Real-World Clinical Characteristics and Outcomes with Daptomycin Use in Pediatric Patients: A Retrospective Case Series
Abstract
1. Introduction
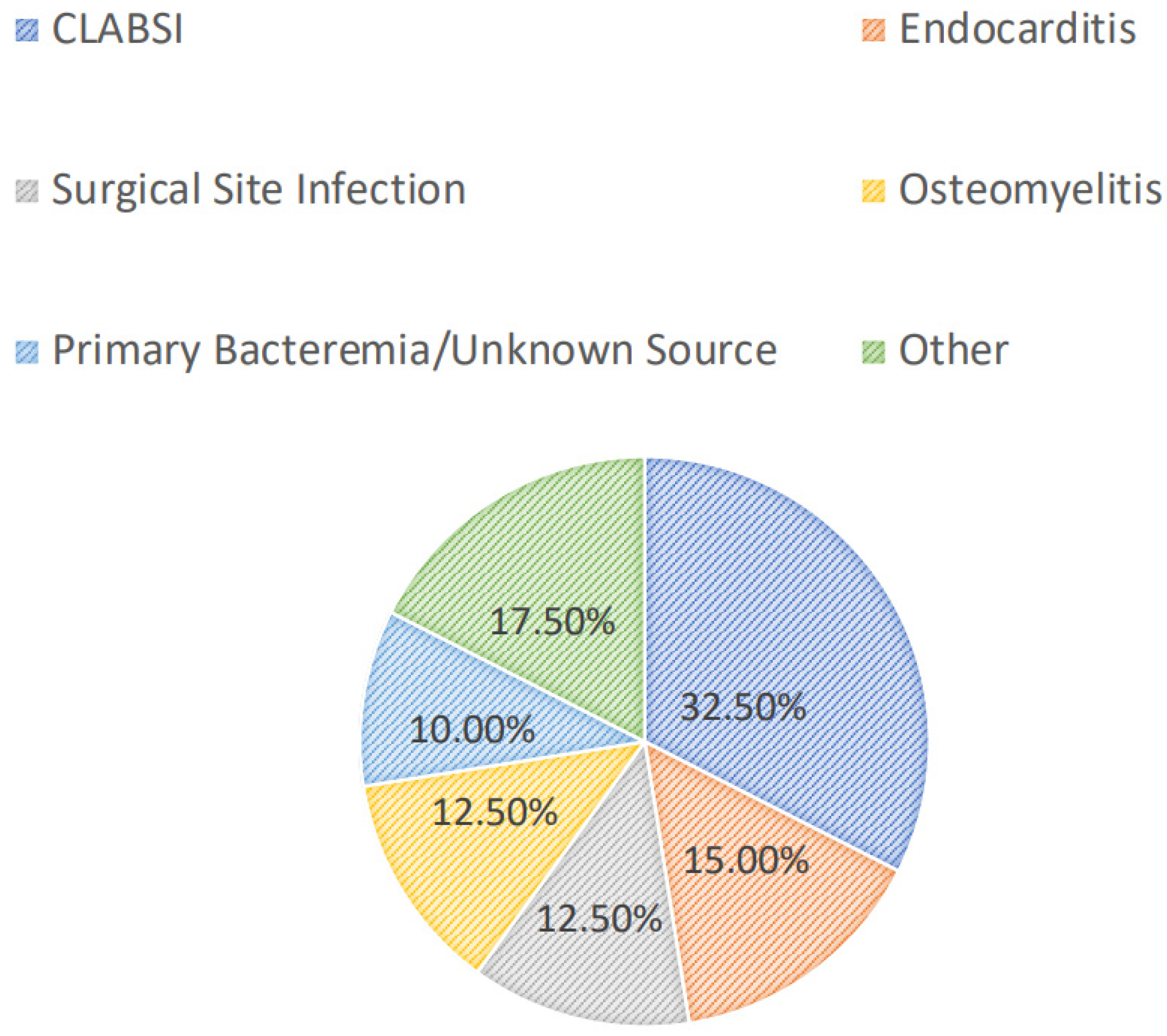
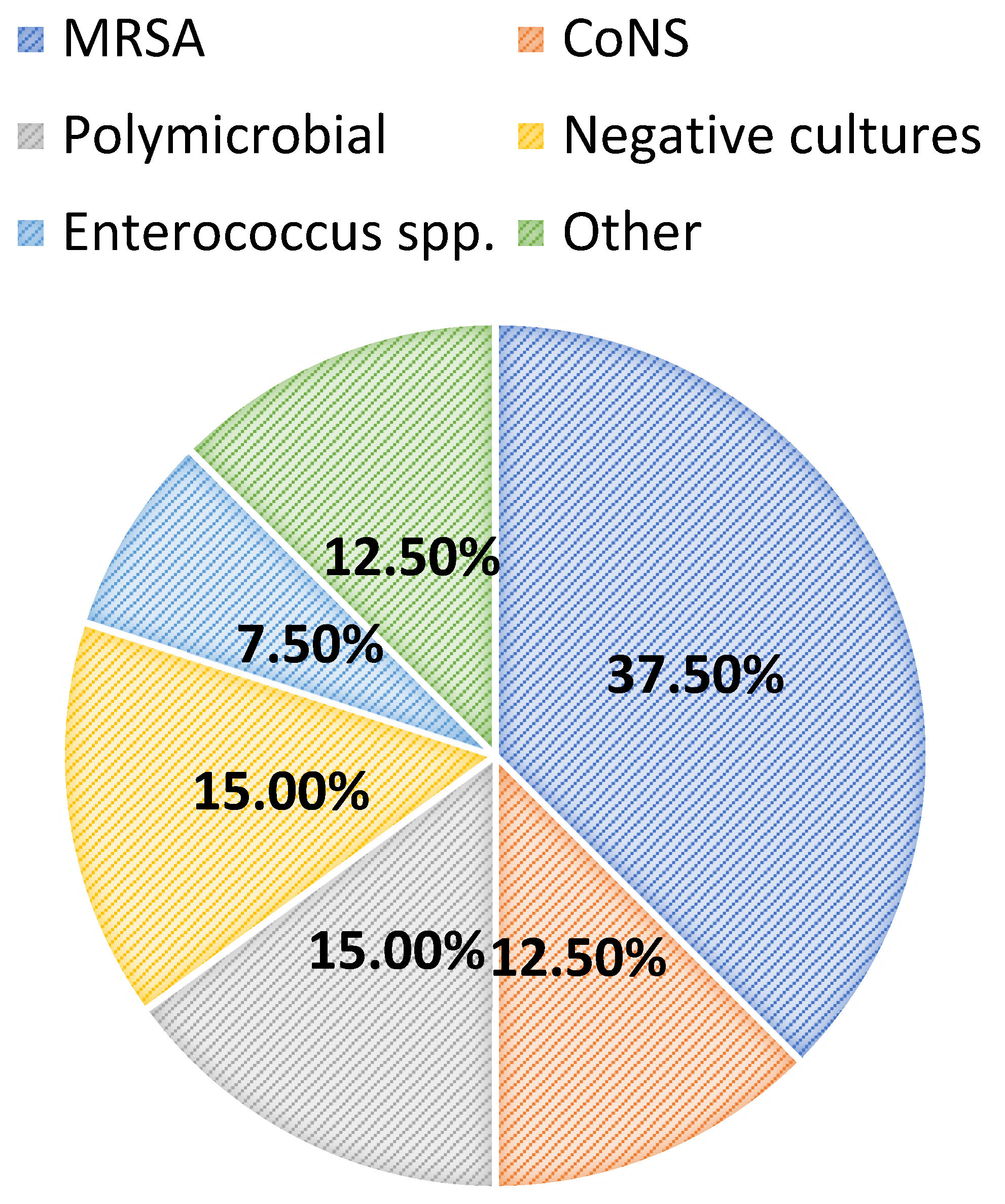
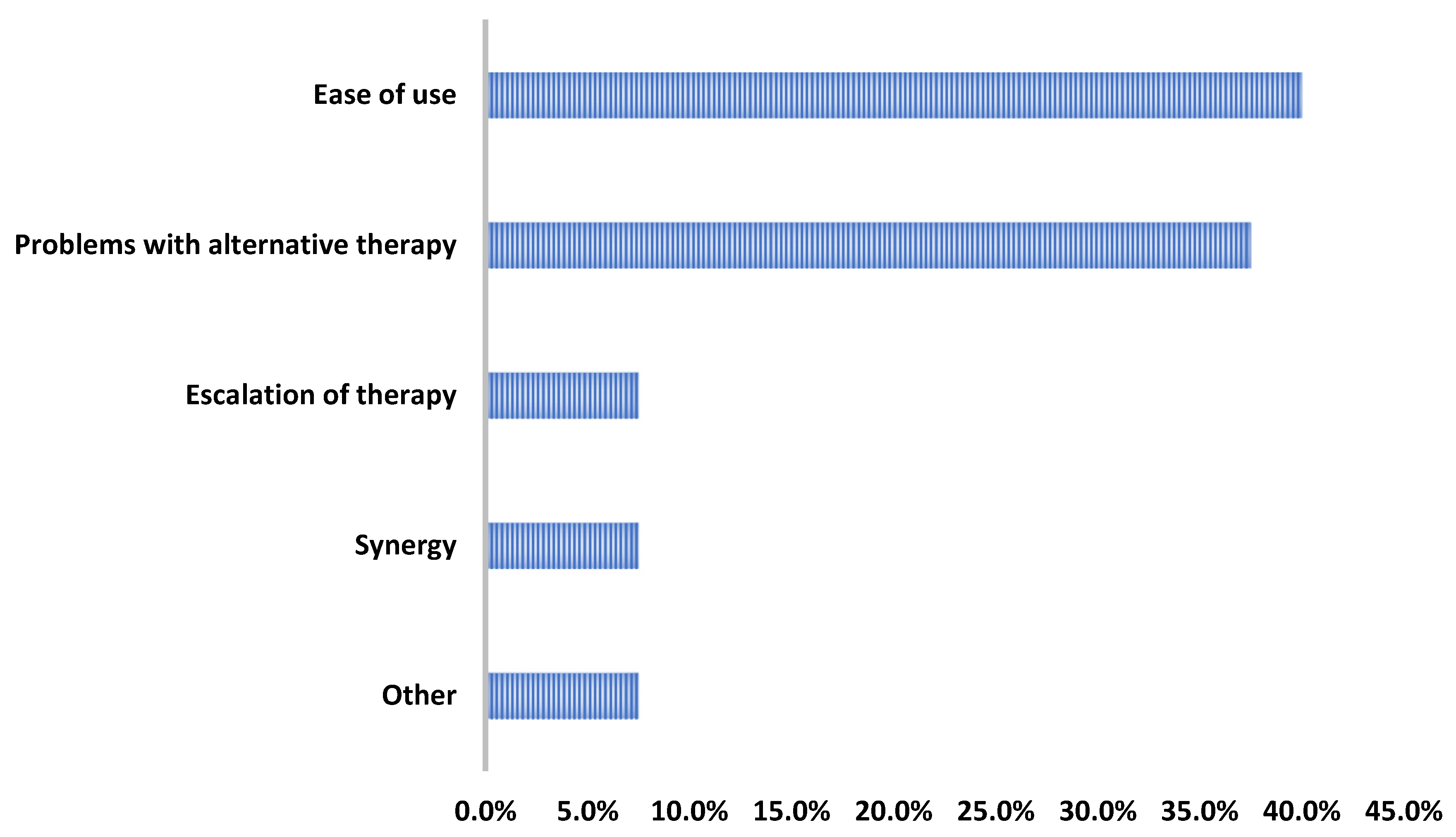
2. Results
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, D.J.; Eberly, M.D.; Goudie, A.; Nylund, C.M. Rising vancomycin-resistant Enterococcus infections in hospitalized children in the United States. Hosp. Pediatr. 2016, 6, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Sutter, D.E.; Milburn, E.; Chukwuma, U.; Dzialowy, N.; Maranich, A.M.; Hospenthal, D.R. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics 2016, 137, e20153099. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, E.C.; Xiao, S.; Tamma, P.D.; Sick-Samuels, A.; Schumacher, C.; Gadala, A.; Carroll, K.C.; Milestone, A.M. Trends in pediatric community-onset Staphylococcus aureus antibiotics susceptibilities over a five-year period in a multihospital health system. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e12. [Google Scholar] [CrossRef]
- Chiusaroli, L.; Liberati, C.; Rulli, L.; Barbieri, E.; De Pieri, M.; Chiara, C.D.; Mengato, D.; Giaquinto, C.; Donà, D. Therapeutic options and outcomes for the treatment of children with gram-positive bacteria with resistances of concern: A systematic review. Antibiotics 2023, 12, 261. [Google Scholar] [CrossRef]
- World Health Organization. WHO Releases Priorities for Research and Development of Age-Appropriate Antibiotics. 24 March 2023. Available online: https://www.who.int/news/item/24-03-2023-who-releases-priorities-for-research-and-development-of-age-appropriate-antibiotics (accessed on 19 June 2024).
- World Health Organization. Shaping the Global Innovation and Access Landscape for Better Paediatric Medicines: Global Accelerator for Paediatric Formulations 2022–2024 Strategy; World Health Organization: Geneva, Switzerland, 2022; License: CC BY-NC-SA 3.0 IGO; Available online: https://www.who.int/publications/i/item/9789240044647 (accessed on 8 January 2024).
- Daptomycin [Package Insert]; Xellia Pharmaceuticals LLC: Buffalo Grove, IL, USA, 2023.
- Rybak, M.J.; Hershberger, E.; Moldovan, T.; Grucz, R.G. In vitro activities of daptomycin, vancomycin, linezolid, and quinopristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 2000, 44, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Rybak, M.J.; Kaatz, G.W. Evaluation of daptomycin treatment of Staphylococcus aureus bacterial endocarditis: An in vitro and in vivo simulation using historical and current dosing strategies. J. Antimicrob. Chemother. 2007, 60, 334–340. [Google Scholar] [CrossRef][Green Version]
- Abdel-Rahman, S.M.; Benziger, D.P.; Jacobs, R.F.; Jafri, H.S.; Hong, E.F.; Kearns, G.L. Single-dose pharmacokinetics of daptomycin in children with suspected or proven gram-positive infections. Pediatr. Infect. Dis. J. 2008, 27, 330–334. [Google Scholar] [CrossRef]
- Shoemaker, D.M.; Simou, J.; Roland, W.E. A review of daptomycin for injection (Cubicin) in the treatment of complicated skin and skin structure infections. Ther. Clin. Risk Manag. 2006, 2, 169–174. [Google Scholar] [CrossRef]
- Syriopoulou, V.; Dailiana, Z.; Dmitriy, N.; Utili, R.; Pathan, R.; Hamed, K. Clinical experience with daptomycin for the treatment of gram-positive infections in children and adolescents. Pediatr. Infect. Dis. J. 2016, 35, 511–516. [Google Scholar] [CrossRef]
- Papachatzi, E.; Gkentzi, D.; Tzifas, S.; Dassios, T.; Dimitrio, G. Daptomycin use for persistent coagulase-negative staphylococcal bacteremia in a neonatal intensive care unit. Antibiotics 2024, 13, 254. [Google Scholar] [CrossRef]
- Karageorgos, S.A.; Miligkos, M.; Dakoutrou, M.; Tsioutis, C. Clinical effectiveness, safety profile, and pharmacokinetics of daptomycin in pediatric patients: A systematic review. J. Pediatric Infect. Dis. Soc. 2016, 5, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Vonasek, B.J.; Samuel, A.M.; Henderson, S.L.; Strayer, J.R.; Bogenschutz, M.C. Safety and treatment outcomes of infants and children treated with daptomycin: Six-year experience from a pediatric academic medical center. Clin. Pediatr. 2024. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.S.; Maples, H.; Parker, S. Time for a change: Considering vancomycin alternatives for pediatric methicillin-resistant Staphylococcus aureus bacteremia. J. Pediatric Infect. Dis. Soc. 2023, 12, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; McConeghy, K.W. Methicillin-resistant Staphylococcus aureus therapy: Past, present, and future. Clin. Infect. Dis. 2014, 58 (Suppl. S1), S20–S27. [Google Scholar] [CrossRef]
- Kollef, M.H. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin. Infect. Dis. 2007, 45 (Suppl. S3), S191–S195. [Google Scholar] [CrossRef]
- Clemett, D.; Markham, A. Linezolid. Drugs 2000, 59, 815–827. [Google Scholar] [CrossRef]
- Woytowish, M.R.; Maynor, L.M. Clinical relevance of linezolid-associated serotonin toxicity. Ann. Pharmacother. 2013, 47, 388–397. [Google Scholar] [CrossRef]
- Linezolid [Package Insert]; Pfizer: New York, NY, USA, 2013.
- Bradley, J.; Glasser, C.; Patino, H.; Arnold, S.R.; Arrieta, A.; Congeni, B.; Daum, R.S.; Kojaoghlanian, T.; Yoon, M.; Anastasiou, D.; et al. Daptomycin for complicated skin infections: A randomized trial. Pediatrics 2017, 139, e20162477. [Google Scholar] [CrossRef]
- Arrieta, A.C.; Bradley, J.S.; Popejoy, M.W.; Bensaci, M.; Grandhi, A.; Bokesch, P.; Glasser, C.; Du, L.; Patino, H.; Kartsonis, N.A. Randomized multicenter study comparing safety and efficacy of daptomycin versus standard-of-care in pediatric patients with staphylococcal bacteremia. Pediatr. Infect. Dis. J. 2018, 37, 893–900. [Google Scholar] [CrossRef]
- Ardura, M.I.; Mejías, A.; Katz, K.S.; Revell, P.; McCracken, G.H., Jr.; Sánchez, P.J. Daptomycin therapy for invasive gram-positive bacterial infections in children. Pediatr. Infect. Dis. J. 2007, 26, 1128–1132. [Google Scholar] [CrossRef]
- Larru, B.; Cowdden, C.L.; Zaoutis, T.E.; Gerber, J.S. Daptomycin use in United States children’s hospitals. J. Pediatr. Infect. Dis. Soc. 2015, 4, 60–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Namtu, K.C.; Crain, J.C.; Messina, A.F.; Dumois, J.A.; Berman, D.M. Clinical experience with daptomycin in pediatrics. Pharmacotherapy 2017, 37, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; Basuino, L.; Diep, B.A.; Steenbergen, J.; Zhang, S.; Tattevin, P.; Alder, J. Relationship between susceptibility to daptomycin in vitro and in vivo activity in a rabbit model of aortic valve endocarditis. Antimicrob. Agents Chemother. 2009, 53, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Leonard, S.N.; Rybak, M.J. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilies to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 2008, 52, 3061–3067. [Google Scholar] [CrossRef]
- Hall, A.D.; Steed, M.E.; Arias, C.A.; Murray, B.E.; Rybak, M.J. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 2012, 56, 3174–3180. [Google Scholar] [CrossRef]
- Timbrook, T.T.; Gaffrey, A.R.; Luther, M.K.; Lopes, V.; LaPlante, K.L. Association of higher daptomycin dose (7 mg/kg or greater) with improved survival in patients with methicillin-resistant Staphylococcus aureus bacteremia. Pharmacotherapy 2018, 38, 189–196. [Google Scholar] [CrossRef]
- Britt, N.S.; Potter, E.M.; Patel, N.; Steed, M.E. Comparative effectiveness and safety of standard-, medium-, and high-dose daptomycin strategies for the treatment of vancomycin-resistant enterococcal bacteremia among veterans affairs patients. Clin. Infect. Dis. 2017, 64, 605–613. [Google Scholar] [CrossRef]
- Bradley, J.S.; Benziger, D.; Bokesch, P.; Jacobs, R. Single-dose pharmacokinetics of daptomycin in pediatric patients 3–24 months of age. Pediatr. Infect. Dis. J. 2014, 33, 936–939. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.M.; Chandorkar, G.; Akins, R.L.; Bradley, J.S.; Jacobs, R.F.; Donovan, J.; Benziger, D.P. Single-dose pharmacokinetics and tolerability of daptomycin 8 to 10 mg/kg in children aged 2 to 6 years with suspected or proved gram-positive infections. Pediatr. Infect. Dis. J. 2011, 30, 712–714. [Google Scholar] [CrossRef]
- Olney, K.B.; Howard, J.I.; Burgess, D.S. Daptomycin use optimization in pediatric Staphylococcus aureus bacteremia: A pharmacokinetic/pharmacodynamic investigation. J. Clin. Pharmacol. 2024, 64, 860–865. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Sader, H.S.; Fritsche, T.R.; Jones, R.N. Antimicrobial activity of daptomycin and selected comparators tested against bloodstream Staphylococcus aureus isolates from hemodialysis patients. Int. J. Infect. Dis. 2009, 13, 291–295. [Google Scholar] [CrossRef]
| N = 40 | |
|---|---|
| Male | 25 (62.5%) |
| Age: years, median (IQR) | 9 (4–16) |
| White | 21 (52.5%) |
| Black | 16 (40.0%) |
| ICU admission | 27 (67.5%) |
| PICU | 16 (40.0%) |
| PCICU | 11 (27.5%) |
| MRSA risk factors | -- |
| -Hospitalization within 12 months | 32 (80.0%) |
| -Antibiotic exposure within 12 months | 26 (65.0%) |
| -Invasive procedures within 12 months | 18 (45.0%) |
| -Long-term central venous access within 12 months | 14 (35.0%) |
| -Prior ABSSSI within 12 months | 1 (2.5%) |
| Source control | 21 (52.5%) |
| -Incision and drainage | 7 (33.0%) |
| -Debridement | 3 (14.3%) |
| -Central line or port removal | 3 (14.3%) |
| Duration of hospitalization: days, median (IQR) | 11 (7–32) |
| Baseline CPK: units/L, median (IQR) | 60 (27–102) * |
| Initial temperature: degrees Celsius, median (IQR) | 37.2 (36.8–38.3) |
| Initial heart rate: beats per minute, median (IQR) | 115 (103–141) |
| Initial respiratory rate: breaths per minute, median (IQR) | 23 (20–31) |
| Initial white blood cell count: median (IQR) | 11.9 (6.4–20.9) ** |
| Initial CRP: mg/dL, median (IQR) | 7.6 (3.2–13.3) *** |
| Initial procalcitonin: ng/mL, median (IQR) | 0.6 (0.2–8.6) **** |
| Initial serum creatinine: mg/dL, median (IQR) | 0.6 (0.3–0.7) ***** |
| Results (n = 40) | |
|---|---|
| Pediatric ID consult | 37 (92.5%) |
| DAP recommended by pediatric ID | 36 (90.0%) |
| Antibiotics prior to DAP initiation | 38 (95.0%) |
| Total duration of antibiotics prior to DAP initiation: days, median (IQR) | 4 (2–7) |
| DAP dosing: mg/kg, median (IQR) | 8 (6–10) |
| Length of DAP duration: days, median (IQR) | 12 (5–19) |
| Concomitant antibiotics | 25 (62.5%) |
| DAP at discharge | 21 (52.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persha, H.; Thacker, S.A.; Hornback, K.M.; Alvira-Arill, G.R.; Lueking, R.; Morrisette, T. Real-World Clinical Characteristics and Outcomes with Daptomycin Use in Pediatric Patients: A Retrospective Case Series. Antibiotics 2024, 13, 833. https://doi.org/10.3390/antibiotics13090833
Persha H, Thacker SA, Hornback KM, Alvira-Arill GR, Lueking R, Morrisette T. Real-World Clinical Characteristics and Outcomes with Daptomycin Use in Pediatric Patients: A Retrospective Case Series. Antibiotics. 2024; 13(9):833. https://doi.org/10.3390/antibiotics13090833
Chicago/Turabian StylePersha, Hanna, Stephen A. Thacker, Krutika Mediwala Hornback, Gustavo R. Alvira-Arill, Richard Lueking, and Taylor Morrisette. 2024. "Real-World Clinical Characteristics and Outcomes with Daptomycin Use in Pediatric Patients: A Retrospective Case Series" Antibiotics 13, no. 9: 833. https://doi.org/10.3390/antibiotics13090833
APA StylePersha, H., Thacker, S. A., Hornback, K. M., Alvira-Arill, G. R., Lueking, R., & Morrisette, T. (2024). Real-World Clinical Characteristics and Outcomes with Daptomycin Use in Pediatric Patients: A Retrospective Case Series. Antibiotics, 13(9), 833. https://doi.org/10.3390/antibiotics13090833






