Droplet Digital PCR for Acinetobacter baumannii Diagnosis in Bronchoalveolar Lavage Samples from Patients with Ventilator-Associated Pneumonia
Abstract
1. Introduction
2. Results
2.1. Microbiological Analysis
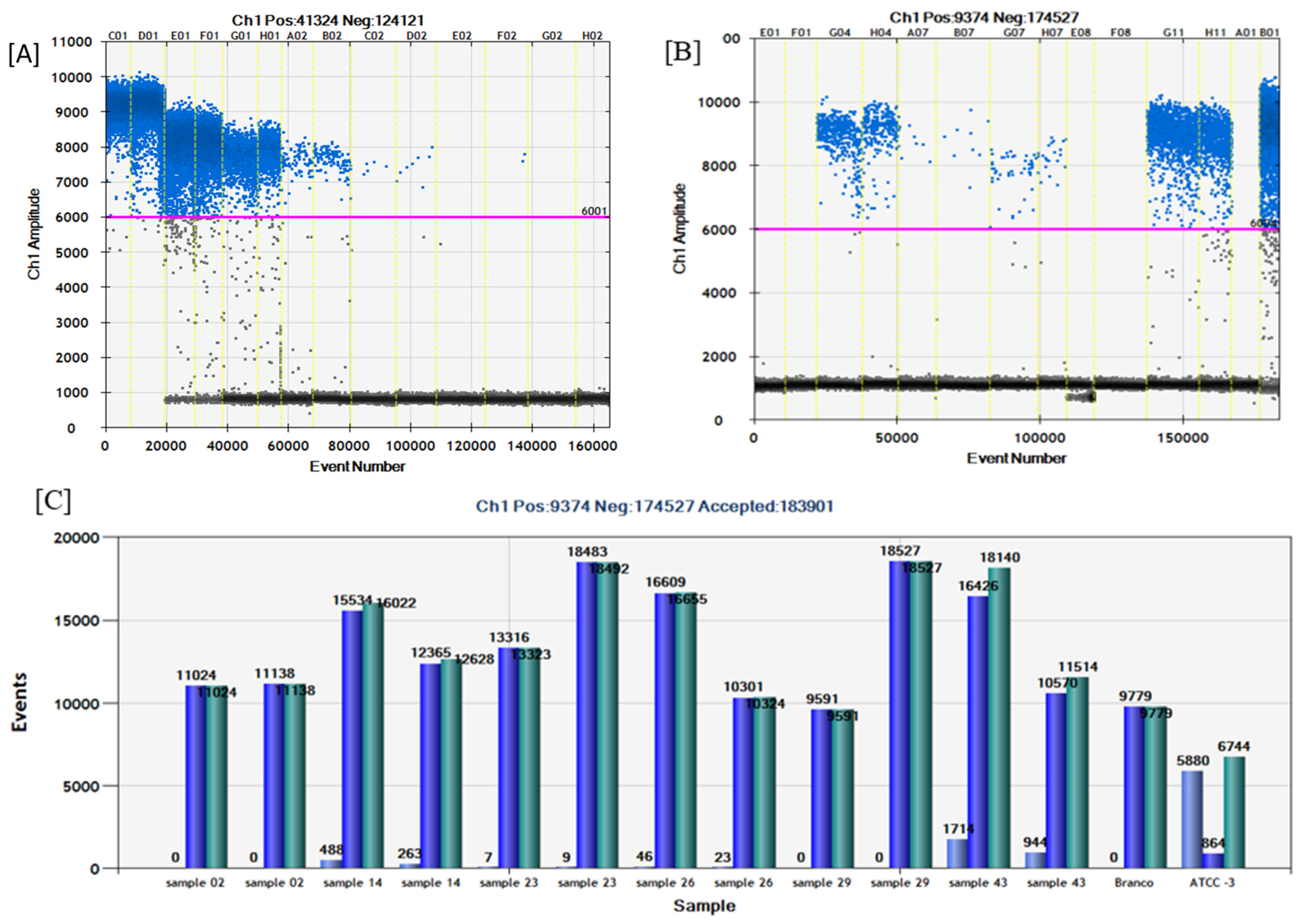
2.2. Analytical Sensitivity
2.3. Analytical Specificity
2.4. Comparison of Conventional PCR, Real-Time PCR (SYBR® Green and TaqMan®), and ddPCR on Detection of A. baumannii in Clinical Samples
3. Discussion
4. Materials and Methods
4.1. Criteria for Diagnosis of VAP
- A mini-BAL culture showing a count of ≥104 CFU/mL;
- An axillary temperature of ≥38.3 °C;
- Total leukocytes of ≥109/L;
- Aspiration of purulent sputum through the orotracheal tube (OT) or tracheostomy; worsening of gas exchange, such as a PaO2/FiO2 ratio of less than 300;
- Increased need for supplemental oxygen or heightened ventilatory demand.
4.2. Sample Collection and Microbiological Analysis
- <10 colonies per plate <104 CFU/mL and, presumably, colonization.
- ≥10 colonies per plate ≥104 CFU/mL and should be interpreted as infectious disease.
4.3. Nucleic Acid Extraction
4.4. Conventional PCR Assay
4.5. SYBR® Green qPCR Assay
4.6. TaqMan® Real-Time PCR Assay (qPCR)
4.7. Droplet Digital PCR (ddPCR) Assay
4.8. Analytical Sensitivity
4.9. Analytical Specificity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.L.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Ciginskienė, A.; Dambrauskienė, A.; Rello, J.; Adukauskienė, D. Ventilator-Associated Pneumonia due to Drug-Resistant Acinetobacter baumannii: Risk Factors and Mortality Relation with Resistance Profiles, and Independent Predictors of In-Hospital Mortality. Medicina 2019, 55, 49. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, J.; Kinoshita, T.; Yamakawa, K.; Matsushima, A.; Nakamoto, N.; Hamasaki, T.F.S. Impact of Gram stain results on initial treatment selection in patients with ventilator-associated pneumonia: A retrospective analysis of two treatment algorithms. Crit. Care 2017, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, D.; Singh, D.; Loomba, P.; Kaur, A.; Tandon, M.; Bishnoi, I. Assessment of surgical risk factors in the development of ventilator-associated pneumonia in neurosurgical intensive care unit patients: Alarming observations. Neurol. India 2017, 65, 779–784. [Google Scholar] [CrossRef]
- Philippart, F.; Gaudry, S.; Quinquis, L.; Lau, N.; Ouanes, I.; Touati, S.; Nguyen, J.C.; Branger, C.; Faibis, F.; Mastouri, M.; et al. Randomized intubation with polyurethane or conical cuffs to prevent pneumonia in ventilated patients. Am. J. Respir. Crit. Care Med. 2015, 191, 637–645. [Google Scholar] [CrossRef]
- Haas, D.J.; Torres, D.A.C. Aplicações das técnicas de PCR no diagnóstico de doenças infecciosas dos animais. Rev. Científica Med. Veterinária 2016, 16, 26. [Google Scholar]
- Anbazhagan, D.; Mui, W.S.; Mansor, M.; Yan, G.O.S.; Yusof, M.Y.; Sekaran, S.D. Development of conventional and real-time multiplex PCR assays for the detection of nosocomial pathogens. Braz. J. Microbiol. 2011, 42, 448–458. [Google Scholar] [CrossRef]
- Zheng, Y.; Jin, J.; Shao, Z.; Liu, J.; Zhang, R.; Sun, R.; Hu, B. Development and clinical validation of a droplet digital PCR assay for detecting Acinetobacter baumannii and Klebsiella pneumoniae in patients with suspected bloodstream infections. Microbiology 2021, 10, e1247. [Google Scholar] [CrossRef]
- Jitmuang, A.; Nititammaluk, A.; Boonsong, T.; Sarasombath, P.T.; Sompradeekul, S.; Chayakulkeeree, M. A novel droplet digital polymerase chain reaction for diagnosis of Pneumocystis pneumonia (PCP)-a clinical performance study and survey of sulfamethoxazole-trimethoprim resistant mutations. J. Infect. 2021, 83, 701–708. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ushio, R.; Watanabe, H.; Tachibana, T.; Tanaka, M.; Yokose, T.; Kaneko, T. Detection of Mycobacterium tuberculosis-derived DNA in circulating cell-free DNA from a patient with disseminated infection using digital PCR. Int. J. Infect. Dis. 2018, 66, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, I.; Cajander, S.; Rasmussen, G.; Ennefors, T.; Mölling, P.; Strålin, K. High nuc DNA load in whole blood is associated with sepsis, mortality and immune dysregulation in Staphylococcus aureus bacteraemia. Infect. Dis. 2019, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Merino, I.; de la Fuente, A.; Domínguez-Gil, M.; Eiros, J.M.; Tedim, A.P.; Bermejo-Martín, J.F. Digital PCR applications for the diagnosis and management of infection in critical care medicine. Crit. Care. Mar. 2022, 26, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farkas, K.; Hassard, F.; McDonald, J.E.; Malham, S.K.; Jones, D.L. Evaluation of Molecular Methods for the Detection and Quantification of Pathogen-Derived Nucleic Acids in Sediment. Front. Microbiol. 2017, 8, 53. [Google Scholar] [CrossRef]
- Ou, Y.; Cao, S.; Zhang, J.; Dong, W.; Yang, Z.; Yu, Z. Droplet microfluidics on analysis of pathogenic microbes for wastewater-based epidemiology. Trends Analyt. Chem. 2021, 143, 116333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koenig, S.M.; Truwit, J.D. Ventilator-Associated Pneumonia: Diagnosis, Treatment, and Prevention. Clin. Microbiol. Rev. 2006, 19, 637–657. [Google Scholar] [CrossRef]
- Papazian, L.; Klompas, M.; Luyt, C. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Berton, D.C.; Kalil, A.C.; Teixeira, P.J. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2014, 10, CD006482. [Google Scholar] [CrossRef]
- Wałaszek, M.; Różańska, A.; Wałaszek, M.Z.; Wójkowska-Mach, J. Epidemiology of Ventilator-Associated Pneumonia, microbiological diagnostics and the length of antimicrobial treatment in the Polish Intensive Care Units in the years 2013–2015. BMC Infect. Dis. 2018, 18, 308. [Google Scholar] [CrossRef]
- Rose, D.D.; Pezzotti, P.; Fortunato, E.; Sordillo, P.; Gini, S.; Boros, S.; Meledandri, M.; Gallo, M.T.; Prignano, G.; Caccese, R.; et al. Clinical predictors and microbiology of ventilator-associated pneumonia in the intensive care unit: A retrospective analysis in six Italian hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1531–1539. [Google Scholar] [CrossRef]
- Sandiumenge, A.; Rello, J. Ventilator-associated pneumonia caused by E.S.K.A.P.E. organisms. Curr. Opin. Pulm. Med. 2012, 18, 187–193. [Google Scholar] [CrossRef]
- Denys, G.A.; Relich, R.F. Antibiotic resistance in nosocomial respiratory infections. Clin. Lab. Med. 2014, 34, 257–270. [Google Scholar] [CrossRef]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Vignon-Whaley, J.J.J.; Vaamonde, J.A.A.; Alonzo, L.A.P.; Reséndiz, A.R.; Álvarez, M.M.; López, E.N.V.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Woodford, N.; Glover, J.; Yarde, S.; Kaufmann, M.E.; Pitt, T.L. Identification of Acinetobacter baumannii by Detection of the blaOXA-51-like Carbapenemase Gene Intrinsic to This Species. J. Clin. Microbiol. 2006, 44, 2974–2976. [Google Scholar] [CrossRef] [PubMed]
- Wattal, C.; Goel, N.; Oberoi, J.K.; Datta, S.; Raveendran, R. Performance of three commercial assays for colistin susceptibility in clinical isolates and Mcr-1 carrying reference strain. Indian J. Med. Microbiol. 2019, 37, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Biswas, I.; Veeraraghavan, B. Accurate identification of clinically important Acinetobacter spp.: An update. Future Sci. 2019, 5, FSO395. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.; Eickmeyer, H.; Heininger, A.; Juretzek, S.; Karrasch, M.; Denis, O.; Roisin, S.; Pletz, M.W.; Klein, M.; Barth, S.; et al. Detection of pneumonia-associated pathogens using a prototype multiplexed pneumonia test in hospitalized patients with severe pneumonia. PLoS ONE 2014, 9, e110566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clavel, M.; Barraud, O.; Moucadel, V.; Meynier, F.; Karam, E.; Ploy, M.-C.; François, B. Molecular quantification of bacteria from respiratory samples in patients with suspected ventilator-associated pneumonia. Clin. Microbiol. Infect. 2016, 22, 812.e1–812.e7. [Google Scholar] [CrossRef]
- Mansour, M.G.E.; Albendary, S. Multiplex polymerase chain reaction: Could change diagnosis of Ventilator-associated pneumonia in pediatric critical care units to the fast track? Egypt. J. Med. Hum. Genet. 2018, 19, 135–139. [Google Scholar] [CrossRef]
- Kwon, S.J.; Jeon, T.; Seo, D.; Na, M.; Choi, E.G.; Son, J.W.; Yoo, E.H.; Park, C.G.; Lee, H.Y.; Kim, J.O.; et al. Quantitative PCR for Etiologic Diagnosis of Methicillin-Resistant Staphylococcus aureus Pneumonia in Intensive Care Unit. Tuberc. Respir. Dis. 2012, 72, 293–301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharifi, A.; Kavoosi, F.; Hosseini, S.M.J.; Mosavat, A.; Ahmadi, A. Prevalence of Streptococcus pneumoniae in Ventilator-Associated Pneumonia by Real-time PCR. Arch. Clin. Infect. Dis. 2019, 14, e86416. [Google Scholar] [CrossRef]
- Pinheiro-de-Oliveira, T.F.; Fonseca, A.A., Jr.; Camargos, M.F.; Laguardia-Nascimento, M.; de Oliveira, A.M.; Cottorello, A.C.P.; Goes-Neto, A.; Barbosa-Stancioli, E.F. Development of a droplet digital RT-PCR for the quantification of foot-and-mouth virus R.N.A. J. Virol. Methods 2018, 259, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Shieh, K.-J.; Chen, G.; Qiao, X.T.; Chuang, M.-Y. Application of Real-time Polymerase Chain Reaction (RT-PCR). J. Am. Sci. 2006, 2, 1–15. [Google Scholar]
- Gadsby, N.J.; McHugh, M.P.; Russell, C.D.; Mark, H.; Conway Morris, A.; Laurenson, I.F.; Hill, A.T.; Templeton, K.E. Development of two real-time multiplex PCR assays for the detection and quantification of eight key bacterial pathogens in lower respiratory tract infections. Clin. Microbiol. Infect. 2015, 21, 788.e1–788.e13. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. Quantification Strategies in Real-Time PCR; International University Line: La Jolla, CA, USA, 2004; Chapter 3; pp. 87–112. [Google Scholar]
- Wong, M.L.; Medrano, J.F. Real-time PCR for mRNA quantitation. BioTechniques 2005, 38, 75–85. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, G.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Second Informational Supplement. CLSI Document VET01-S2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014; Volume 33, 70p. [Google Scholar]
- Moreira, M.G.; Camargo, C.H.; Vasconcellos, F.M.; Barreto, L.M.; Nobre, V.; Santos, S.G. Diversity of blaOXA-51 variants and its clonal complexes in multidrug-resistant Acinetobacter baumannii strains in patients with ventilator-associated pneumonia. J. Glob. Antimicrob. Resist. 2017, 9, 94–95. [Google Scholar] [CrossRef]
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent O.X.A. carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2006, 27, 351–353. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, 71–74. [Google Scholar] [CrossRef]
- Cohen, J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]


| Sample | Dilutions | SYBR Cq | SYBR CV | TaqMan Cq | TaqMan CV | ddPCR Copies | ddPCR CV |
|---|---|---|---|---|---|---|---|
| ATCC 19606 | 105 | 35.61 | 0.6 | 30.46 | 0.5 | 194 | 8.0 |
| 35.17 | 30.59 | 228 | |||||
| 36.06 | 30.93 | - | |||||
| 106 | - | - | 33.53 | 1.7 | 9.6 | 19.3 | |
| - | 33.72 | 14.2 | |||||
| - | 34.93 | - | |||||
| 107 | - | - | - | 1.4 | - | ||
| - | - | - | |||||
| - | - | - | |||||
| Clinical sample | 105 | 36.48 | 1.7 | 31.42 | 0.4 | 156 | 11.4 |
| 37.08 | 31.62 | 124 | |||||
| 38.28 | 31.80 | - | |||||
| 106 | - | - | 35.22 | 0.5 | 14 | 23.9 | |
| - | 35.22 | 8.6 | |||||
| - | 35.68 | - | |||||
| 107 | N.E | - | - | - | - | - | |
| N.A | - | - | |||||
| N.A | - | - |
| No | Microbiological Analysis | Conventional PCR | SYBR®Green | TaqMan® | ddPCR | ddPCR |
|---|---|---|---|---|---|---|
| Quantitative Culture (CFU/mL Count) | Electrophoresis’s Result | Cq Value | Cq Value | Number of + Droplets (Average) | Number of Copies/µL (Average) | |
| 1 | - | - | 28.80 | - | - | - |
| 2 | - | - | 32.21 | - | - | - |
| 3 | - | - | 29.90 | - | - | - |
| 4 | - | - | 29.10 | - | - | - |
| 5 | 40 | - | 28.95 | - | - | - |
| 6 | - | - | 30.70 | - | - | - |
| 7 | - | - | 29.20 | - | - | - |
| 8 | - | - | 29.37 | - | - | - |
| 9 | 350–400 | + | 10.10 | 25.14 | 1640 | 126.1 |
| 10 | 350–400 | + | 19.52 | 32.87 | 107 | 9.65 |
| 11 | - | - | 28.98 | - | 4 | 0.265 |
| 12 | - | - | 28.80 | - | - | - |
| 13 | - | - | 30.87 | - | - | - |
| 14 | 100 | + | 25.62 | 27.99 | 376 | 30.6 |
| 15 | - | - | 30.41 | - | - | - |
| 16 | - | - | 34.19 | - | - | - |
| 17 | - | - | 31.66 | - | - | - |
| 18 | - | - | 29.76 | - | - | - |
| 19 | - | - | - | - | - | - |
| 20 | - | - | 30.01 | - | - | - |
| 21 | - | - | 30.03 | - | - | - |
| 22 | - | - | 30.88 | - | - | - |
| 23 | - | - | 28.97 | - | 8 | 0.595 |
| 24 | - | - | 29.46 | - | - | - |
| 25 | - | - | 29.98 | - | - | - |
| 26 | 350–500 | + | 10.08 | 30.82 | 35 | 2.95 |
| 27 | - | - | 29.09 | - | 5 | 0.33 |
| 28 | - | - | 27.92 | 34.52 | 11 | 0.815 |
| 29 | - | - | 30.50 | - | - | - |
| 30 | - | - | 29.84 | 34.29 | 5 | 0.4 |
| 31 | - | - | 30.33 | 31.33 | 80 | 5.7 |
| 32 | - | - | 30.22 | - | - | - |
| 33 | - | - | 28.85 | - | - | - |
| 34 | 200 | + | 23.30 | 30.26 | 570 | 42.75 |
| 35 | 79 | - | 30.02 | 29.58 | 94 | 9.35 |
| 36 | - | - | 30.37 | - | - | - |
| 37 | - | - | 28.51 | - | 8 | 0.525 |
| 38 | - | - | 31.12 | - | - | - |
| 39 | - | - | 28.33 | - | - | - |
| 40 | - | - | 28.45 | - | 5 | 0.3 |
| 41 | - | + | 28.42 | 33.04 | 17 | 18.2 |
| 42 | - | - | 23.55 | - | - | - |
| 43 | 135 | + | 29.15 | 26.04 | 1329 | 108.7 |
| 44 | - | + | 21.27 | - | 11 | 0.895 |
| Molecular Assays | Quantitative Culture | Total | Kappa (%)/Error | ||
|---|---|---|---|---|---|
| Detected | Non-Detected | ||||
| Conventional PCR | Detected | 6 | 2 | 8 | 69.44/0.1183 |
| Not detected | 2 | 34 | 46 | ||
| Total | 8 | 36 | 44 | ||
| Taqman | Detected | 7 | 4 | 11 | 66.67/0.1125 |
| Not detected | 1 | 32 | 33 | ||
| Total | 8 | 36 | 44 | ||
| ddPCR | Detected | 7 | 10 | 17 | 41.55/0.1096 |
| Not detected | 1 | 26 | 27 | ||
| Total | 8 | 36 | 44 | ||
| Molecular Assays | ddPCR | Total | Kappa(%) Error | ||
|---|---|---|---|---|---|
| Detected | Not Detected | ||||
| Conventional PCR | Detected | 8 | 0 | 8 | |
| Not detected | 9 | 27 | 36 | 52.17/ | |
| Total | 17 | 27 | 44 | 0.1036 | |
| Taqman® | Detected | 11 | 0 | 11 | |
| Not detected | 6 | 27 | 33 | 69.23/ | |
| Total | 17 | 27 | 44 | 0.0921 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giselle Moreira, M.; Guimarães Oliveira, A.G.; Ul Haq, I.; Pinheiro de Oliveira, T.F.; Alonazi, W.B.; Fonseca Júnior, A.A.; Nobre Junior, V.A.; Santos, S.G.d. Droplet Digital PCR for Acinetobacter baumannii Diagnosis in Bronchoalveolar Lavage Samples from Patients with Ventilator-Associated Pneumonia. Antibiotics 2024, 13, 878. https://doi.org/10.3390/antibiotics13090878
Giselle Moreira M, Guimarães Oliveira AG, Ul Haq I, Pinheiro de Oliveira TF, Alonazi WB, Fonseca Júnior AA, Nobre Junior VA, Santos SGd. Droplet Digital PCR for Acinetobacter baumannii Diagnosis in Bronchoalveolar Lavage Samples from Patients with Ventilator-Associated Pneumonia. Antibiotics. 2024; 13(9):878. https://doi.org/10.3390/antibiotics13090878
Chicago/Turabian StyleGiselle Moreira, Mirna, Anna Gabriella Guimarães Oliveira, Ihtisham Ul Haq, Tatiana Flávia Pinheiro de Oliveira, Wadi B. Alonazi, Antônio Augusto Fonseca Júnior, Vandack Alencar Nobre Junior, and Simone Gonçalves dos Santos. 2024. "Droplet Digital PCR for Acinetobacter baumannii Diagnosis in Bronchoalveolar Lavage Samples from Patients with Ventilator-Associated Pneumonia" Antibiotics 13, no. 9: 878. https://doi.org/10.3390/antibiotics13090878
APA StyleGiselle Moreira, M., Guimarães Oliveira, A. G., Ul Haq, I., Pinheiro de Oliveira, T. F., Alonazi, W. B., Fonseca Júnior, A. A., Nobre Junior, V. A., & Santos, S. G. d. (2024). Droplet Digital PCR for Acinetobacter baumannii Diagnosis in Bronchoalveolar Lavage Samples from Patients with Ventilator-Associated Pneumonia. Antibiotics, 13(9), 878. https://doi.org/10.3390/antibiotics13090878







