MIC, significantly disrupted bacterial PMF, potentially leading to reduced ATP synthesis and metabolic disorders. Disrupted membrane homeostasis often leads to the accumulation of reactive oxygen species (ROS). Evidence suggests that ROS formation is crucial in bactericidal antibiotic-mediated killing [48]. Using 2′,7′-dichlorofluorescein diacetate (DCFH-DA) to label bacteria, we found that RH17 significantly increased ROS production in S. aureus. Overall, our results indicate that RH17 disrupts membrane homeostasis, affects metabolism, and promotes the production of ROS.
3. Discussion
MRSA and Enterococcus species are increasingly recognized as significant public health threats due to their capacity to develop resistance to antibiotics and cause severe infections [49,50]. Among Enterococcus species, E. faecalis and E. faecium are particularly concerning due to their multidrug-resistant profiles, contributing to severe conditions such as bacteremia and endocarditis [51]. The current rise in resistance among Gram-positive pathogens highlights the need for new antimicrobial agents with novel mechanisms of action [11,52].
In this study, RH17, a rhein-based derivative, demonstrated a superior antibacterial activity when compared to the parent compound RH against both S. aureus and Enterococcus species, including drug-resistant strains like MRSA and VRE. The MIC and MBC values of RH17 were consistently lower than those of RH, demonstrating RH17’s enhanced potency. For instance, RH17 exhibited MIC values between 8 and 16 μg/mL for S. aureus isolates, while the parent RH compound showed significantly higher MIC values, confirming RH17’s greater efficacy. The ability of RH17 to perform well against both S. aureus and Enterococcus species indicates its broad-spectrum potential against critical Gram-positive pathogens.
Furthermore, our results indicate that RH17 operates through a multifaceted mechanism of action. It disrupts bacterial membrane stability, causing leakage of intracellular components while simultaneously hindering metabolic processes and increasing the production of ROS. This multifaceted mode of action, targeting both membrane integrity and intracellular metabolic processes, accelerates bacterial death and may also reduce the likelihood of resistance development, as it differs from traditional antibiotics that typically focus on a single target pathway [53].
While these in vitro findings are promising, several limitations exist. First, although RH17 has shown potent activity against both S. aureus and Enterococcus species, the exact molecular targets within these pathogens remain unidentified. Future studies should focus on elucidating these targets through proteomic and metabolomic analyses. Additionally, the current study did not assess RH17’s efficacy in vivo, making it necessary to conduct animal model studies to evaluate its pharmacokinetics, bioavailability, and safety profile. These preclinical evaluations are essential for determining the therapeutic potential of RH17. Furthermore, the potential applications of RH17 may extend beyond S. aureus and Enterococcus species. Given its novel mechanism of action and strong efficacy against Gram-positive pathogens, RH17 could be investigated for activity against other multidrug-resistant organisms. Expanding the spectrum of testing to include various resistant Gram-positive bacteria will help establish RH17’s role as a broad-spectrum antimicrobial agent.
In conclusion, we have developed RH17, a potent rhein derivative with a significant antimicrobial activity against both S. aureus and Enterococcus species. The dual mechanism of action of RH17, which disrupts both bacterial membrane integrity and metabolism, makes it a promising candidate for further development. However, additional studies, including in vivo testing and target identification, are necessary to explore its therapeutic potential fully and address our study’s current limitations.
4. Materials and Methods
4.1. Materials
Rhein and other chemical reagents and solvents were sourced from Shanghai Titan Scientific Co., Ltd., Shanghai, China. Nuclear magnetic resonance (NMR) spectra were obtained in deuterated dimethyl sulfoxide (DMSO-d6) and deuterated methanol (CD3OD) using a Bruker Avance 400 MHz or 600 MHz instrument (Billerica, MA, USA) with TMS as the internal standard. High-resolution mass spectra (HRMS) were recorded on an Agilent LC-MS/MS (Santa Clara, CA, USA). Thin-layer chromatography (TLC) analyses were conducted on precoated silica-gel F254 plates with solvent systems of petroleum/ethyl acetate (6:4) and dichloromethane/methanol (9:1). Silica-gel (100–200 mesh) was used in column chromatography for purification of derivatives. Additionally, a preparative Shimadzu HPLC equipped with a C18 column (Shim-pack GIST, C18, 5 μm, 10 × 250 mm, Kyoto, Japan) was used to purify the derivatives, employing a methanol/distilled water (10–100%) mobile phase containing 0.1% formic acid.
4.2. Synthesis of RH Derivatives
N-pentyl-4,5-dihydroxyl-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (RH1): a solution of RH (400 mg, 1.41 mmol) and dimethylformamide (DMF, 0.1 mM) in 6 mL dichloromethane (DCM) was added to 1.8 mL oxalyl chloride (2.0 M in dichloromethane, 2 mL) under nitrogen at 0 °C. The reaction mixture was then brought to room temperature and refluxed at 42 °C for eight hours. The resulting crude product (RH0) was unstable and directly used in the next reaction without further purification. The crude product (100 mg, 0.33 mmol) was dissolved in anhydrous THF (3 mL) along with amylamine (3 mL) and stirred for 12 h at room temperature. The reaction mixture was then cooled to room temperature and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography and eluted with a gradient of DCM/methanol (100:1, v/v) to obtain RH1 (42 mg). 1H NMR (600 MHz, DMSO-d6) δ: 8.88 (t, 1H, J = 5.5 Hz, -NH-), 8.12 (d, 1H, J = 1.7 Hz, Ar-H), 7.83 (m, 1H, Ar-H), 7.76 (m, 2H, 2×Ar-H), 7.41 (dd, 1H, J = 8.3, 1.2 Hz, Ar-H), 3.27 (m, 2H, -CH2-), 1.55 (m, 2H, -CH2-), 1.31 (m, 4H, 2×-CH2-), and 0.87 (m, 3H, CH3). HR-ESI-MS m/z: calculated for C20H20NO5 [M+H]+ 354.1336; 354.1336 was observed.
N-hexyl-4,5-dihydroxyl-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (RH2): the title compound was prepared using a procedure analogous to that used for compound RH1, starting from compound RH0 and hexylamine. The resultant compound RH2 was isolated as a yellow powder, with a yield of 67%. 1H NMR (600 MHz, DMSO-d6) δ: 8.89 (t, 1H, J = 5.5 Hz, -NH-), 8.13(d, 1H, J = 1.5 Hz, Ar-H), 7.84 (m, 1H, Ar-H), 7.76 (m, 2H, 2×Ar-H), 7.41 (dd, 1H, J = 8.3, 1.1 Hz, Ar-H), 3.28 (m, 2H, -CH2-), 1.98 (m, 2H, -CH2-), 1.54 (m, 2H, -CH2-), 1.29 (m, 4H, 2×-CH2-), and 0.86 (m, 3H, CH3). HR-ESI-MS m/z: calculated for C21H22NO5 [M+H]+ 368.1492; 368.1492 was observed.
N-heptyl-4,5-dihydroxyl-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (RH3): the title compound was prepared using a method similar to that for compound RH1, starting from compound RH0 and octylamine. Compound RH3 was isolated as a yellow powder with a yield of 56%. 1H NMR (600 MHz, DMSO-d6) δ: 8.87 (t, 1H, J = 5.4 Hz, -NH-), 8.12 (s, 1H, Ar-H), 7.83 (t, 1H, J = 7.9 Hz, Ar-H), 7.75 (m, 2H, 2×Ar-H), 7.41 (d, 1H, J = 8.4 Hz, Ar-H), 3.28 (m, 2H, -CH2-), 1.99(m, 2H, -CH2-), 1.54 (m, 2H, -CH2-), 1.28(m, 8H, 4×-CH2-), and 0.85 (m, 3H, CH3). HR-ESI-MS m/z: calculated for C23H26NO5 [M+H]+ 396.1805; 396.1802 was observed.
N-ethyl-4,5-dihydroxy-9,10-dioxo-N-propyl-9,10-dihydroanthracene-2-carboxamide (RH4): the title compound was prepared using the same method as that for RH1, starting with compound RH0 and N-ethylpropan-1-amine. Compound RH4 was obtained as a yellow powder with a yield of 62%. 1H NMR (400 MHz, CD3OD) δ: 7.91 (s, 1H, Ar-H), 7.64 (s, 1H, Ar-H), 7.55 (m, 2H, 2×Ar-H), 7.20 (dd, 1H, J = 5.4, 2.1 Hz, Ar-H), 3.31 (m, 2H, -CH2-), 3.18 (m, 2H, -CH2-), 1.64 (m, 2H, -CH2-), 1.16 (t, 3H, J = 8.0 Hz, CH3), and 0.87 (t, 3H, J = 7.5 Hz, CH3). 13C NMR (100 MHz, CD3OD) δ: 193.3, 181.8, 167.4, 163.6, 163.3, 142.6, 138.8, 134.9, 134.4, 125.8, 123.8, 120.9, 118.7, 118.5, 116.8, 50.8, 48.3, 38.2, 25.2, and 9.1. HR-ESI-MS m/z: calculated for C20H20NO5 [M+H]+ 354.1336; 354.1328 was observed.
N-propyl-4,5-dihydroxy-9,10-dioxo-N-propyl-9,10-dihydroanthracene-2-carbox-amide (RH5): the title compound was prepared using the same method as that for RH1, starting from compound RH0 and N-methylpropylamine. Compound RH5 was obtained as a yellow powder with a yield of 58%. 1H NMR (400 MHz, CD3OD) δ: 7.74 (m, 3H, 3×Ar-H), 7.31 (m, 1H, 2×Ar-H), 3.56, 3.27 (m, 0.8H and 1.2 H, -CH2-), 3.10, 3.00 (s, 1.5H and 1.5H, CH3), 1.75 and 1.65 (m, 1H and 1H, -CH2-), and 1.02 and 0.81 (m, 1.5H and 1.5H, CH3). 13C NMR (100 MHz, CD3OD) δ: 193.8, 182.3, 170.8, 163.7, 163.6, 146.2, 138.7, 135.6, 134.9, 125.8, 122.9, 120.9, 118.3, 117.7, 117.1, 37.7, 33.1, 22.4, and 11.5. HR-ESI-MS m/z: calculated for C19H18NO5 [M+H]+ 340.1179; 340.1165 was observed.
N,N-dimethyl-4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (RH6): the title compound was prepared following the same method as that for RH1, starting from compound RH0 and dimethylamine. Compound RH6 was obtained as a yellow powder with a yield of 64%. 1H NMR (400 MHz, DMSO-d6) δ: 11.94 (brs, 2H, 2×-OH), 7.83 (t, 1H, J = 7.8 Hz, Ar-H), 7.73 (d, 1H, J = 7.2 Hz, Ar-H), 7.63 (d, 1H, J = 1.3 Hz, Ar-H), 7.41 (d, 1H, J = 7.4 Hz, Ar-H), 7.38 (d, 1H, J = 1.2 Hz, Ar-H), 3.02 (s, 3H, CH3), and 2.92 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ: 191.37, 181.0, 167.5, 161.3, 161.1, 144.7, 137.4, 133.7, 133.2, 124.5, 121.7, 119.3, 117.0, 116.3, 116.0, 38.6, and 34.6. HR-ESI-MS m/z: calculated for C17H14NO5 [M+H]+ 312.0866; 312.0864 was observed.
N-(3-aminopropyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carbox-amide (RH7): the title compound was prepared using the same synthetic procedure as that for compound RH1, starting from compound RH0 and 1,3-diaminopropane. Compound RH7 was obtained as a yellow powder with a yield of 36%. 1H NMR (400 MHz, CD3OD) δ: 8.20 (d, 1H, J = 1.6 Hz, Ar-H), 7.84 (dd, 1H, J = 7.7, 1.5 Hz, Ar-H), 7.79 (m, 1H, Ar-H), 7.75 (d, 1H, J = 1.6 Hz, Ar-H), 7.37 (dd, 1H, J = 8.1, 1.5 Hz, Ar-H), 3.53 (t, 2H, J = 6.8 Hz, -CH2-), 3.02 (t, 2H, J = 7.0 Hz, -CH2-), and 1.99 (m, 2H, -CH2-). HR-ESI-MS m/z: calculated for C18H17N2O5 [M+H]+ 341.1132; 341.1135 was observed.
N-(3-(methylamino)propyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (RH8): the title compound was prepared following the same synthetic procedure as that for RH1, starting from compound RH0 and N-methyl-1,3-propanediamine. The final product, compound RH8, was obtained as a yellow powder with a yield of 31%. 1H NMR (400 MHz, CD3OD) δ: 8.15 (s, 1H, Ar-H), 7.77 (m, 2H, 2×Ar-H), 7.71 (s, 1H, Ar-H), 7.34 (dd, 1H, J = 7.2, 2.1 Hz, Ar-H), 3.53 (t, 2H, J = 6.8 Hz, -CH2-), 3.08 (t, 2H, J = 7.4 Hz, -CH2-), 2.74 (s, 3H, -CH3), and 2.02 (m, 2H, -CH2-). 13C NMR (100 MHz, CD3OD) δ: 193.9, 182.4, 168.2, 163.8, 163.6, 142.8, 138.7, 135.4, 134.9, 125.8, 123.9, 120.9, 119.0, 118.7, 117.3, 48.0, 37.8, 33.7, and 27.6. HR-ESI-MS m/z: calculated for C19H19N2O5 [M+H]+ 355.1288; 355.1280 was observed.
N-(3-(dimethylamino)propyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (RH9): the title compound was prepared using the same synthetic procedure as that for RH1, starting from compound RH0 and N,N-dimethyl-1,3-propanediamine. The final product, RH9, was obtained as a yellow powder with a yield of 33%. 1H NMR (400 MHz, DMSO-d6) δ: 9.03 (t, 1H, -NH-), 8.13 (d, 1H, J = 1.6 Hz, Ar-H), 7.84 (t, 1H, J = 7.6 Hz, Ar-H), 7.77 (d, 1H, J = 1.6 Hz, Ar-H), 7.76 (dd, 1H, J = 7.6, 1.0 Hz, Ar-H), 7.42 (dd, 1H, J = 7.6, 1.0 Hz, Ar-H), 3.07 (m, 2H, -CH2-), 2.76 (s, 6H, 2×CH3), and 1.90 (m, 2H, -CH2-). 13C NMR (150 MHz, DMSO-d6) δ: 191.5, 181.3, 164.4, 161.5, 161.2, 141.5, 137.7, 133.7, 133.4, 124.7, 122.5, 119.5, 117.8, 117.5, 116.2, 54.9, 46.4, 2 × 42.5, 36.7, and 24.4. HR-ESI-MS m/z: calculated for C20H21N2O5 [M+H]+ 369.1445; 369.1441 was observed.
N-(3-(diethylamino)propyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (RH10): the title compound was synthesized following the same procedure as that for RH1, using RH0 and 3-diethylaminopropylamine. The resulting product, RH10, was acquored as a yellow powder with a yield of 42%. 1H NMR (400 MHz, CD3OD) δ: 7.94 (s, 1H, Ar-H), 7.64 (m, 1H, Ar-H), 7.58 (m, 2H, 2×Ar-H), 7.20 (d, 1H, J = 7.0 Hz, Ar-H), 3.53 (m, 2H, -CH2-), 3.31 (m, 6H, 2×-CH2-), 2.11 (m, 2H, -CH2-), and 1.37 (t, 6H, J = 7.3 Hz, 2×CH3). 13C NMR (100 MHz, CD3OD) δ: 193.3, 181.8, 167.4, 163.6, 163.3, 142.5, 138.7, 135.0, 134.4, 130.8, 125.8, 123.8, 120.9, 118.7, 118.5, 116.8, 50.9, 38.2, 30.7, 2 × 25.3, and 2 × 9.2. HR-ESI-MS m/z: calculated for C22H25N2O5 [M+H]+ 397.1758; 397.1750 was observed.
N-(3-(diethylamino)propyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxamide (RH11): the title compound was synthesized following the same procedure as that for RH1, starting from RH0 and 1-(3-aminopropyl) pyrrolidine. The resulting product, RH11, was obtained as a yellow powder with a yield of 37%. 1H NMR (600 MHz, DMSO-d6) δ: 8.98 (m, 1H, -NH-), 8.28 (s, 1H, Ar-H), 8.03 (s, 1H, Ar-H), 7.77 (m, 1H, Ar-H), 7.68 (m, 2H, 2×Ar-H), 3.34 (m, 2H, -CH2-), 2.82 (m, 4H, 2×-CH2-), 2.79 (m, 2H, -CH2-), 1.83 (m, 2H, -CH2-), and 1.80 (m, 4H, 2×-CH2-). HR-ESI-MS m/z: calculated for C22H23N2O5 [M+H]+ 395.1601 and observed 395.1611.
N-(3-(4-methylpiperazin-1-yl)propyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydro-anthracene-2-carboxamide (RH12): the title compound was synthesized following the same procedure as that for RH1, starting from RH0 and 1-(3-aminopropyl)-4-methylpiperazine. The resulting product, RH12, was obtained as a yellow powder with a yield of 41%. 1H NMR (400 MHz, CD3OD) δ: 8.50 (s, 2H, -NH- and -OH), 8.15 (d, 1H, J = 1.7 Hz, Ar-H), 7.82 (m, 1H, Ar-H), 7.78 (m, 1H, Ar-H), 7.72 (d, 1H, J = 1.7 Hz, Ar-H), 7.36 (dd, 1H, J = 8.0, 1.6 Hz, Ar-H), 3.49 (m, 2H, -CH2-), 2.92 (m, 4H, 2× -CH2-), 2.74 (m, 4H, 2× -CH2-), 2.64 (m, 2H, -CH2-), 2.60 (s, 3H, CH3), and 1.88 (m, 2H, -CH2-). HR-ESI-MS m/z: calculated for C23H26N3O5 [M+H]+ 424.1867; 424.1863 was observed.
N-(3-(1H-imidazol-1-yl)propyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydro-anthra-cene-2-carboxamide (RH13): the title compound was synthesized by applying the same method used for RH1, starting from RH0 and 1-(3-aminopropyl)-4-methylpiperazine. The product, RH13, was obtained as a yellow powder with a yield of 36%. 1H NMR (400 MHz, CD3OD) δ: 8.18 (d, 1H, J = 1.6 Hz, Ar-H), 7.92 (s, 1H, Ar-H), 7.83 (m, 1H, Ar-H), 7.79 (m, 1H, Ar-H), 7.72 (d, 1H, J = 1.6 Hz, Ar-H), 7.36 (dd, 1H, J = 8.1, 1.3 Hz, Ar-H), 7.29 (s, 1H, Ar-H), 7.08 (s, 1H, Ar-H), 4.18 (t, 2H, J = 7.0 Hz, -CH2-), 3.44 (t, 2H, J = 6.7 Hz, -CH2-), and 2.02 (m, 2H, -CH2-). HR-ESI-MS m/z: calculated for C21H18N3O5 [M+H]+ 392.1241; 392.1238 was observed.
N-(3-((3-aminopropyl)amino)propyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydro-anthracene-2-carboxamide (RH14): the title compound was synthesized following the same procedure as that for compound RH1, using RH0 and bis(3-aminopropyl)amine. The resulting compound, RH14, was obtained as a yellow powder with a yield of 31%. 1H NMR (400 MHz, CD3OD) δ: 8.15 (d, 1H, J = 1.4 Hz, Ar-H), 7.78 (m, 2H, 2×Ar-H), 7.72 (d, 1H, J = 1.4 Hz, Ar-H), 7.34 (dd, 1H, J = 7.6, 1.4 Hz, Ar-H), 3.54 (t, 2H, J = 6.8 Hz, -CH2-), 3.06 (q, 4H, J = 7.5 Hz, 2×-CH2-), 3.01 (t, 2H, J = 7.5 Hz, -CH2-), and 2.01 (m, 4H, 2×-CH2-). HR-ESI-MS m/z: calculated for C21H24N3O5 [M+H]+ 398.1710 and observed 398.1710.
N-(3-hydroxypropyl)-4,5-dihydroxy-9,10-dioxo-9,10-dihydro-anthracene-2-carboxamide (RH15): the title compound was synthesized using the same procedure as that for compound RH1, starting from compound RH0 and 3-aminopropan-1-ol. Compound RH15 was obtained as a yellow powder with a yield of 52%. 1H NMR (600 MHz, DMSO-d6) δ: 8.87 (t, 1H, J = 5.6 Hz, -NH-), 8.12 (d, 1H, J = 1.5 Hz, Ar-H), 7.83 (t, 1H, J = 5.2 Hz, Ar-H), 7.75 (m, 2H, 2×Ar-H), 7.41 (d, 1H, J = 5.2 Hz, Ar-H), 3.48 (t, 2H, J = 4.1 Hz, -CH2-), 3.35 (t, 2H, J = 6.7 Hz, -CH2-), and 1.71 (m, 2H, -CH2-). 13C NMR (150 MHz, DMSO-d6) δ: 191.4, 181.2, 164.0, 161.5, 161.3, 141.8, 137.5, 133.6, 133.4, 129.6, 124.6, 122.5, 119.4, 117.5, 116.2, 58.5, 36.9, and 32.2. HR-ESI-MS m/z: calculated for C18H16NO6 [M+H]+ 342.0972; 342.0968 was observed.
2-Aminoethyl-4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylate (RH17): N-Boc-ethanolamine (81 mg, 0.5 mmol) was introduced to a solution containing RH0 (100 mg, 0.33 mmol) in DCM. The reaction mixture was stirred at room temperature, and triethylamine (TEA, 51 mg, 0.5 mmol) was gradually added. After three hours, the solvent was evaporated under reduced pressure, and the product was purified using column chromatography on a silica gel with a petroleum ether (PE)/ethyl ether (EA) (4:1, v/v) solvent system, resulting in RH16 (115 mg) with an 82% yield. To remove the Boc-protected group, 4 M HCl in dioxane (1.0 mL) was added dropwise to a solution of RH16 (43 mg, 0.10 mmol) in dioxane (2.0 mL) with vigorous stirring at room temperature. After five hours, the solvent was evaporated under reduced pressure, and the crude product was washed with EA, yielding RH17 (30 mg) with a 92% yield. 1H NMR (400 MHz, DMSO-d6) δ: 11.97 (br s, 1H, -OH), 11.86 (s, 1H, -OH), 8.29 (br s, 2H, -NH), 8.20 (m, 1H, Ar-H), 8.05 (m, 1H, Ar-H), 7.84 (s, 1H, Ar-H), 7.74 (m, 1H, Ar-H), 7.43 (m, 1H, Ar-H), 4.54 (t, 2H, J = 4.9 Hz, -CH2-), and 3.27 (m, 2H, -CH2-). 13C NMR (150 MHz, DMSO-d6) δ: 191.3, 180.9, 163.9, 161.4, 161.0, 137.7, 136.2, 133.8, 133.2, 124.8, 124.7, 119.5, 119.1, 118.9, 116.2, 62.4, and 37.8. HR-ESI-MS m/z: calculated for C17H14NO6 [M+H]+ 328.0816; 328.0813 was observed.
4.3. Antibacterial Susceptibility Assay
Broth microdilution method: derivatives were dissolved in DMSO, followed by a two-fold serial dilution in Mueller–Hinton Broth (MHB) to achieve final concentrations ranging from 128 to 0.25 μg/mL. Each well in the microtiter plate contained 100 μL of the diluted compounds and 100 μL of a bacterial suspension at a final concentration of 106 colony-forming units (CFU)/mL, prepared from an overnight culture in Brain Heart Infusion Broth (BHI). Plates were incubated at 37 °C for 16 h. The negative control well contained 100 μL of MHB alone, while the positive well contained 100 μL of MHB with the bacterial suspension. The MIC values were identified as the lowest concentrations without visible bacterial growth. MICs were verified through three independent biological experiments.
Well diffusion method: a cell suspension of the tested pathogenic cultures (final concentration 106 CFU/mL) was spread over the surface of a Mueller–Hinton (MH) agar plate. Each well (with an inner diameter of 6 mm) was filled with 100 μL of the RH17 at 1×, 2×, 4×, and 8 × MIC. Plates were incubated at 37 °C for 16 h. Antimicrobial activity was assessed by measuring the diameter of the clear inhibition zone around each well.
4.4. Minimum Bactericidal Concentration
Minimum bactericidal concentrations (MBC) are the minimum concentration of a compound required to kill an organism. Tested strains were cultured, and cell density was adjusted before exposing the cultures to varying concentrations of RH17 for 24 h. Post incubation, the samples were plated on MH agar and further incubated at 37 °C for 24 h to monitor growth. The MBC of RH17 was determined as the lowest concentration at which no organism growth was observed. Experiments were conducted in triplicates.
4.5. Bactericidal Kinetics Assay
The procedure was adapted from previously reported methods. Briefly, bacterial suspensions were prepared and diluted to a final concentration of 106 CFUs/mL in MHB. RH17 solutions were then added to the bacterial suspensions to achieve final concentrations of 0 × MIC (as the negative control, using PBS), 1 × MIC, 4 × MIC, and 10 × MIC. Vancomycin at 10 × MIC was used as the positive control. The cultures were incubated in a shaking incubator at 37 °C. At time points 0, 0.25, 0.5, 1, 2, 4, 8, 12, and 24 h, 100 μL aliquots of the culture were diluted appropriately and spread on agar plates. Following an 18 h incubation at 37 °C, colonies on the plates were counted and quantified as CFU/mL.
To assess the time-kill kinetics under metabolic inhibition, the bacterial suspension was pre-incubated at 0 °C in an ice-water bath to inhibit metabolism. Afterward, the prepared bacterial suspension was treated with RH17 at concentrations of 0 × MIC (as the negative control, using PBS), 1 × MIC, 4 × MIC, and 10 × MIC. The cultures were maintained at 0 °C in the ice-water bath throughout the experiment. Sampling and subsequent colony counting were conducted as described previously for the standard time-kill assay.
4.6. Anti-Biofilm Assay
The anti-biofilm assay was performed following the literature with minor modifications [54,55]. A biofilm inhibition assay was performed in a 96-well microplate. Bacterial isolates were cultured overnight in tryptic soy broth (TSB) containing 0.25% glucose at 37 °C. The bacterial suspension was then adjusted to match the turbidity of a 0.5 McFarland standard. Bacterial aliquots of 200 μL with different concentrations of RH17 were added to each microplate well and incubated at 37 °C for 24 h. Negative control wells contained only broth. After incubation, the wells were centrifugated, gently washed with PBS, fixed with 99% methanol, and allowed to dry at room temperature. The attached biofilm cells were stained using 0.1% crystal violet, and the dye was subsequently dissolved with 33% glacial acetic acid. Optical density (OD) at 570 nm was measured using a microplate reader. The biofilm eradication assay is similar to the biofilm inhibition assay. First, a mature biofilm was formed using the above method; the spent culture medium was removed, and the biofilm was washed with PBS three times. Then the biofilms were exposed to different concentrations of RH17 for 24 h. The control group was added with the same amount of PBS solution. Finally, staining and measuring were carried out according to the above protocols.
4.7. Membrane Fluidity Assay
Membrane fluidity was evaluated using Laurdan generalized polarization (GP) (MCE, Monmouth Junction, NJ, USA, Cat No. HY-D0080) based on previously published protocols with modifications [56]. Overnight cultures of S. aureus were diluted to an OD600 of 0.5 and treated with RH17 at 1×, 4×, and 10 × MIC. After one hour of incubation, the cells were washed twice with PBS, resuspended to the original volume, and stained with 10 μM Laurdan for 20 min at room temperature. Subsequently, the cells were washed three times with PBS and transferred to clear, flat-bottomed 96-well black plates. Laurdan fluorescence was recorded at 460 and 500 nm with excitation at 330 nm. Laurdan GP values were calculated using the formula: GP = (I460 − I500)/ (I460 + I500). PBS was used as the negative control, while 50 mM benzyl alcohol (BZ) was employed as the positive control.
4.8. Membrane Permeability Assay
Propidium iodide (PI, Sigma-Aldrich, St. Louis, MO, USA, Cat No. 537079) staining was used to assess bacterial membrane permeability as described in previous studies [57]. Briefly, bacterial cultures were diluted to OD600 = 0.5 and exposed to RH17 at 1 ×, 4 ×, and 10 × MIC concentrations. Negative controls (PBS) and positive controls (200 μg/mL nisin) were included in parallel. After one hour of treatment, 10 μM PI stain was added and incubated for 15 min. The cultures were washed twice with PBS, and fluorescence (Ex/Em = 540/610 nm) was measured within one hour using a microplate reader (TECAN Infinite 200 pro, Mennedorf, Switzerland).
4.9. ATP Determination Assay
The ATP levels of S. aureus ATCC 29213 were assessed using an Enhanced ATP Assay Kit (Beyotime, Shanghai, China, Cat No. S0027) as described previously [58]. In brief, overnight bacterial cultures were sub-cultured for 2–3 h at 37 °C. The cultures were then washed and resuspended in PBS to reach an OD600 of 0.5. Following this, the bacterial suspensions were exposed to different concentrations of RH17 for one hour. After treatment, the suspensions were centrifuged, and the supernatants were collected to measure extracellular ATP levels. Concurrently, the bacterial pellets were lysed, centrifuged, and the resulting supernatants were used to determine intracellular ATP levels. Chemiluminescence was measured using the TECAN Infinite 200 pro microplate reader.
4.10. Membrane Depolarization Assay
Membrane depolarization was assessed using 3,3′-dipropylthiadicarbocyanine iodide [DiSC3(5)] (MCE, Cat No. HY-D0085), following previously described methods with modifications [59]. Briefly, overnight bacterial cultures were diluted to an OD600 of 0.5 and treated with RH17 at concentrations of 1×, 4×, and 10 × MIC. HEPES buffer (5 mM, 20 mM glucose, pH 7.4) was used as the negative control, and 200 μg/mL nisin served as the positive control. After one hour of incubation, DiSC3(5) was added to a final concentration of 10 μM and incubated for 30 min. Fluorescence (Ex/Em = 622/670 nm) was measured using a microplate reader (TECAN Infinite 200 pro) to assess membrane depolarization.
4.11. Reactive Oxygen Species (ROS) Determination Assay
The ROS Assay Kit (Beyotime, Cat No. S0033S) measured the ROS levels. Bacterial cells were diluted to an OD600 of 0.5, treated with RH17 at 1×, 4×, and 10 × MIC, and incubated for one hour. After centrifugation, the supernatant was collected and DCFDA (100 μM) was added. Following a 30 min incubation, fluorescence was measured at Ex/Em = 485/535 nm. ROS UP was the positive control, and PBS was the negative control.
4.12. Statistical Analysis
Statistical analyses were conducted using Prism version 9.0. Data were expressed as mean ± standard deviation (SD). Comparisons between groups were performed using two-way ANOVA, with the level of significance set at a p-value of 0.05 or less.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics13090882/s1, Figures S1–S30: the NMR spectrum data and HR-ESI-MS spectrum data of compounds RH1–17; Figure S31: Cytotoxicity of RH and RH17; Table S1: MIC and MBC values of RH and RH17 against S. aureus isolates (n = 40).
Author Contributions
Conceptualization, X.L. and Y.L.; methodology, X.L. and Y.L.; validation, X.L., Y.L. and M.S.; formal analysis, X.L., Y.L. and M.S.; investigation, X.L. and M.S.; resources, X.L. and Y.L.; data curation, X.L.; writing—original draft preparation, X.L.; writing—review and editing, X.L.; visualization, X.L.; supervision, X.L. and K.Z.; project administration, J.S.; funding acquisition, K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology of the People’s Republic of China (National Key Research and Development Program of China, grant number 2022YFD1801600) and the National Natural Science Foundation of China (Key Projects, grant number 32230106).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Bakkeren, E.; Diard, M.; Hardt, W.D. Evolutionary causes and consequences of bacterial antibiotic persistence. Nat. Rev. Microbiol. 2020, 18, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Le, P.; Kunold, E.; Macsics, R.; Rox, K.; Jennings, M.C.; Ugur, I.; Reinecke, M.; Chaves-Moreno, D.; Hackl, M.W.; Fetzer, C.; et al. Repurposing human kinase inhibitors to create an antibiotic active against drug-resistant Staphylococcus aureus, persisters and biofilms. Nat. Chem. 2020, 12, 145–158. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.O.; Hoiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Peyrusson, F.; Varet, H.; Nguyen, T.K.; Legendre, R.; Sismeiro, O.; Coppee, J.Y.; Wolz, C.; Tenson, T.; Van Bambeke, F. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat. Commun. 2020, 11, 2200. [Google Scholar] [CrossRef]
- Zhang, K.; Du, Y.; Si, Z.; Liu, Y.; Turvey, M.E.; Raju, C.; Keogh, D.; Ruan, L.; Jothy, S.L.; Reghu, S.; et al. Enantiomeric glycosylated cationic block co-beta-peptides eradicate Staphylococcus aureus biofilms and antibiotic-tolerant persisters. Nat. Commun. 2019, 10, 4792. [Google Scholar] [CrossRef]
- Heim, C.E.; Bosch, M.E.; Yamada, K.J.; Aldrich, A.L.; Chaudhari, S.S.; Klinkebiel, D.; Gries, C.M.; Alqarzaee, A.A.; Li, Y.; Thomas, V.C.; et al. Lactate production by Staphylococcus aureus biofilm inhibits HDAC11 to reprogramme the host immune response during persistent infection. Nat. Microbiol. 2020, 5, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Bush, K.; Harbarth, S.; Paul, M.; Rex, J.H.; Tacconelli, E.; Thwaites, G.E. Critical analysis of antibacterial agents in clinical development. Nat. Rev. Microbiol. 2020, 18, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Kalelkar, P.P.; Riddick, M.; Garcia, A.J. Biomaterial-based delivery of antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 2022, 7, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Basak, A.K.; Melander, C. Natural products as inspiration for the development of bacterial antibiofilm agents. Nat. Prod. Rep. 2020, 37, 1454–1477. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Pena, M.A.; Massaro, N.P.; Lin, Y.C.; Pierce, J.G. Leveraging marine natural products as a platform to tackle bacterial resistance and persistence. Acc. Chem. Res. 2021, 54, 1866–1877. [Google Scholar] [CrossRef] [PubMed]
- Abouelhassan, Y.; Garrison, A.T.; Yang, H.; Chavez-Riveros, A.; Burch, G.M.; Huigens, R.W., 3rd. Recent progress in natural-product-inspired programs aimed to address antibiotic resistance and tolerance. J. Med. Chem. 2019, 62, 7618–7642. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Q.; Pi, R.; Chen, J. A research update on the therapeutic potential of rhein and its derivatives. Eur. J. Pharmacol. 2021, 899, 173908. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Du, H.; Zhang, M.; Xu, H.; Pu, X.; Chen, Q.; Luo, R.; Hu, Y.; Wang, Y.; Tu, H.; et al. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytother. Res. 2022, 36, 2081–2094. [Google Scholar] [CrossRef]
- Junfang, F.; Ou, C.; Yibiao, W. Anti-inflammatory mechanism of rhein in treating asthma based on network pharmacology. J. Tradit. Chin. Med. 2022, 42, 296–303. [Google Scholar] [CrossRef]
- Huang, Q.; Lu, G.; Shen, H.M.; Chung, M.C.; Ong, C.N. Anti-cancer properties of anthraquinones from rhubarb. Med. Res. Rev. 2007, 27, 609–630. [Google Scholar] [CrossRef]
- Fernand, V.E.; Losso, J.N.; Truax, R.E.; Villar, E.E.; Bwambok, D.K.; Fakayode, S.O.; Lowry, M.; Warner, I.M. Rhein inhibits angiogenesis and the viability of hormone-dependent and -independent cancer cells under normoxic or hypoxic conditions in vitro. Chem. Biol. Interact. 2011, 192, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, C.S.; Wang, T.; Shen, J.L.; Ling, F.; Jiang, H.F.; Li, P.F.; Wang, G.X. Antiviral, antioxidant, and anti-inflammatory activities of rhein against white spot syndrome virus infection in red swamp crayfish (Procambarus clarkii). Microbiol. Spectr. 2023, 11, e0104723. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, T.X.; Wang, H.; Hu, L.; Liu, L.; Chen, J. Application of rhein as an immunostimulant controls spring viremia of carp virus infection. Fish. Shellfish. Immunol. 2023, 142, 109128. [Google Scholar] [CrossRef] [PubMed]
- Folliero, V.; Dell’Annunziata, F.; Roscetto, E.; Amato, A.; Gasparro, R.; Zannella, C.; Casolaro, V.; De Filippis, A.; Catania, M.R.; Franci, G.; et al. Rhein: A novel antibacterial compound against Streptococcus mutans infection. Microbiol. Res. 2022, 261, 127062. [Google Scholar] [CrossRef] [PubMed]
- Muller-Heupt, L.K.; Vierengel, N.; Gross, J.; Opatz, T.; Deschner, J.; von Loewenich, F.D. Antimicrobial activity of Eucalyptus globulus, Azadirachta indica, Glycyrrhiza glabra, Rheum palmatum Extracts and Rhein against Porphyromonas gingivalis. Antibiotics 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, Q.; Chen, W.; Mai, Z.; Mo, L.; Su, X.; Ou, J.; Lan, Y.; Zheng, H.; Xue, Y. Rhein inhibits Chlamydia trachomatis infection by regulating pathogen-host cell. Front. Public. Health 2022, 10, 1002029. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.; Nicorescu, I.M.; Olaru, O.T.; Ungurianu, A.; Mihai, D.P.; Zanfirescu, A.; Nitulescu, G.M.; Margina, D. Molecular docking and screening studies of new natural sortase A inhibitors. Int. J. Mol. Sci. 2017, 18, 2217. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Jiang, Y.; Lei, G.; Chen, J.; Liu, M.; Liu, S. Rhein Derivatives, A Promising Pivot? Mini Rev. Med. Chem. 2021, 21, 554–575. [Google Scholar] [CrossRef]
- Deng, T.; Du, J.; Yin, Y.; Cao, B.; Wang, Z.; Zhang, Z.; Yang, M.; Han, J. Rhein for treating diabetes mellitus: A pharmacological and mechanistic overview. Front. Pharmacol. 2022, 13, 1106260. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Du, F.; Sun, L.; Shi, J.; Long, M.; Chen, Z. Activity and mechanism of action of antifungal peptides from microorganisms: A Review. Molecules 2021, 26, 3438. [Google Scholar] [CrossRef]
- van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Q.; Xie, J.; Cong, Z.; Cao, C.; Zhang, W.; Zhang, D.; Chen, S.; Gu, J.; Deng, S.; et al. Switching from membrane disrupting to membrane crossing, an effective strategy in designing antibacterial polypeptide. Sci. Adv. 2023, 9, eabn0771. [Google Scholar] [CrossRef]
- Lin, S.; Wade, J.D.; Liu, S. De novo design of flavonoid-based mimetics of cationic antimicrobial peptides: Discovery, development, and applications. Acc. Chem. Res. 2021, 54, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Kim, S.C.; Choi, D.Y.; Jung, W.K.; Moon, M.J. Basic amino acid-mediated cationic amphiphilic surfaces for antimicrobial pH monitoring sensor with wound healing effects. Biomater. Res. 2023, 27, 14. [Google Scholar] [CrossRef] [PubMed]
- Logviniuk, D.; Fridman, M. Serum prevents interactions between antimicrobial amphiphilic aminoglycosides and plasma membranes. ACS Infect. Dis. 2020, 6, 3212–3223. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, X.; Yu, Z.; Yang, F.; Liu, H.; Xue, R.; Luan, S.; Tang, H. Facile synthesis of imidazolium-based block copolypeptides with excellent antimicrobial activity. Biomacromolecules 2021, 22, 2373–2381. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Janson, H.; Wold, H.; Fugelli, A.; Andersson, K.; Hakangard, C.; Olsson, P.; Olsen, W.M. LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 145–151. [Google Scholar] [CrossRef]
- Hu, Y.; Jo, H.; DeGrado, W.F.; Wang, J. Brilacidin, a COVID-19 drug candidate, demonstrates broad-spectrum antiviral activity against human coronaviruses OC43, 229E, and NL63 through targeting both the virus and the host cell. J. Med. Virol. 2022, 94, 2188–2200. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI: Wayne, PA, USA, 2022; pp. 1–250. [Google Scholar]
- Gohrbandt, M.; Lipski, A.; Grimshaw, J.W.; Buttress, J.A.; Baig, Z.; Herkenhoff, B.; Walter, S.; Kurre, R.; Deckers-Hebestreit, G.; Strahl, H. Low membrane fluidity triggers lipid phase separation and protein segregation in living bacteria. EMBO J. 2022, 41, e109800. [Google Scholar] [CrossRef] [PubMed]
- Mascio, C.T.; Alder, J.D.; Silverman, J.A. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 2007, 51, 4255–4260. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 16051. [Google Scholar] [CrossRef]
- Knox, B.E.; Tsong, T.Y. Voltage-driven ATP synthesis by beef heart mitochondrial F0F1-ATPase. J. Biol. Chem. 1984, 259, 4757–4763. [Google Scholar] [CrossRef]
- Fillingame, R.H. Coupling H+ transport and ATP synthesis in F1F0-ATP synthases: Glimpses of interacting parts in a dynamic molecular machine. J. Exp. Biol. 1997, 200, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.P.; Mangerich, W.E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J. Bacteriol. 1981, 147, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Goto, T.; Ogami, S.; Morimoto, H.; Yamazaki, K.; Inoue, N.; Matsuyama, H.; Yoshimune, K.; Yumoto, I. Formation of proton motive force under low-aeration alkaline conditions in alkaliphilic bacteria. Front. Microbiol. 2018, 9, 2331. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Walsh, M.L.; Bockus, B.J.; Chen, L.B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 1981, 88, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; Verschoor, C.P.; Bowdish, D.; Brown, E.D. Collapsing the proton motive force to identify synergistic combinations against Staphylococcus aureus. Chem. Biol. 2013, 20, 1168–1178. [Google Scholar] [CrossRef]
- Hong, Y.; Li, L.; Luan, G.; Drlica, K.; Zhao, X. Contribution of reactive oxygen species to thymineless death in Escherichia coli. Nat. Microbiol. 2017, 2, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, Y. Strategies for managing today’s infections. Clin. Microbiol. Infect. 2008, 14 (Suppl. 3), 22–31. [Google Scholar] [CrossRef][Green Version]
- Safdar, N.; Maki, D.G. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, Gram-negative bacilli, Clostridium difficile, and Candida. Ann. Intern. Med. 2002, 136, 834–844. [Google Scholar] [CrossRef]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A Review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updates 2023, 68, 100954. [Google Scholar] [CrossRef]
- Lopatkin, A.J.; Bening, S.C.; Manson, A.L.; Stokes, J.M.; Kohanski, M.A.; Badran, A.H.; Earl, A.M.; Cheney, N.J.; Yang, J.H.; Collins, J.J. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021, 371, eaba0862. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Hou, X.; Guo, S.; Zhang, L.; Wei, C.; Peng, T.; Hu, X. Nanohole-boosted electron transport between nanomaterials and bacteria as a concept for nano-bio interactions. Nat. Commun. 2021, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Mehershahi, K.S.; Chen, S.L. DNA methylation by three Type I restriction modification systems of Escherichia coli does not influence gene regulation of the host bacterium. Nucleic Acids Res. 2021, 49, 7375–7388. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zou, G.; Hari, T.P.A.; Wilt, I.K.; Zhu, W.; Galle, N.; Faizi, H.A.; Hendricks, G.L.; Tori, K.; Pan, W.; et al. A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2019, 116, 16529–16534. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. 2021, 8, e2100749. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Huang, X.; Ding, S.; Wang, Y.; Shen, J.; Zhu, K. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020, 5, 1040–1050. [Google Scholar] [CrossRef]
- Elliott, A.G.; Huang, J.X.; Neve, S.; Zuegg, J.; Edwards, I.A.; Cain, A.K.; Boinett, C.J.; Barquist, L.; Lundberg, C.V.; Steen, J.; et al. An amphipathic peptide with antibiotic activity against multidrug-resistant Gram-negative bacteria. Nat. Commun. 2020, 11, 3184. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).