An Institutional Febrile Neutropenia Protocol Improved the Antibacterial Treatment and Encouraged the Development of a Computerized Clinical Decision Support System
Abstract
1. Introduction
2. Results
2.1. Patient Baseline Characteristics
2.2. Prophylaxis
2.3. Diagnostic Stewardship
2.4. Antibacterial Stewardship
2.5. Compliance with the CDSS
3. Discussion
Study Limitations
4. Material and Methods
4.1. Establishment of the Local Guideline
4.2. Definitions, Inclusion Criteria, and Exclusion Criteria
4.3. Guideline Recommendations
4.3.1. Antibacterial and Antifungal Prophylaxis
4.3.2. Diagnostic Stewardship
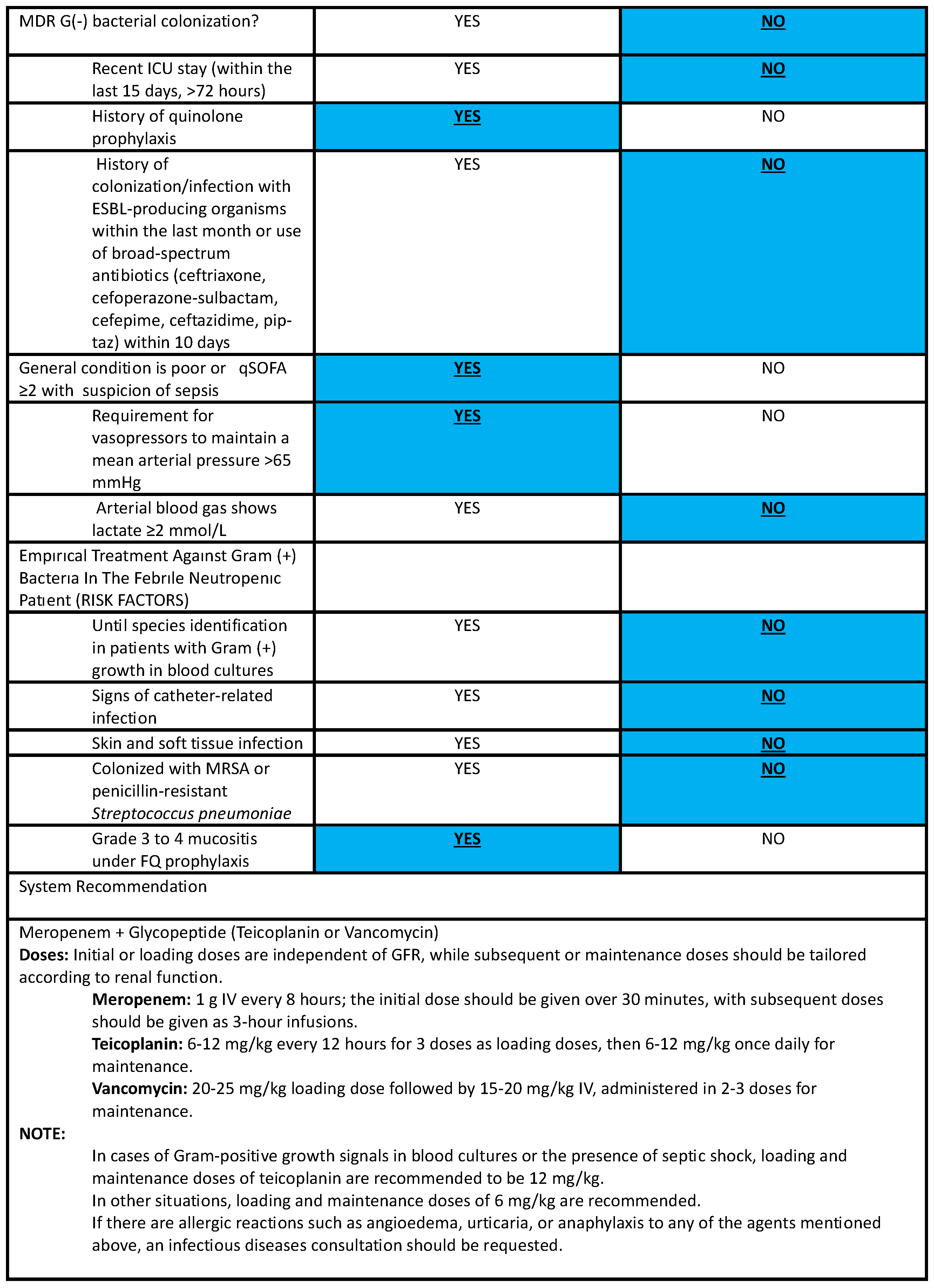
4.3.3. Antibacterial Stewardship
4.4. Development of a Computerized Clinical Decision Support System
4.5. Evaluation of the Applicability of the CDSS
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Power for Unmatched Case–Control Studies | |
|---|---|
| Input Data | |
| Two-sided confidence interval (%) | 95 |
| Number of cases | 91 |
| Percent of exposure among cases (%) | 58 |
| Number of controls | 136 |
| Percent of exposure among controls (%) | 88 |
| Odds Ratio | 0.19 |
| Power based on: | |
| Normal approximation | 99.93% |
| Normal approximation with continuity correction | 99.87% |
References
- Keck, J.M.; Wingler, M.J.B.; Cretella, D.A.; Vijayvargiya, P.; Wagner, J.L.; Barber, K.E.; Jhaveri, T.A.; Stover, K.R. Approach to fever in patients with neutropenia: A review of diagnosis and management. Ther. Adv. Infect. Dis. 2022, 9, 20499361221138346. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Swaminathan, S.; Angarone, M.; Blouin, G.; Camins, B.C.; Casper, C.; Cooper, B.; Dubberke, E.R.; Engemann, A.M.; Freifeld, A.G.; et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2016, 14, 882–913. [Google Scholar] [CrossRef] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Lingaratnam, S.; Slavin, M.A.; Koczwara, B.; Seymour, J.F.; Szer, J.; Underhill, C.; Prince, M.; Mileshkin, L.; O’Reilly, M.; Kirsa, S.W.; et al. Introduction to the Australian consensus guidelines for the management of neutropenic fever in adult cancer patients, 2010/2011. Australian Consensus Guidelines 2011 Steering Committee. Intern. Med. J. 2011, 41, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, G.; Bullinger, L.; Garcia-Vidal, C.; Herbrecht, R.; Maertens, J.; Menna, P.; Pagano, L.; Thiebaut-Bertrand, A.; Calandra, T. Infectious complications of targeted drugs and biotherapies in acute leukemia. Clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Leukemia 2022, 36, 1215–1226. [Google Scholar] [CrossRef]
- Contejean, A.; Abbara, S.; Chentouh, R.; Alviset, S.; Grignano, E.; Gastli, N.; Casetta, A.; Willems, L.; Canouï, E.; Charlier, C.; et al. Antimicrobial stewardship in high-risk febrile neutropenia patients. Antimicrob. Resist. Infect. Control 2022, 11, 52. [Google Scholar] [CrossRef]
- Trinh, T.D.; Strnad, L.; Damon, L.; Dzundza, J.H.; Graff, L.R.; Griffith, L.M.; Hilts-Horeczko, A.; Olin, R.; Shenoy, S.; DeVoe, C.; et al. Reductions in vancomycin and meropenem following the implementation of a febrile neutropenia management algorithm in hospitalized adults: An interrupted time series analysis. Infect. Control Hosp. Epidemiol. 2021, 42, 1090–1097. [Google Scholar] [CrossRef]
- Metan, G.; Kaynar, L.; Yozgat, N.; Elmali, F.; Kürkçüoglu, C.A.; Alp, E.; Çetin, M. A change for the antibacterial treatment policy to decrease carbapenem consumption at a haematopoietic stem cell transplantation centre. Infez. Med. 2017, 25, 33–37. [Google Scholar]
- Ranuhardy, D. The Role of Febrile Neutropenia Guideline’s Implementation on Mortality Rate in Dharmais Hospital-National Cancer Center. Indones. J. Cancer 2019, 12, 71–75. [Google Scholar] [CrossRef]
- Verlinden, A.; Mikulska, M.; Knelange, N.S.; Averbuch, D.; Styczynski, J. Current antimicrobial practice in febrile neutropenia across Europe and Asia: The EBMT Infectious Disease Working Party survey. Bone Marrow Transplant. 2020, 55, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Akpan, M.R.; Ahmad, R.; Shebl, N.A.; Ashiru-Oredope, D. A Review of Quality Measures for Assessing the Impact of Antimicrobial Stewardship Programs in Hospitals. Antibiotics 2016, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Puerta-Alcalde, P.; Moreno-García, E.; Soriano, A. Artificial intelligence to support clinical decision-making processes. eBioMedicine 2019, 46, 27–29. [Google Scholar] [CrossRef]
- Gillum, R.F. From papyrus to the electronic tablet: A brief history of the clinical medical record with lessons for the digital age. Am. J. Med. 2013, 126, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Georgiou, P.; Lescure, F.X.; Birgand, G.; Holmes, A.H. Machine learning for clinical decision support in infectious diseases: A narrative review of current applications. Clin. Microbiol. Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Rittmann, B.; Stevens, M.P. Clinical Decision Support Systems and Their Role in Antibiotic Stewardship: A Systematic Review. Curr. Infect. Dis. Rep. 2019, 21, 29. [Google Scholar] [CrossRef]
- Carracedo-Martinez, E.; Gonzalez-Gonzalez, C.; Teixeira-Rodrigues, A.; Prego-Dominguez, J.; Takkouche, B.; Herdeiro, M.T.; Figueiras, A. Computerized Clinical Decision Support Systems and Antibiotic Prescribing: A Systematic Review and Meta-analysis. Clin. Ther. 2019, 41, 552–581. [Google Scholar] [CrossRef]
- Graham, T.A.; Kushniruk, A.W.; Bullard, M.J.; Holroyd, B.R.; Meurer, D.P.; Rowe, B.H. How usability of a web-based clinical decision support system has the potential to contribute to adverse medical events. AMIA Annu. Symp. Proc. 2008, 2008, 257–261. [Google Scholar]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Metan, G.; Uzun, Ö.; Dizman, G.T.; Sönmezer, M.Ç.; İnkaya, A.Ç.; Hazırolan, G.; Akova, M.; Ünal, S. Is there still a room for improvement in antimicrobial use in a setting where use of broad-spectrum antibiotics require approval of an infectious diseases physician? Infect. Control Hosp. Epidemiol. 2022, 43, 802–804. [Google Scholar] [CrossRef]
- Ishikawa, K.; Masaki, T.; Kawai, F.; Ota, E.; Mori, N. Systematic Review of the Short-Term versus Long-Term Duration of Antibiotic Management for Neutropenic Fever in Patients with Cancer. Cancers 2023, 15, 1611. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.A.; Stocking, K.; Bowden, S.; Poynton, M.H.; White, P.L. Prevention and diagnosis of invasive fungal disease in high-risk patients within an integrative care pathway. J. Infect. 2013, 67, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Rojas, L.; Cervera, C.; Garrido, G.; Fariñas, M.C.; Valerio, M.; Giannella, M.; Bouza, E. Poor compliance with antifungal drug use guidelines by transplant physicians: A framework for educational guidelines and an international consensus on patient safety. Clin. Transplant. 2012, 26, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.T.; Wong, J.; Young, B.; Tay, H.L.; Tan, S.H.; Yap, M.Y.; Teng, C.B.; Ang, B.; Lee, T.H.; Tan, H.L.; et al. Effective Antimicrobial StewaRdship StrategIES (ARIES): Cluster Randomized Trial of Computerized Decision Support System and Prospective Review and Feedback. Open Forum Infect. Dis. 2020, 7, ofaa254. [Google Scholar] [CrossRef] [PubMed]
- Catho, G.; Centemero, N.S.; Catho, H.; Ranzani, A.; Balmelli, C.; Landelle, C.; Zanichelli, V.; Huttner, B.D. Factors determining the adherence to antimicrobial guidelines and the adoption of computerised decision support systems by physicians: A qualitative study in three European hospitals. Int. J. Med. Inform. 2020, 141, 104233. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Erdem, H.; Kocoglu, E.; Ankarali, H.; El-Sokkary, R.; Hakamifard, A.; Karaali, R.; Kulzhanova, S.; El-Kholy, A.; Tehrani, H.A.; Khedr, R.; et al. Prospective analysis of febrile neutropenia patients with bacteraemia: The results of an international ID-IRI study. Int. J. Antimicrob. Agents 2023, 62, 106919. [Google Scholar] [CrossRef]

| First Period (n = 91) (%) | Second Period (n = 136) (%) | All Patients (n = 227) (%) | p | |
|---|---|---|---|---|
| Age (median, minimum–maximum) (years) | 50 (18–82) | 55 (18–82) | 53 (18–82) | 0.16 |
| Sex. n (%) | 0.71 | |||
| Female | 35 (38.5) | 49 (36.0) | 84 (37.0) | |
| Male | 56 (61.5) | 87 (64.0) | 143 (63.0) | |
| Hematological malignancy | 69 (75.8) | 106 (77.9) | 175 (7.1) | 0.35 |
| Acute myeloid leukemia | 14 (20.3) | 36 (34.0) | 50 (28.6) | |
| Acute lymphocytic leukemia | 11 (15.9) | 17 (16.0) | 28 (16) | |
| Multiple myeloma | 8 (11.6) | 19 (17.9) | 27 (15.4) | |
| Non-Hodgkin lymphoma | 30 (43.5) | 27 (25.5) | 57 (32.6) | |
| Hodgkin’s lymphoma | 3 (4.3) | 1 (0.9) | 4 (2.3) | |
| Myelodysplastic syndrome | 2 (2.9) | 3 (2.8) | 5 (2.9) | |
| Chronic lymphocytic leukemia | 0 (0.0) | 3 (2.8) | 3 (1.7) | |
| Biphenotypic leukemia | 1 (1.4) | 0 (0.0) | 1 (0.6) | |
| Solid tumors * | 22 (24.2) | 27 (19.9) | 49 (21.6) | |
| None-malignant conditions ** | 0 (0.0) | 3 (2.2) | 3 (1.3) | |
| HSCT | 22 (100.0) | 44 (100.0) | 66 (100.0) | 0.21 |
| Allogeneic HSCT | 11 (50.0) | 15 (34.1) | 26 (39.4) | |
| Autologous HSCT | 11 (100.0) | 15 (100.0) | 40 (60.6) | |
| Duration of neutropenia (median, minimum-maximum) days | 6 (1–70) | 7 (0–112) | 7 (0–112) | 0.01 |
| Patients who did not recover from neutropenia | 5 (5.5) | 16 (11.8) | 21 (9.3) | 0.54 |
| Compliance Rate | |||
|---|---|---|---|
| First Period (P1) | Second Period (P2) | p | |
| Antibacterial prophylaxis * | |||
| Full compliance n (%) | 11/33 (33.3) | 25/61 (41.0) | 0.53 |
| Type of drug and dosage n, (%) | 27/27 (100) | 46/46 (100) | |
| Time of initiation and cessation n, (%) | 11/33 (33.3) | 25/61 (41.0) | 0.53 |
| Antifungal prophylaxis ** | |||
| Full compliance n, (%) | 7/33 (21.2) | 8/74 (10.8) | 0.19 |
| Type of antifungal drug n, (%) | 29/33 (87.9) | 65/71 (91.5) | 0.72 |
| Compliance with the dosage n, (%) | 25/28 (89.3) | 64/65 (98.5) | 0.08 |
| Time of initiation and cessation n, (%) | 3/24 (23.1) | 7/59 (17.5) | 0.54 |
| First Period (P1) (n = 91)% | Second Period (P2) (n = 136)% | All Patients (n = 227)% | p | |
|---|---|---|---|---|
| Duration of neutropenia (median, minimum–maximum) days | 6 (1–70) | 7 (0–112) | 7 (0–112) | 0.01 |
| Patients who did not recover from neutropenia | 5 (5.5) | 16 (11.8) | 21 (9.3) | 0.54 |
| Risk factors for antibacterial resistance | ||||
| Levofloxacin prophylaxis | 39 (42.9) | 71 (52.2) | 110 (48.5) | 0.18 |
| Broad spectrum antibiotic consumption in the last month | 63 (69.2) | 98 (72.1) | 161(70.9) | 0.64 |
| Septic shock | 5 (5.5) | 10 (7.4) | 15 (6.6) | 0.58 |
| Previous colonization with a multidrug resistant bacterium | 3 (3.3) | 1 (0.7) | 4 (1.8) | 0.3 |
| Presence with hospital acquired pneumonia | 5 (5.5) | 4 (2.9) | 9 (4) | 0.49 |
| Receipt of care in the intensive care unit >72 h in the last 6 months | 4 (4.4) | 2 (1.5) | 6 (2.6) | 0.22 |
| Indications for empirical glycopeptide treatment | ||||
| Grade 3 or 4 mucositis | 4 (4.4) | 8 (5.9) | 12 (5.3) | 0.76 |
| Cellulitis | 2 (2.2) | 9 (6.6) | 11 (4.8) | 0.20 |
| Previous Methicillin-resistant Staphylococcus aureus colonization | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Pain around central venous line | 0 (0.0) | 1 (0.7) | 1 (0.4) | 1.0 |
| Erythema around central venous line | 0 (0.0) | 5 (3.7) | 5 (2.2) | 0.09 |
| Perianal pain | 3 (3.3) | 6 (4.4) | 9 (4) | 0.75 |
| Number of patients with bacteremia | 24 (25) | 42 (30.9) | 66 (29.1) | 0.46 |
| Bacteremia with a multidrug resistant Gram-negative bacilli | 10 (10.9) | 12 (8.8) | 22 (9.7) | 0.59 |
| 3rd-generation cephalosporin-resistant Enterobacterales | 8 | 11 | ||
| 3rd-generation cephalosporin-resistant non-fermentating gram-negative bacilli | 1 | 0 | ||
| Carbapenem-resistant Gram-negative bacilli | 1 | 1 | ||
| Bacteremia with a multidrug-resistant gram-positive bacteria | 5 (5.5) | 3 (2.2) | 8 (3.5) | 0.21 |
| Methicillin-resistant Staphylococci | 4 | 2 | 6 | |
| Ampicillin-resistant Enterococcus faecium | 0 | 0 | 1 | |
| Penicillin-resistant Streptococcus viridans | 1 | 1 | 1 |
| First Period (n = 91)(%) | Second Period (n = 136)(%) | All Patients (n = 227)(%) | p Value | |
|---|---|---|---|---|
| Empirical antibacterial treatment compliance with the local guideline | 72 (79.1) | 118 (86.8) | 190 (83.7) | 0.13 |
| Appropriate empirical antibacterial treatment in patients with positive blood cultures (n = 66) | 14/24 (58.3%) | 37/42 (88.1%) | 51/66 (77.3%) | 0.006 |
| Escalation of empirical antibacterial treatment | 45 (49.5) | 48 (35.3) | 93 (41.0) | 0.03 |
| Reasons for escalation of empirical antibacterial treatment | 0.35 | |||
| Persistent fever | 22 (48.9) | 27 (56.3) | 49 (52.7) | |
| Clinical deterioration | 11 (12.1) | 14 (29.2) | 25 (26.9) | |
| Bacteremia by a resistant bacterium | 12 (26.7) | 7 (14.6) | 19 (20.4) | |
| Duration between the day of empirical antibacterial treatment and escalation (Median, minimum-maximum) days | 3 (1–9) | 4 (1–13) | 4 (1–13) | 0.03 |
| De-escalation of empirical antibacterial treatment | 12 (13.2) | 22 (16.2) | 34 (15) | 0.54 |
| Defervence with first line antibacterial treatment | 32 (35.2) | 79 (58.1) | 111 (48.9) | 0.001 |
| Duration of antibacterial treatment ≤ 7 days | 20 (21.9) | 47 (34.5) | 67 (29.5) | 0.02 |
| Number of patients who received empirical antifungal treatment due to persistent fever | 8 (8.8) | 19 (14) | 27 (11.8) | 0.24 |
| Number of patients who received antifungal treatment with a diagnosis of invasive fungal disease | 6 (6.6) | 12 (8.8) | 18 (7.99) | 0.54 |
| Invasive aspergillosis * | 4 | 9 | 13 | |
| Fungemia | 0 | 2 | 2 | |
| Candida esophagitis | 1 | 0 | 1 | |
| Fungal sinusitis | 1 | 2 | 3 | |
| 30-day mortality | 16 (17.6) | 22 (16.2) | 38 (16.7) | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taş, Z.; Metan, G.; Telli Dizman, G.; Yavuz, E.; Dizdar, Ö.; Büyükaşık, Y.; Uzun, Ö.; Akova, M. An Institutional Febrile Neutropenia Protocol Improved the Antibacterial Treatment and Encouraged the Development of a Computerized Clinical Decision Support System. Antibiotics 2024, 13, 832. https://doi.org/10.3390/antibiotics13090832
Taş Z, Metan G, Telli Dizman G, Yavuz E, Dizdar Ö, Büyükaşık Y, Uzun Ö, Akova M. An Institutional Febrile Neutropenia Protocol Improved the Antibacterial Treatment and Encouraged the Development of a Computerized Clinical Decision Support System. Antibiotics. 2024; 13(9):832. https://doi.org/10.3390/antibiotics13090832
Chicago/Turabian StyleTaş, Zahit, Gökhan Metan, Gülçin Telli Dizman, Eren Yavuz, Ömer Dizdar, Yahya Büyükaşık, Ömrüm Uzun, and Murat Akova. 2024. "An Institutional Febrile Neutropenia Protocol Improved the Antibacterial Treatment and Encouraged the Development of a Computerized Clinical Decision Support System" Antibiotics 13, no. 9: 832. https://doi.org/10.3390/antibiotics13090832
APA StyleTaş, Z., Metan, G., Telli Dizman, G., Yavuz, E., Dizdar, Ö., Büyükaşık, Y., Uzun, Ö., & Akova, M. (2024). An Institutional Febrile Neutropenia Protocol Improved the Antibacterial Treatment and Encouraged the Development of a Computerized Clinical Decision Support System. Antibiotics, 13(9), 832. https://doi.org/10.3390/antibiotics13090832







