Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- The following virtual trial settings were used to replicate studies investigating ceftazidime PK in young adult and elderly subjects after i.v. administration.
| Trial (Ref.) | Trial Code: Virtual Trial Settings |
| Trial A [26] | Trial A1: Healthy-Pop (18–39 years): a bolus of 2 g to 200 individuals (40% female) Trial A2: Healthy-Pop (40–59 years): a bolus of 2 g to 200 individuals (50% female) Trial A3: Healthy-Pop (60–79 years): a bolus of 2 g to 200 individuals (23% female) Trial A4: Healthy-Pop (80–95 years): a bolus of 2 g to 200 individuals (44% female) |
| Trial B [27] | Trial B1: Healthy-Pop (23–31 years): a bolus of 2 g to 200 individuals (0% female) Trial B2: Healthy-Pop (63–76 years): a bolus of 2 g to 200 individuals (0% female) |
| Trial C [25] | Trial C1: Healthy-Pop (24–32 years): a bolus of 2 g to 200 individuals (0.5% female) Trial C2: Healthy-Pop (65–83 years): a bolus of 2 g to 200 individuals (0.15% female) |
| Trial D [30] | Trial D1: Healthy-Pop (19–29 years): a bolus of 1 g to 200 individuals (50% female) Trial D2: Healthy-Pop (57–73 years): a bolus of 1 g to 200 individuals (0% female) |
| Trial E [28] | Trial E: Healthy-Pop (67–75 years): a 30-min infusion of 2 g to 200 individuals (50% female) |
| Trial F [29] | Trial F: Healthy-Pop (69–91 years): a 30-min infusion dose of 2 g to 200 individuals (0% female). |
| Trial G [31] | Trial G: Healthy-Pop (68–82 years): a bolus of 1 g to 200 individuals (0.33% female) |
- 2.
- The following trial designs were used for predicting ceftazidime PK in individuals with varying degrees of renal impairment after i.v. administration.
| Trial (Ref.) | Trial Code: Virtual Trial Settings |
| Trial A (Ohkawa [32]) | Trial A1: Healthy-Pop: a bolus of 0.5 g to 200 individuals (43% female) aged 20–65 years, with CLcr of 105–133 mL/min/1.73 m2. Trial A2: Mild-RI-Pop: a bolus of 0.5 g to 200 individuals (43% female) aged 20–87 years, with CLcr of 63–89 mL/min/1.73 m2. Trial A3: Moderate-RI-Pop: a bolus of 0.5 g to 200 individuals (43% female) aged 20–87 years, with CLcr of 30–57 mL/min/1.73 m2. Trial A4: Severe-RI-Pop: a bolus of 0.5 g to 200 individuals (43% female) aged 20–87 years, with CLcr of 8–29 mL/min/1.73 m2. |
| Trial B (Saito [33]) | Trial B1: Healthy-Pop: a bolus of 1 g to 200 individuals (0% female) aged 20–50 years, with CLcr of >90 mL/min. Trial B2: Mild-RI-Pop: a bolus of 1 g to 200 individuals (0% female) aged 20–50 years, with CLcr of 60–89 mL/min. Trial B3: Moderate-RI-Pop: a bolus of 1 g to 200 individuals (0% female) aged 20–50 years, with CLcr of 30–59 mL/min. Trial B4: Severe-RI-Pop: a bolus of 1 g to 200 individuals (0% female) aged 20–50 years, with CLcr of 10–29 mL/min. |
| Trial C (Ackerman [34]) | Trial C1: Healthy-Pop: a bolus of 1 g to 200 individuals (40% female) aged 26–27 years, with CLcr of 97–113 mL/min. Trial C2: Mild-RI-Pop: a bolus of 1 g to 200 individuals (40% female) aged 27 years with, CLcr of 75 mL/min. Trial C3: Moderate-RI-Pop: a bolus of 1 g to 200 individuals (40% female) aged 33–74 years with, CLcr of 34–45 mL/min. Trial C4: Severe-RI-Pop: a bolus of 1 g to 200 individuals (40% female) aged 78 years with, CLcr of 6 mL/min using lowest limit CLcr of 15 mL/min). |
| Trial D (Leroy [35]) | Trial D1: Healthy-Pop: a bolus of 15 mg/kg to 200 individuals (32% female) aged 22–31 years, with CLcr of 110–141 mL/min. Trial D2: Moderate-RI-Pop: a bolus of 15 mg/kg to 200 individuals (32% female) aged 26–74 years, with CLcr of 39–73 mL/min. Trial D3: Severe-RI-Pop: a bolus of 15 mg/kg to 200 individuals (32% female) aged 26–74 years, with CLcr of 14–27 mL/min. Trial D4: Severe-RI-Pop: a bolus of 15 mg/kg to 200 individuals (32% female) aged 26–74 years, with CLR reset to zero. |
| Trial E (Norrby [37]) | Trial E1: Healthy-Pop: a 20-min infusion of 1 g to 200 individuals (33% female) aged 57–77 years, with CLEDTA 92–146 mL/min/1.73 m2. Trial E2: Mild-RI-Pop: a 20-min infusion of 1 g to 200 individuals (60% female) aged 69–84 years, with CLEDTA 60–76 mL/min/1.73 m2. Trial E3: Moderate-RI-Pop: a 20-min infusion of 1 g to 200 individuals (33% female) aged 57–77 years, with CLEDTA 47–54 mL/min/1.73 m2. |
| Trial F (Welage [40]) | Trial F1: Healthy-Pop: a bolus of 1 g to 200 individuals (0% female) aged 30–36 years, with CLcr of 110–122 mL/min. Trial F2: Moderate-RI-Pop: a bolus of 1 g to 200 individuals (20% female) aged 49–69 years, with CLcr of 34–53 mL/min. Trial F3: Severe-RI-Pop: a bolus of 1 g to 200 individuals (0%female) aged 27–91 years, with CLcr of 21–29.5 mL/min. |
| Trial G (van Dalen [38]) | Trial G1: Healthy-Pop: a bolus of 1 g to 200 individuals (30% female) aged 34–65 years, with CLcr of 93–134 mL/min. Trial G2: Mild-RI-Pop: a bolus of 1 g to 200 individuals (30% female) aged 34–88 years, with CLcr of 72–86 mL/min. Trial G3: Moderate-RI-Pop: an i.v. bolus of 1 g to 200 individuals (30% female) aged 34–88 years, with CLcr of 30–59 mL/min. Trial G4: Severe-RI-Pop: an i.v. bolus of 1 g to 200 individuals (30% female) aged 34–88 years, with CLcr of 9–20 mL/min. |
| Trial H (Walstad [39]) | Trial H1: Mild-RI-Pop: a bolus of 1 g to 200 individuals (57% female) aged 28–89 years, with CLcr ≥50 mL/min. Trial H2: Moderate-RI-Pop: a bolus of 1 g to 200 individuals (57% female) aged 28–89 years, with CLcr 31–50 mL/min. Trial H3: Severe-RI-Pop: a bolus of 0.5 g to 200 individuals (57% female) aged 28–89 years, with CLcr 16–30 mL/min. |
| Trial I (Lin [36]) | Trial I1: Mild-RI-Pop: two 30-min infusions of 2 g each to 200 individuals (33% female) aged 21–74 years, with CLcr of 51–94 mL/min. Trial I2: Severe-RI-Pop: two 30-min infusions of 2 g each to 200 individuals (38% female) aged 58–75 years, with CLcr of 10–35 mL/min. |
References
- United Nations. Population Divisions. World Population Ageing 2017—Highlights. United Nations, Department of Economic and Social Affairs. 2017. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf (accessed on 24 January 2024).
- Avorn, J. Medication use and the elderly: Current status and opportunities. Health Aff. 1995, 14, 276–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lassiter, J.; Bennett, W.M.; Olyaei, A.J. Drug dosing in elderly patients with chronic kidney disease. Clin. Geriatr. Med. 2013, 29, 657–705. [Google Scholar] [CrossRef]
- Tian, F.; Chen, Z.; Zeng, Y.; Feng, Q.; Chen, X. Prevalence of Use of Potentially Inappropriate Medications Among Older Adults Worldwide: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2326910. [Google Scholar] [CrossRef]
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical consequences of polypharmacy in elderly. Expert. Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef]
- Florisson, S.; Aagesen, E.K.; Bertelsen, A.S.; Nielsen, L.P.; Rosholm, J.U. Are older adults insufficiently included in clinical trials?-An umbrella review. Basic. Clin. Pharmacol. Toxicol. 2021, 128, 213–223. [Google Scholar] [CrossRef]
- The International Conference on Harmonization (ICH). Studies in Support of Special Populations: Geriatrics E7. 1994. Available online: https://database.ich.org/sites/default/files/E7_Guideline.pdf (accessed on 23 January 2024).
- Food and Drug Administration (FDA). Guidance for Industry. E7 Studies in Support of Special Populations: Geriatrics Questions and Answers. 2012. Available online: https://www.fda.gov/files/drugs/published/E7-Studies-in-Support-of-Special-Populations--Geriatrics--Questions-and-Answers.pdf (accessed on 5 March 2024).
- Hilmer, S.N.; Gnjidic, D.; Abernethy, D.R. Pharmacoepidemiology in the postmarketing assessment of the safety and efficacy of drugs in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 181–188. [Google Scholar] [CrossRef]
- Coetzee, E.; Absalom, A.R. Pharmacokinetic and Pharmacodynamic Changes in the Elderly: Impact on Anesthetics. Anesth. Clin. 2023, 41, 549–565. [Google Scholar] [CrossRef]
- Jamei, M.; Dickinson, G.L.; Rostami-Hodjegan, A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: A tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab. Pharmacokinet. 2009, 24, 53–75. [Google Scholar] [CrossRef]
- Turnheim, K. Pharmacokinetic dosage guidelines for elderly subjects. Expert Opin Drug Metab. Toxicol. 2005, 1, 33–48. [Google Scholar] [CrossRef]
- Mangoni, A.A. Cardiovascular drug therapy in elderly patients: Specific age-related pharmacokinetic, pharmacodynamic and therapeutic considerations. Drugs Aging 2005, 22, 913–941. [Google Scholar] [CrossRef]
- Sobamowo, H.; Prabhakar, S.S. The Kidney in Aging: Physiological Changes and Pathological Implications. Prog. Mol. Biol. Transl. Sci. 2017, 146, 303–340. [Google Scholar] [CrossRef]
- Davies, D.F.; Shock, N.W. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J. Clin. Invest. 1950, 29, 496–507. [Google Scholar] [CrossRef]
- DeSanto, N.G.; Anastasio, P.; Coppola, S.; Barba, G.; Jadanza, A.; Capasso, G. Age-related changes in renal reserve and renal tubular function in healthy humans. Child. Nephrol. Urol. 1991, 11, 33–40. [Google Scholar] [PubMed]
- European Medicines Agency (EMA). Guideline on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Decreased Renal Function. 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-decreased-renal-function_en.pdf (accessed on 15 February 2024).
- US Food and Drug Administration (FDA). Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing. Guidance for Industry. March 2024. Available online: https://www.fda.gov/media/78573/download (accessed on 5 March 2024).
- Chetty, M.; Johnson, T.N.; Polak, S.; Salem, F.; Doki, K.; Rostami-Hodjegan, A. Physiologically based pharmacokinetic modelling to guide drug delivery in older people. Adv. Drug Deliv. Rev. 2018, 135, 85–96. [Google Scholar] [CrossRef]
- Polasek, T.M.; Patel, F.; Jensen, B.P.; Sorich, M.J.; Wiese, M.D.; Doogue, M.P. Predicted metabolic drug clearance with increasing adult age. Br. J. Clin. Pharmacol. 2013, 75, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Mendes, M.; Chetty, M. Are Standard Doses of Renally-Excreted Antiretrovirals in Older Patients Appropriate: A PBPK Study Comparing Exposures in the Elderly Population With Those in Renal Impairment. Drugs R D 2019, 19, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Schlender, J.F.; Meyer, M.; Thelen, K.; Krauss, M.; Willmann, S.; Eissing, T.; Jaehde, U. Development of a Whole-Body Physiologically Based Pharmacokinetic Approach to Assess the Pharmacokinetics of Drugs in Elderly Individuals. Clin. Pharmacokinet. 2016, 55, 1573–1589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tong, X.; Sharma, P.; Xu, H.; Al-Huniti, N.; Zhou, D. Physiologically based pharmacokinetic modelling to predict exposure differences in healthy volunteers and subjects with renal impairment: Ceftazidime case study. Basic. Clin. Pharmacol. Toxicol. 2019, 125, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Abduljalil, K.; Gardner, I.; Jamei, M. An Application of a Physiologically Based Pharmacokinetic Approach to Predict Ceftazidime Pharmacokinetics in a Pregnant Population. Pharmaceutics 2024, 16, 474. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Kees, F.; Grobecker, H. Ceftazidime: Pharmacokinetics in young volunteers versus elderly patients and therapeutic efficacy with complicated urinary tract infections. J. Antimicrob. Chemother. 1983, 12 (Suppl. SA), 41–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ljungberg, B.; Nilsson-Ehle, I. Influence of age on the pharmacokinetics of ceftazidime in acutely ill, adult patients. Eur. J. Clin. Pharmacol. 1988, 34, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Nilsson-Ehle, I. Advancing age and acute infection influence the kinetics of ceftazidime. Scand. J. Infect. Dis. 1989, 21, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Deeter, R.G.; Weinstein, M.P.; Swanson, K.A.; Gross, J.S.; Bailey, L.C. Crossover assessment of serum bactericidal activity and pharmacokinetics of five broad-spectrum cephalosporins in the elderly. Antimicrob. Agents Chemother. 1990, 34, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Higbee, M.D.; Swenson, E.; Gooch, W.M., 3rd. Pharmacokinetics of ceftazidime in elderly patients. Clin. Pharm. 1989, 8, 59–62. [Google Scholar]
- LeBel, M.; Barbeau, G.; Vallee, F.; Bergeron, M.G. Pharmacokinetics of ceftazidime in elderly volunteers. Antimicrob. Agents Chemother. 1985, 28, 713–715. [Google Scholar] [CrossRef]
- Shimada, K.; Inamatsu, T.; Urayama, K.; Ikuma, K. Ceftazidime pharmacokinetics and clinical experiences in the aged patients. Chemotherapy 1983, 31, 251–257. [Google Scholar]
- Ohkawa, M.; Nakashima, T.; Shoda, R.; Ikeda, A.; Orito, M.; Sawaki, M.; Sugata, T.; Shimamura, M.; Hirano, S.; Okumura, K. Pharmacokinetics of ceftazidime in patients with renal insufficiency and in those undergoing hemodialysis. Chemotherapy 1985, 31, 410–416. [Google Scholar] [CrossRef]
- Saito, A. Studies on absorption, distribution, metabolism and excretion of ceftazidime in Japan. J. Antimicrob. Chemother. 1983, 12 (Suppl. SA), 255–262. [Google Scholar] [CrossRef]
- Ackerman, B.H.; Ross, J.; Tofte, R.W.; Rotschafer, J.C. Effect of decreased renal function on the pharmacokinetics of ceftazidime. Antimicrob. Agents Chemother. 1984, 25, 785–786. [Google Scholar] [CrossRef][Green Version]
- Leroy, A.; Leguy, F.; Borsa, F.; Spencer, G.R.; Fillastre, J.P.; Humbert, G. Pharmacokinetics of ceftazidime in normal and uremic subjects. Antimicrob. Agents Chemother. 1984, 25, 638–642. [Google Scholar] [CrossRef]
- Lin, M.S.; Wang, L.S.; Huang, J.D. Single- and multiple-dose pharmacokinetics of ceftazidime in infected patients with varying degrees of renal function. J. Clin. Pharmacol. 1989, 29, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S.R.; Burman, L.A.; Linderholm, H.; Trollfors, B. Ceftazidime: Pharmacokinetics in patients and effects on the renal function. J. Antimicrob. Chemother. 1982, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, R.; Vree, T.B.; Baars, A.M.; Termond, E. Dosage adjustment for ceftazidime in patients with impaired renal function. Eur. J. Clin. Pharmacol. 1986, 30, 597–605. [Google Scholar] [CrossRef]
- Walstad, R.A.; Dahl, K.; Hellum, K.B.; Thurmann-Nielsen, E. The pharmacokinetics of ceftazidime in patients with impaired renal function and concurrent frusemide therapy. Eur. J. Clin. Pharmacol. 1988, 35, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Welage, L.S.; Schultz, R.W.; Schentag, J.J. Pharmacokinetics of ceftazidime in patients with renal insufficiency. Antimicrob. Agents Chemother. 1984, 25, 201–204. [Google Scholar] [CrossRef]
- GlaxoSmithKline. FORTAZ®: Ceftazidime for Injection. 2007. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/050578s053,050634s020lbl.pdf (accessed on 5 March 2024).
- Conil, J.M.; Georges, B.; Lavit, M.; Laguerre, J.; Samii, K.; Houin, G.; Saivin, S. A population pharmacokinetic approach to ceftazidime use in burn patients: Influence of glomerular filtration, gender and mechanical ventilation. Br. J. Clin. Pharmacol. 2007, 64, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, T.; Rowland, M. Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef]
- Harding, S.M.; Harper, P.B. The pharmacokinetic behaviour of ceftazidime in man and the relationship between serum levels and the in vitro susceptibility of clinical isolates. Infection 1983, 11 (Suppl. S1), S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Rowland Yeo, K.; Aarabi, M.; Jamei, M.; Rostami-Hodjegan, A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev. Clin. Pharmacol. 2011, 4, 261–274. [Google Scholar] [CrossRef]
- Rostoker, G.; Andrivet, P.; Pham, I.; Griuncelli, M.; Adnot, S. A modified Cockcroft-Gault formula taking into account the body surface area gives a more accurate estimation of the glomerular filtration rate. J. Nephrol. 2007, 20, 576–585. [Google Scholar]
- National Kidney, F. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
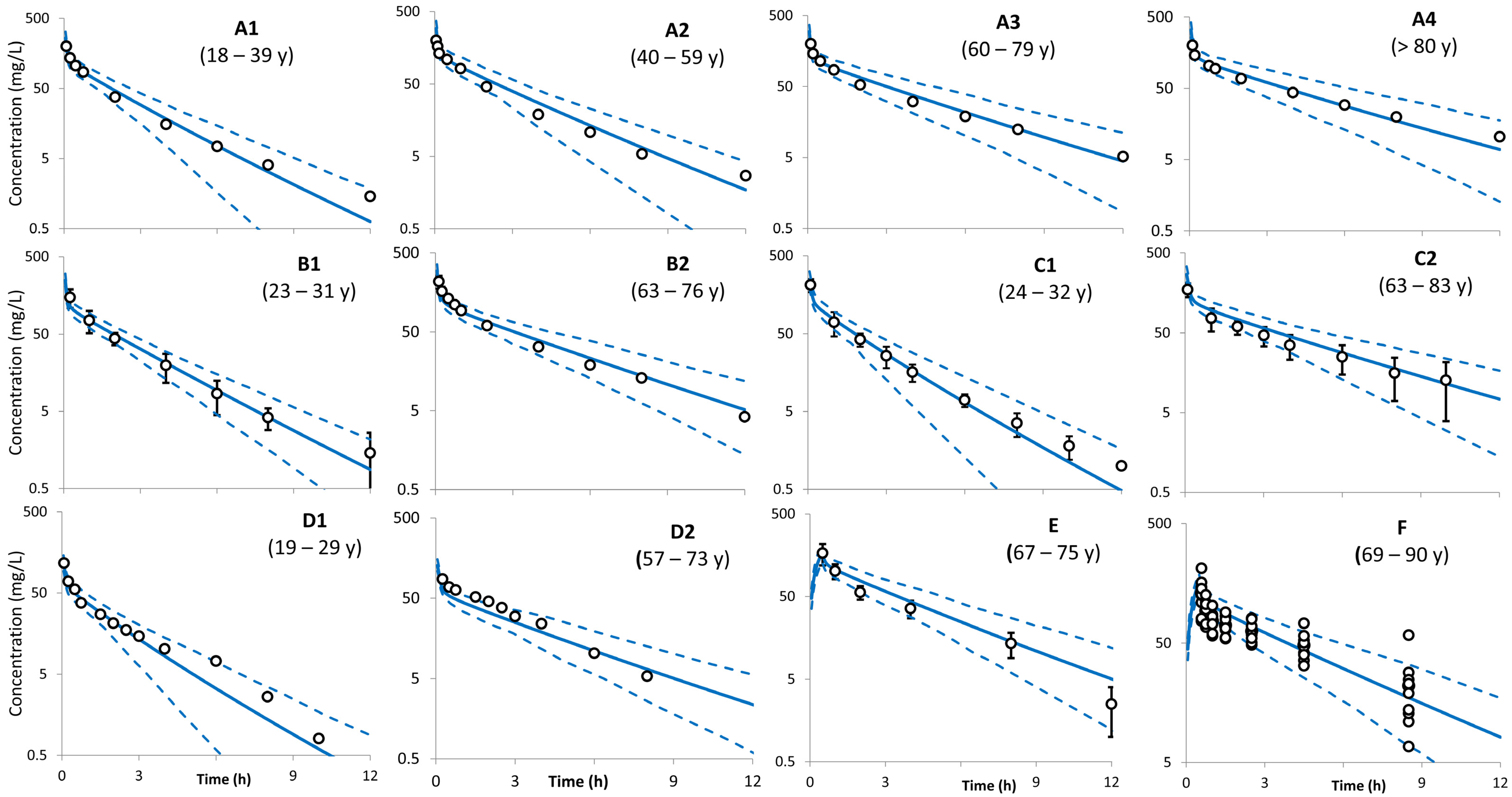
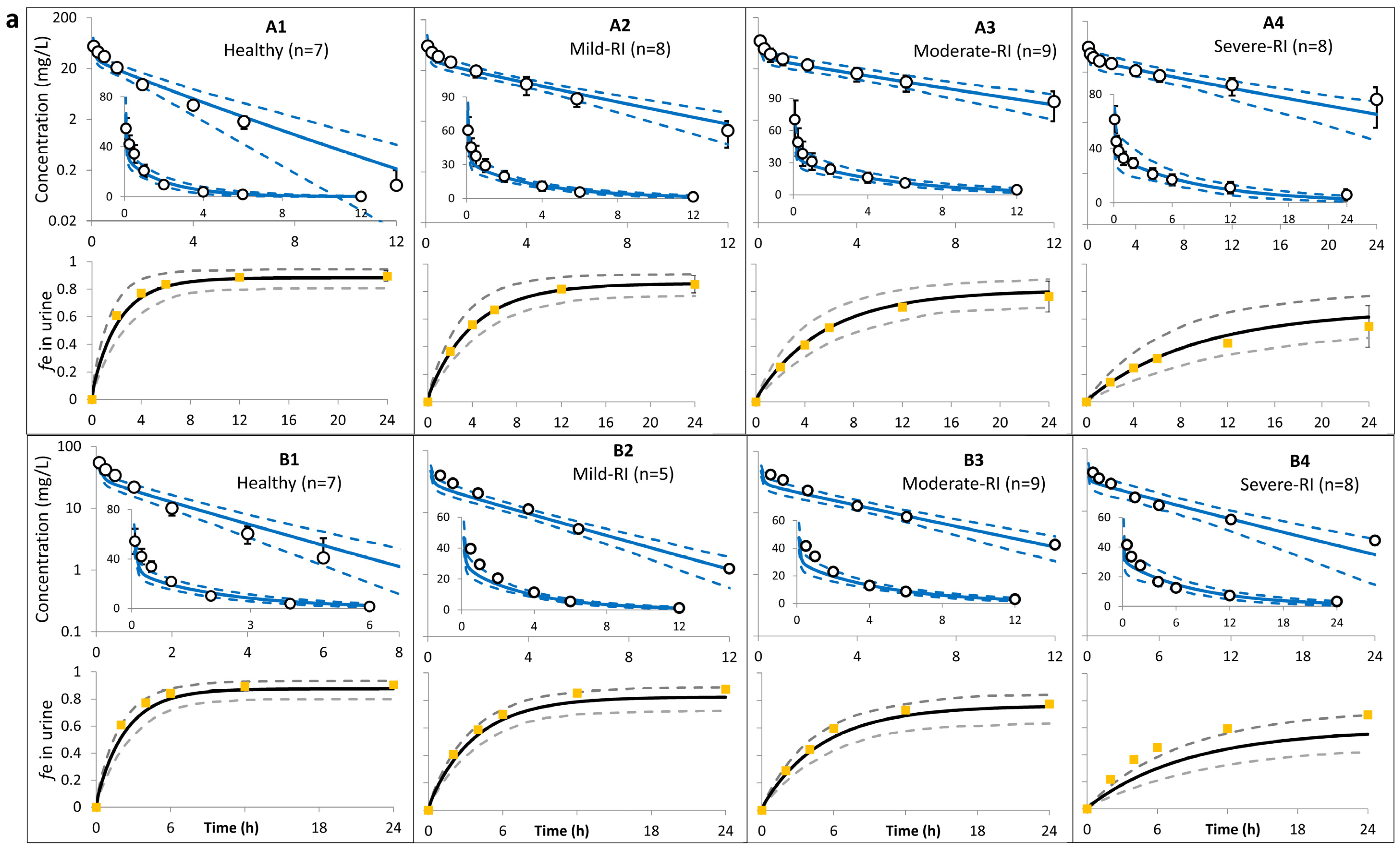
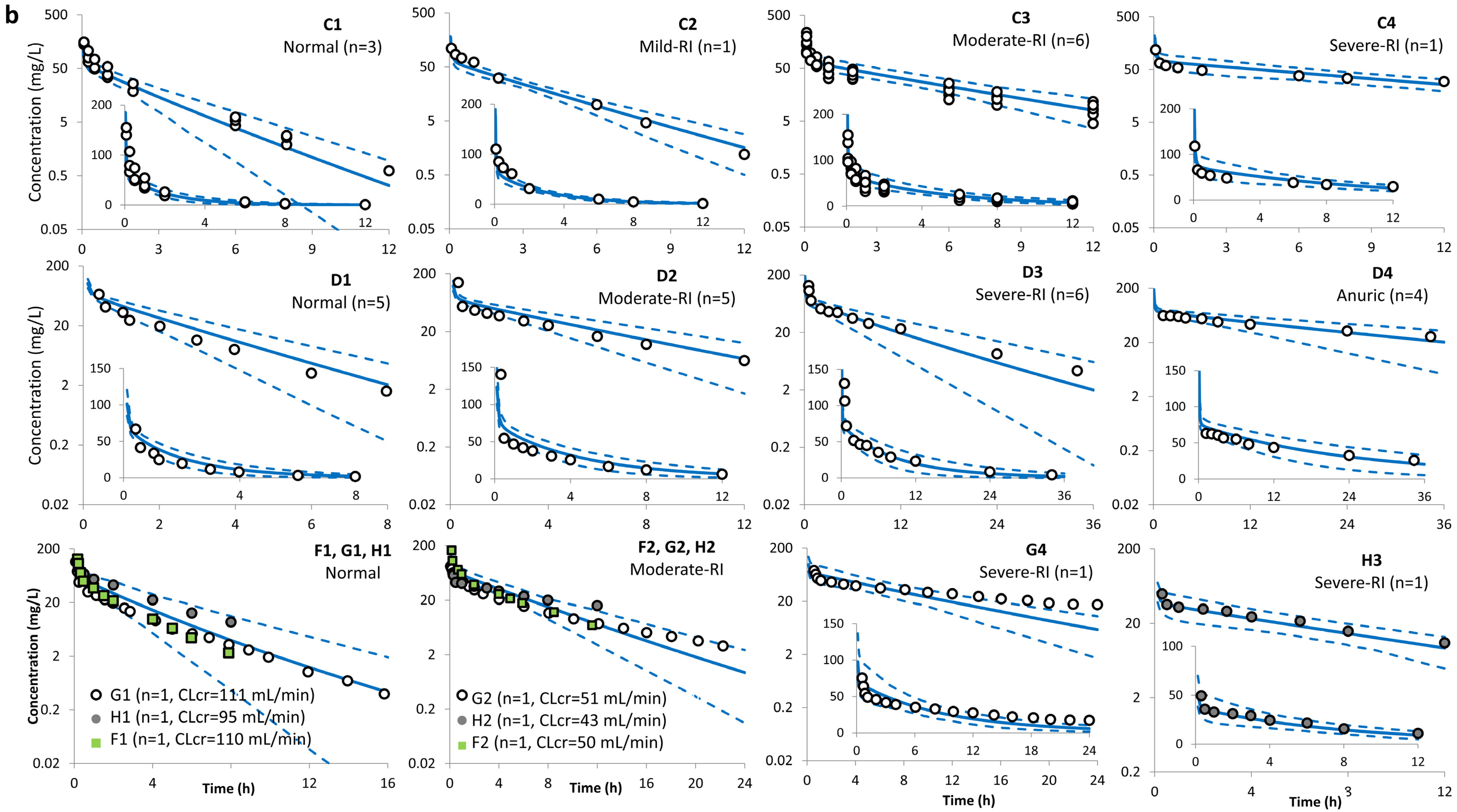
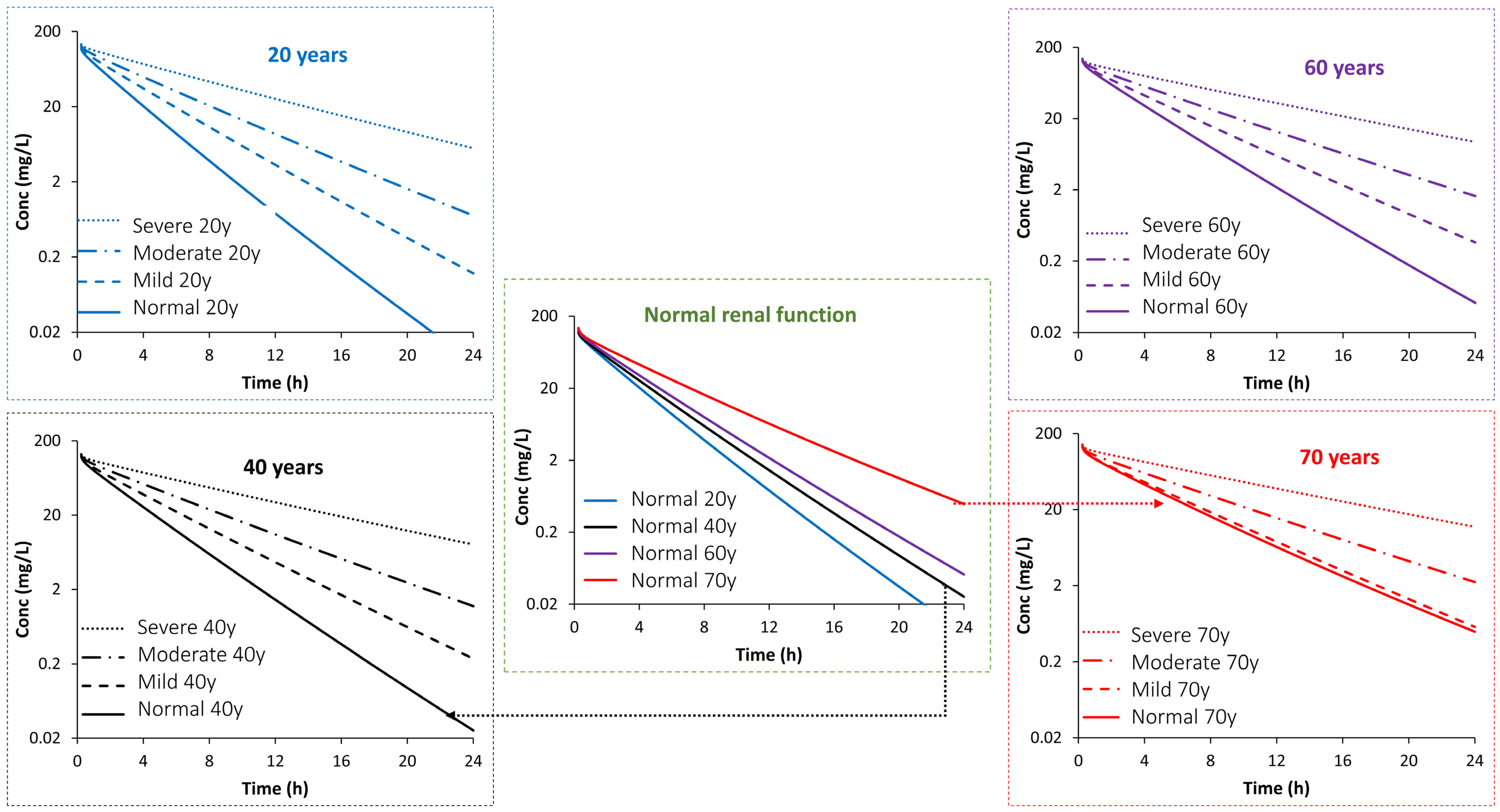
| Study (Dose) | Age (Years) | Sample Size | Sex | Weight (kg) | CLcr (mL/min) | SerCr (µmol/L) | Additional Notes |
|---|---|---|---|---|---|---|---|
| |||||||
| Ljungberg et al. [26] (2 g i.v. bolus) | 18–39 | 7 | 5M/2F | NA | 112 ± 19 | 74.3 ± 17.7 | GFR determined by 15Cr-EDTA; unit of mL/min/1.73 m2 |
| 40–59 | 8 | 4M/4F | NA | 105 ± 26 | 71.6 ±17.7 | ||
| 60–79 | 13 | 10M/3F | NA | 79 ± 18 | 90.2 ± 19.5 | ||
| 84 ± 3.6 | 9 | 5M/4F | NA | 56 ± 16 | 92.0 ± 19.5 | ||
| Ljungberg et al. [27] (2 g i.v. bolus) | 23–31 | 9 | M | NA | 101 ± 6.5 | 84.9 ± 10.6 | GFR determined by 15Cr-EDTA; unit of mL/min/1.73 m2 |
| 63–76 | 10 | M | NA | 77 ± 9.8 | 90.2 ± 11.5 | ||
| Naber et al. [25] (2 g i.v. bolus) | 24–32 | 6 | 3M/3F | 54–81 | NA | 35.4–79.6 | |
| 63–83 | 13 | 11M/2F | 55–96 | 61.9–132.6 | |||
| LeBel et al. [30] (2 g i.v. bolus) | 19–29 | 12 | 6M/6F | 44–78.5 | 76.6–124 | 70.74–88.42 | CLcr calculated using Cockcroft–Gault equation; unit of mL/min. Individual demographics available |
| 57–73 | 5 | M | 50–84 | 56.9–89.8 | 70.74–97.26 | ||
| Deeter et al. [28] (2 g infused over 30 min) | 70.7 ± 3.5 | 6 | 3M/3F | 75 ± 16 | 55.9 ± 13.5 | 88.4 ± 26.5 | CLcr calculated using Cockcroft–Gault equation; unit of mL/min/1.73 m2 |
| Higbee et al. [29] (2 g infused over 30 min) | 69–90 | 10 | M | 43.6–81.4 | 24–80 | <221.05 | Individual demographic available; CLcr calculated using Cockcroft–Gault equation; unit of mL/min |
| Shimada et al. [31] (1 g i.v. bolus) | 68–82 | 3 | 2M/1F | 35–55 | 30–70 | 61.9–88.4 | |
| |||||||
| Ohkawa et al. [32] (0.5 g bolus) | 20–87 | 7 | 29 M/10 F | 38–79 | 105.2–133 | NA | CLcr determined from endogenous creatinine clearance corrected for a normalized body surface area (per 1.73 m2) |
| 8 | 63.1–89.1 | ||||||
| 9 | 30–56.8 | ||||||
| 8 | 8.3–29.2 | ||||||
| Saito et al. [33] (0.5 g bolus) | NA | 7 | M | NA | >90 | NA | Determination of CLcr not described |
| 5 | 60–90 | ||||||
| 9 | 30–60 | ||||||
| 8 | 10–30 | ||||||
| 10 | <10 | ||||||
| Ackerman et al. [34] (1 g bolus) | 26–92 | 11 | 7M/4F | NA | 6–113 | NA | Individual conc and PK data available, but not for sex and weight. Determination of CLcr not described |
| Leroy et al. [35] (15 mg/kg bolus) | 22–31 | 5 | NA | 64–78 | 110–141 | NA | CLcr determined from measurement of endogenous creatinine over time |
| 26–74 | 5 | NA NA NA NA | 41–83 | 39–72.5 | NA | ||
| 6 | 13.8–27 | ||||||
| 4 | 2.0–12 | ||||||
| 4 | Anuric | ||||||
| Norrby et al. [37] (1 g; 20-min inf) | 57–88 | 14 | 8M/6F | NA | 47–146 | 54.8–122 | No conc profiles. GFR determined (51Cr-EDTA Clearance); individual data for PK, CLEDTA, demographics reported |
| Welage et al. [40] (1 g bolus) | 30–91 | 14 | 12M/2F | 57–95 | 4.5–122.3 | 88.4–751.6 | Individual data for PK, measured CLcr (urine collection), demographics. Conc profiles from 3 individuals only |
| Van Dalen et al. [38] (1 g bolus) | 34–88 | 20 | 14M/9F | NA | 0–133.8 | NA | Individual PK data and CLcr (urine collection) available, but not demographics. Conc profiles from 3 individuals only |
| Walstad et al. [39] (1 g, but 0.5 g for severe RI patients) | 28–89 (26 of them > 75 years) | 9 | 16M/21F | NA | >50 | NA | CLcr estimated using Cockcroft and Gault’s method |
| 10 | 50–31 | ||||||
| 10 | 30–16 | ||||||
| 8 | 5.0–15 | ||||||
| Lin et al. [36] (2 g b.i.d. bolus) | 21–74 | 6 | 4M/2F | 50–65 | 51–94 | NA | CLcr estimated using Bjornsson’s method using serum creatinine, age, and weight |
| 58–75 | 8 | 5M/3F | 42–74 | 10–35 | |||
| Study Design * | AUC (h·mg/L) | Half-Life (h) | Clearance (L/h) | fe_12h (%) ** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Population Age (N) | (Trial Code) | Obs | Pred | Ratio | Obs | Pred | Ratio | Obs | Pred | Ratio | Obs | Pred | Ratio |
| Ljungberg et al. [26] 2 g i.v. bolus | 18–39 y (7) | A1 | 248 ± 61 | 278 ± 53 | 1.12 | 2.0 | 1.5 ± 0.3 | 0.74 | 8.06 | 7.48 ± 1.5 | 0.93 | 84 ± 7 | 88 ± 4 | 1.05 |
| 40–59 y (8) | A2 | 287 ± 121 | 323 ± 54 | 1.13 | 2.0 | 1.7 ± 0.4 | 0.84 | 6.97 | 6.38 ± 1.21 | 0.92 | 85 ± 7.5 | 88 ± 5 | 1.04 | |
| Ratio (40–59 y/18–39 y) | 1.16 | 1.16 | 1.00 | 0.99 | 1.12 | 1.13 | 0.86 | 0.85 | 0.99 | 1.01 | 1.00 | 0.99 | ||
| 60–79 y (13) | A3 | 392 ± 115 | 481 ± 118 | 1.23 | 2.73 | 2.64 ± 0.65 | 0.97 | 5.1 | 4.4 ± 1.1 | 0.86 | 74 ± 14 | 83 ± 6 | 1.12 | |
| Ratio (60–79 y/18–39 y) | 1.58 | 1.73 | 1.09 | 1.35 | 1.77 | 1.31 | 0.63 | 0.59 | 0.93 | 0.88 | 0.94 | 1.07 | ||
| >80 y (9) | A4 | 536 ± 142 | 626 ± 164 | 1.17 | 3.54 | 3.08 ± 0.8 | 0.87 | 5.73 | 3.4 ± 0.85 | 0.59 | 67 ± 16 | 82 ± 7 | 1.22 | |
| Ratio (>80 y/18–39 y) | 2.16 | 2.25 | 1.04 | 1.75 | 2.07 | 1.18 | 0.71 | 0.45 | 0.64 | 0.80 | 0.93 | 1.17 | ||
| Ljungberg et al. [27] 2 g i.v. bolus | 23–31 y (9) | B1 | 277 ± 29 | 291 ± 49 | 1.05 | 1.94 | 1.67 ± 0.27 | 0.86 | 7.22 ± 0.8 | 7.05 ± 1.2 | 0.98 | 87 ± 10 | 87 ± 4.4 | 1.00 |
| 63–76 y (10) | B2 | 418 ± 52 | 503 ± 119 | 1.20 | 2.63 | 2.84 ± 0.63 | 1.08 | 4.78 | 4.19 ± 0.9 | 0.88 | 72 ± 8.6 | 82 ± 6.3 | 1.14 | |
| Ratio (63–76 y/23–31) | 1.51 | 1.73 | 1.15 | 1.36 | 1.70 | 1.25 | 0.66 | 0.59 | 0.90 | 0.82 | 0.94 | 1.15 | ||
| Naber et al. [25] 2 g i.v. bolus | 24–32 y (6) | C1 | 271 | 270 ± 55 | 1.0 | 1.75 ± 0.14 | 1.4 ± 0.33 | 0.80 | 7.38 ± 0.7 | 7.71 ± 1.6 | 1.0 | 87 ± 8.4 | 89 ± 4.0 | 1.0 |
| 65–83 y (13) | C2 | 422 | 515 ± 128 | 1.2 | 2.9 ± 0.5 | 2.85 ± 0.69 | 0.98 | 4.74 ± 1.0 | 4.1 ± 0.95 | 0.86 | 57 ± 18 | 82 ± 6.9 | 1.43 | |
| Ratio (65–83 y/24–32 y) | 1.56 | 1.91 | 1.2 | 1.66 | 2.04 | 1.23 | 0.64 | 0.53 | 0.83 | 0.66 | 0.92 | 0.14 | ||
| Le Bel et al. [30] 1 g i.v. bolus | 19–29 y (12) | D1 | 134 ± 13 | 133 ± 27 | 0.99 | 1.9 ± 0.3 | 1.41 ± 0.35 | 0.74 | 7.50 ± 0.7 | 7.86 ± 1.6 | 1.05 | 77 ± 8.6 | 89 ± 4.3 | 1.16 |
| 57–73 y (5) | D2 | 224 ± 79 | 224 ± 54 | 1.00 | 1.9 ± 0.7 | 2.54 ± 0.58 | 1.34 | 4.99 ± 2.0 | 4.71 ± 1.1 | 0.94 | 76 ± 13 | 86 ± 5 | 1.14 | |
| Ratio (19–29 y/19–29 y) | 1.67 | 1.68 | 1.0 | 1 | 1.8 | 1.8 | 0.67 | 0.60 | 0.90 | 0.98 | 0.97 | 0.98 | ||
| Deeter et al. [28] 2 g infusion | 70.7 ± 3.5 y (6) | E | 409 ± 62 | 483 ± 107 | 1.18 | 3.7 ± 2.0 | 2.47 ± 0.59 | 0.67 | 4.89 ± 0.80 | 4.34 ± 0.92 | 0.89 | NA | 84 ± 6.2 | NA |
| Higbee et al. [29] 2 g infusion | 69–91 y (10) | G | 463 ± 209 | 541 ± 137 | 1.17 | 3.9 ± 1.3 | 3.0 ± 0.65 | 0.77 | 4.9 ± 1.4 | 3.93 ± 0.96 | 0.80 | 71 ± 3 | 70 ± 9 | 0.98 |
| Shimada et al. [31] 1 g i.v. (bolus) | 68–82 y (3) | F | 287 ± 93 | 260 ± 69 | 0.91 | 3.7 ± 1.1 | 2.76 ± 0.10 | 0.74 | NA | 4.08 ± 0.97 | NA | 71 ± 3 | 70 ± 9 | 0.99 |
| Model Predictions 2 g (bolus) | 25–35 y (200) | H1 | 282 ± 55 | 1.5 ± 0.4 | 7.4 ± 1.6 | 88 ± 4 | ||||||||
| 45–55 y (200) | H2 | 328 ± 49 | 1.8 ± 0.4 | 6.2 ± 1.1 | 88 ± 4 | |||||||||
| Ratio (45–55/25–35 y) | 1.16 | 1.18 | 0.85 | 1.0 | ||||||||||
| 65–75 y (200) | H3 | 499 ± 134 | 2.5 ± 0.6 | 4.3 ± 1.1 | 84 ± 6 | |||||||||
| Ratio (65–75 y/25–35 y) | 1.77 | 1.69 | 0.58 | 0.95 | ||||||||||
| 85–95 y (200) | H4 | 722 ± 205 | 3.5 ± 1.0 | 3.0 ± 0.8 | 80 ± 8 | |||||||||
| Ratio (85–95 y/25–35 y) | 2.56 | 2.36 | 0.40 | 0.91 | ||||||||||
| Ref. | Population *; Age (n: CLcr (mL/min) | Trial Code | AUC (h · mg/L) | Half-Life (h) | Clearance (L/h) | fe_24h (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Pred | Ratio | Obs | Pred | Ratio | Obs | Pred | Ratio | Obs | Pred | Ratio | |||
| Ohkawa et al. [32] (0.5 i.v. g bolus) | 20–65 y (7 Normal: 105.2–133 a) | A1 | 72.9 ± 14 | 71.3 ± 14 | 0.98 | 1.55 ± 0.3 | 1.52 ± 0.4 | 0.98 | 8.2 ± 1.5 | 7.3 ± 1.4 | 0.89 | 90 ± 4 | 89 ± 4 | 0.99 |
| 20–87 y (8 Mild RI: 63.1–89.1) | A2 | 133 ± 14 | 134 ± 15 | 1.0 | 2.9 ± 0.6 | 2.9 ± 0.6 | 1.0 | 4.6 ± 1.2 | 3.8 ± 0.4 | 0.82 | 85 ± 6 | 86 ± 5 | 1.0 | |
| Ratio (Mild/Normal) | 1.83 | 1.88 | 1.0 | 1.84 | 1.93 | 1.1 | 0.56 | 0.52 | 0.92 | 0.95 | 0.97 | 1.0 | ||
| 20–87 y (9 Moderate RI: 30–57) | A3 | 192 ± 32 | 192 ± 32 | 1.0 | 3.9 ± 0.9 | 4.1 ± 0.9 | 1.0 | 2.9 ± 1.0 | 2.7 ± 0.4 | 0.92 | 76 ± 11 | 80 ± 6 | 1.04 | |
| Ratio (Moderate/Normal) | 2.64 | 2.69 | 1.0 | 2.54 | 2.67 | 1.1 | 0.36 | 0.37 | 1.0 | 0.85 | 0.90 | 1.1 | ||
| 20–87 y (8 Severe RI: 8.3–29.2) | A4 | 338 ± 65 | 344 ± 66 | 1.0 | 6.7 ± 1.8 | 6.9 ± 1.9 | 1.0 | 1.5 ± 0.6 | 1.5 ± 0.3 | 1.0 | 55 ± 15 | 62 ± 10 | 1.1 | |
| Ratio (Severe/Normal) | 4.64 | 4.82 | 1.0 | 4.34 | 4.51 | 1.0 | 0.18 | 0.21 | 1.1 | 0.61 | 0.70 | 1.1 | ||
| Saito [33] (0.5 g i.v. bolus) | 20–50 y (7 Normal: >=90) | B1 | NA | 73 ± 13 | NA | 1.7 | 1.7 ± 0.3 | 1.00 | NA | 7.0 ± 1.2 | NA | 90 | 89 ± 4 | 0.99 |
| 20–50 y (5 Mild RI: 60–90) | B2 | NA | 111 ± 11 | NA | 2.3 | 2.7 ± 0.4 | 1.16 | NA | 4.6 ± 0.5 | NA | 88 | 83 ± 5 | 0.94 | |
| Ratio (Mild/Normal) | NA | 1.51 | NA | 1.4 | 1.57 | 1.16 | NA | 0.65 | NA | 0.98 | 0.94 | 0.96 | ||
| 20–50 y (9 Moderate RI: 30–60) | B3 | NA | 154 ± 18 | NA | 3.4 | 3.6 ± 0.6 | 1.06 | NA | 3.3 ± 0.4 | NA | 78 | 76 ± 7 | 0.98 | |
| Ratio (Moderate/Normal) | NA | 2.10 | NA | 2.0 | 2.11 | 1.1 | NA | 0.47 | NA | 0.86 | 0.87 | 1.0 | ||
| 20–50 y (8 Severe RI: 10–30) | B4 | NA | 267 ± 49 | NA | 6.1 | 6.2 ± 1.4 | 1.02 | NA | 1.9 ± 0.4 | NA | 70 | 55 ± 9 | 0.79 | |
| Ratio (Severe/Normal) | NA | 3.64 | NA | 3.6 | 3.65 | 1.0 | NA | 0.28 | NA | 0.77 | 0.63 | 0.82 | ||
| Ackerman et al. [34] (1 g i.v. bolus) | 26–27 y (3 Normal: >=90) | C1 | 133 ± 28 | 138 ± 27 | 1.0 | 1.3 ± 0.1 | 1.5 ± 0.3 | 1.14 | 7.8 ± 1.5 | 7.5 ± 1.4 | 0.96 | NA | 88 ± 4 | NA |
| 33–74 y (5 Moderate RI: 34–45) | C3 | 336 ± 64 | 353 ± 56 | 1.16 | 4.7 ± 2 | 3.8 ± 0.8 | 0.87 | 3.1 ± 0.6 | 2.9 ± 0.4 | 0.94 | NA | 72 ± 7 | NA | |
| Ratio (Moderate/Normal) | 2.52 | 2.56 | 1.12 | 3.55 | 2.52 | 0.71 | 0.40 | 0.39 | 0.98 | NA | 0.82 | NA | ||
| Leroy et al. [35] (15 mg/kg i.v. bolus) * | 22–31 y (5 Normal: 110–141) | D1 | 127 ± 15 | 159 ± 36 | 1.25 | 1.6 ± 0.1 | 1.5 ± 0.3 | 0.97 | 7.8 ± 0.8 | 7.5 ± 1.4 | 0.96 | 84 ± 4 | 88 ± 4 | 1.05 |
| 26–74 y (5 Moderate RI: 39–73) | D2 | 314 ± 38 | 376 ± 90 | 1.20 | 3.7 ± 0.8 | 3.5 ± 0.8 | 0.92 | 3.3 ± 0.5 | 3.2 ± 0.4 | 0.97 | 56 ± 7 | 72 ± 7 | 1.28 | |
| Ratio (Moderate/Normal) | 2.47 | 2.36 | 0.96 | 2.38 | 2.27 | 0.95 | 0.42 | 0.43 | 1.01 | 0.67 | 0.82 | 1.21 | ||
| 26–74 y (6 Severe RI: 14–27) | D3 | 773 ± 119 | 708 ± 205 | 0.92 | 9.3 ± 1.1 | 6.5 ± 1.7 | 0.71 | 1.3 ± 0.1 | 1.6 ± 0.3 | 1.25 | 45 ± 13 | 63 ± 8 | 1.42 | |
| Ratio (Severe/Normal) | 6.09 | 4.45 | 0.73 | 5.89 | 4.30 | 0.73 | 0.17 | 0.22 | 1.30 | 0.53 | 0.72 | 1.34 | ||
| 26–74 y (4 Anuric: 0) | D4 | 2313 ± 414 | 2166 ± 849 | 0.94 | 25 ± 4.1 | 19.8 ± 7.3 | 0.78 | 0.4 ± 0.0 | 0.6 ± 0.2 | 1.42 | 0.0 | 0.0 | NA | |
| Ratio (Anuric/Normal) | 18.2 | 13.6 | 0.75 | 16.1 | 13.0 | 0.81 | 0.05 | 0.08 | 1.48 | NA | 0.00 | NA | ||
| Norrby et al. [37] (1 g; 20-min i.v. infusion) | 57–77 y (6 Normal: 92–146) | E1 | 118 ± 38 | 218 ± 54 | 1.84 | 1.5 ± 0.4 | 2.4 ± 0.5 | 1.57 | 9.4 ± 3.3 | 4.9 ± 1.2 | 0.52 | NA | 87 ± 5 | NA |
| 69–84 y (5 Mild RI: 60–76) | E2 | 175 ± 36 | 264 ± 30 | 1.51 | 2.4 ± 0.4 | 2.7 ± 0.5 | 1.13 | 6.0 ± 1.4 | 3.9 ± 0.5 | 0.64 | NA | 87 ± 5 | NA | |
| Ratio (Mild/Normal) | 1.48 | 1.21 | 0.82 | 1.60 | 1.15 | 0.72 | 0.64 | 0.79 | 1.24 | NA | 1 | NA | ||
| 62–78 y (3 Moderate RI: 47–54) | E3 | 228 ± 24 | 368 ± 64 | 1.61 | 3.4 ± 0.3 | 3.9 ± 0.9 | 1.14 | 4.4 ± 0.5 | 2.8 ± 0.5 | 0.64 | NA | 79 ± 7 | NA | |
| Ratio (Moderate/Normal) | 1.93 | 1.69 | 0.88 | 2.27 | 1.66 | 0.73 | 0.47 | 0.57 | 1.23 | NA | 0.91 | NA | ||
| Welage et al. [40] (1 g i.v. bolus) | 30–36 y (2 Normal: 110–122) | F1 | 152 ± 37 | 150 ± 27 | 0.99 | 1.7 ± 0.2 | 1.7 ± 0.3 | 0.99 | 7.0 ± 1.7 | 6.9 ± 1.2 | 0.98 | 78 ± 23 | 87 ± 4 | 1.12 |
| 49–69 y (5 Moderate RI: 30–60) | F2 | 336 ± 39 | 317 ± 45 | 0.94 | 3.6 ± 0.5 | 3.4 ± 0.7 | 0.93 | 3.0 ± 0.3 | 3.2 ± 0.4 | 1.06 | 80 ± 15 | 72 ± 6 | 0.90 | |
| Ratio (Moderate/Normal) | 2.21 | 2.11 | 0.96 | 2.12 | 1.99 | 0.94 | 0.43 | 0.47 | 1.09 | 1.03 | 0.83 | 0.81 | ||
| 27–91 y (4 Severe RI: 21–29.5) | F3 | 582 ± 86 | 548 ± 89 | 0.94 | 6.3 ± 2.4 | 5.6 ± 1.5 | 0.89 | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.06 | 74 ± 11 | 50 ± 8 | 0.68 | |
| Ratio (Severe/Normal) | 3.83 | 3.65 | 0.95 | 3.71 | 3.31 | 0.89 | 0.25 | 0.27 | 1.09 | 0.95 | 0.57 | 0.61 | ||
| Van Dalen et al. [38] (1 g i.v. bolus) | 34–88 y (4 Normal: 93–134) | G1 | 136 ± 36 | 160 ± 26 | 1.18 | 2.5 ± 0.9 | 1.7 ± 0.4 | 0.70 | 7.8 ± 1.7 | 6.4 ± 1.1 | 0.83 | 80 ± 2 | 89 ± 4 | 1.11 |
| 34–88 y (3 Mild RI: 72–86) | G2 | 190 ± 6 | 268 ± 31 | 1.41 | 3.7 ± 1.1 | 2.9 ± 0.6 | 0.78 | 5.3 ± 0.2 | 3.8 ± 0.5 | 0.72 | 88 ± 5 | 86 ± 5 | 0.98 | |
| Ratio (Mild/Normal) | 1.40 | 1.68 | 1.20 | 1.49 | 1.67 | 1.12 | 0.67 | 0.59 | 0.87 | 1.10 | 0.97 | 0.88 | ||
| 34–88 y (4 Moderate RI: 30–59) | G3 | 393 ± 187 | 386 ± 66 | 0.98 | 6.9 ± 3.1 | 4.0 ± 0.9 | 0.58 | 3.0 ± 1.0 | 2.7 ± 0.4 | 0.89 | 69 ± 10 | 81 ± 6 | 1.17 | |
| Ratio (Moderate/Normal) | 2.89 | 2.41 | 0.83 | 2.76 | 2.32 | 0.84 | 0.38 | 0.41 | 1.08 | 0.86 | 0.91 | 1.06 | ||
| 34–88 y (2 Severe RI: 9–20) | G4 | 1140 ± 314 | 681 ± 131 | 0.60 | 15.1 ± 1.0 | 6.9 ± 1.9 | 0.46 | 0.9 ± 0.2 | 1.5 ± 0.3 | 1.63 | 41 ± 12 | 62 ± 9 | 1.51 | |
| Ratio (Severe/Normal) | 8.38 | 4.26 | 0.51 | 6.04 | 3.97 | 0.66 | 0.12 | 0.24 | 1.97 | 0.51 | 0.70 | 1.36 | ||
| Walstad et al. [39] (1 g i.v.) | 28–89 y (9 Mild RI: ≥50) | H1 | 232 ± 34 | 261 ± 32 | 1.13 | 2.8 ± 0.5 | 2.7 ± 0.6 | 0.95 | 4.4 ± 0.7 | 3.9 ± 0.5 | 0.89 | 94 ± 8 | 87 ± 4 | 0.93 |
| 28–89 y (10 Moderate RI: 31–50) | H2 | 359 ± 62 | 382 ± 68 | 1.06 | 5.0 ± 1.2 | 3.8 ± 0.9 | 0.75 | 2.9 ± 0.5 | 2.7 ± 0.5 | 0.95 | 80 ± 12 | 81 ± 6 | 1.01 | |
| 28–89 y (10 Severe RI: 16–30) | H3 | 279 ± 54 | 337 ± 65 | 1.21 | 8.6 ± 1.7 | 6.5 ± 1.8 | 0.75 | 1.9 ± 0.4 | 1.5 ± 0.3 | 0.83 | 58 ± 5 | 64 ± 9 | 1.10 | |
| Lin et al. [36] (2 g i.v. b.i.d. bolus) | 21–74 y (6 Mild RI: 51–94) | I1 | 410 ± 13 | 504 ± 54 | 1.23 | 3.3 ± 1.1 | 2.9 ± 0.6 | 0.86 | 5.7 | 4.0 ± 0.5 | 0.70 | NA | NA | |
| 58–75 y (8 Severe RI: 10–35) | I2 | 990 ± 265 | 1114 ± 213 | 1.13 | 7.6 ± 1.6 | 6.2 ± 1.7 | 0.82 | 2.0 | 1.9 ± 0.4 | 0.97 | NA | NA | ||
| Model prediction (1 g i.v. bolus) | 65–80 y (200 Normal) | 254 ± 63 | 2.9 ± 0.7 | 4.2 ± 1.0 | 86 ± 6 | |||||||||
| 65–80 y (200 Mild RI) | 268 ± 29 | 3.1 ± 0.5 | 3.8 ± 0.4 | 85 ± 5 | ||||||||||
| Ratio (Mild/Normal) | 1.06 | 1.08 | 0.91 | 0.99 | ||||||||||
| 65–80 y (200 Moderate RI) | 376 ± 64 | 4.2 ± 0.8 | 2.7 ± 0.4 | 79 ± 6 | ||||||||||
| Ratio (Moderate/Normal) | 1.48 | 1.46 | 0.66 | 0.92 | ||||||||||
| 65–80 y (200 Severe RI) | 665 ± 126 | 7.2 ± 1.7 | 1.6 ± 0.3 | 60 ± 8 | ||||||||||
| Ratio (Severe/Normal) | 2.62 | 2.50 | 0.38 | 0.70 | ||||||||||
| PK Parameter | Age (Years) | Impact of Age or/and Disease Stage (Fold Change from Predicted Mean PK Value in a Population Aged 20 Years with Normal Function) | Impact of Disease Stage (Fold Change from Predicted Mean PK Value in an Age-Matched Population with Normal Function) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Mild RI | Moderate RI | Severe RI | Normal | Mild RI | Moderate RI | Severe RI | ||
| Half-Life | 20 | 1.00 | 1.42 | 1.93 | 3.22 | 1.0 | 1.42 | 1.93 | 3.22 |
| 30 | 1.08 | 1.52 | 2.07 | 3.57 | 1.0 | 1.41 | 1.92 | 3.31 | |
| 40 | 1.18 | 1.59 | 2.16 | 3.75 | 1.0 | 1.36 | 1.84 | 3.19 | |
| 50 | 1.30 | 1.67 | 2.26 | 3.96 | 1.0 | 1.29 | 1.74 | 3.06 | |
| 60 | 1.44 | 1.72 | 2.32 | 3.96 | 1.0 | 1.19 | 1.61 | 2.75 | |
| 70 | 1.73 | 1.87 | 2.50 | 4.32 | 1.0 | 1.08 | 1.44 | 2.49 | |
| AUCINF | 20 | 1.00 | 1.47 | 2.03 | 3.50 | 1.0 | 1.47 | 2.03 | 3.50 |
| 30 | 1.07 | 1.52 | 2.12 | 3.71 | 1.0 | 1.42 | 1.98 | 3.47 | |
| 40 | 1.17 | 1.58 | 2.19 | 3.84 | 1.0 | 1.34 | 1.87 | 3.27 | |
| 50 | 1.31 | 1.64 | 2.31 | 4.04 | 1.0 | 1.26 | 1.77 | 3.10 | |
| 60 | 1.50 | 1.74 | 2.41 | 4.18 | 1.0 | 1.16 | 1.60 | 2.79 | |
| 70 | 1.83 | 1.90 | 2.62 | 4.67 | 1.0 | 1.04 | 1.43 | 2.55 | |
| Clearance | 20 | 1.00 | 0.67 | 0.48 | 0.29 | 1.0 | 0.67 | 0.48 | 0.29 |
| 30 | 0.93 | 0.64 | 0.46 | 0.27 | 1.0 | 0.69 | 0.50 | 0.29 | |
| 40 | 0.85 | 0.62 | 0.45 | 0.26 | 1.0 | 0.73 | 0.53 | 0.31 | |
| 50 | 0.76 | 0.60 | 0.43 | 0.25 | 1.0 | 0.78 | 0.56 | 0.33 | |
| 60 | 0.67 | 0.56 | 0.41 | 0.24 | 1.0 | 0.85 | 0.62 | 0.36 | |
| 70 | 0.56 | 0.52 | 0.38 | 0.22 | 1.0 | 0.93 | 0.68 | 0.39 | |
| Parameter | Value | Reference |
|---|---|---|
| Physicochemical properties and binding | ||
| Molecular Weight (g/mol) | 546.580 | Zhou et al., 2019 [23] |
| Log P | −3.750 | |
| Compound Type | Diprotic Acid | |
| pKa 1 | 2.430 | |
| pKa 2 | 2.890 | |
| BP | 0.550 | Default |
| Plasma fu (Binding Protein) | 0.9 (Human Serum Albumin) | Predicted and used as input |
| Distribution | ||
| Distribution Model | Full PBPK Model | |
| Vss (L/kg) | 0.20 | (Predicted using Method 2 after [43]) |
| Kp Scalar | 1.0 | |
| Elimination | ||
| Elimination option | Enzyme Kinetics | |
| CLR (L/h) | 6.0 | Zhou et al., 2019 [23] |
| Biliary CLint (µL/min/million hepatocyte) | 0.085 (30% CV) | Adjusted to recover Harding et al., 1983 [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduljalil, K.; Gardner, I.; Jamei, M. Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance. Antibiotics 2024, 13, 862. https://doi.org/10.3390/antibiotics13090862
Abduljalil K, Gardner I, Jamei M. Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance. Antibiotics. 2024; 13(9):862. https://doi.org/10.3390/antibiotics13090862
Chicago/Turabian StyleAbduljalil, Khaled, Iain Gardner, and Masoud Jamei. 2024. "Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance" Antibiotics 13, no. 9: 862. https://doi.org/10.3390/antibiotics13090862
APA StyleAbduljalil, K., Gardner, I., & Jamei, M. (2024). Application of Physiologically Based Pharmacokinetic Model to Delineate the Impact of Aging and Renal Impairment on Ceftazidime Clearance. Antibiotics, 13(9), 862. https://doi.org/10.3390/antibiotics13090862







