Characteristics of Metallic Nanoparticles (Especially Silver Nanoparticles) as Anti-Biofilm Agents
Abstract
1. Introduction
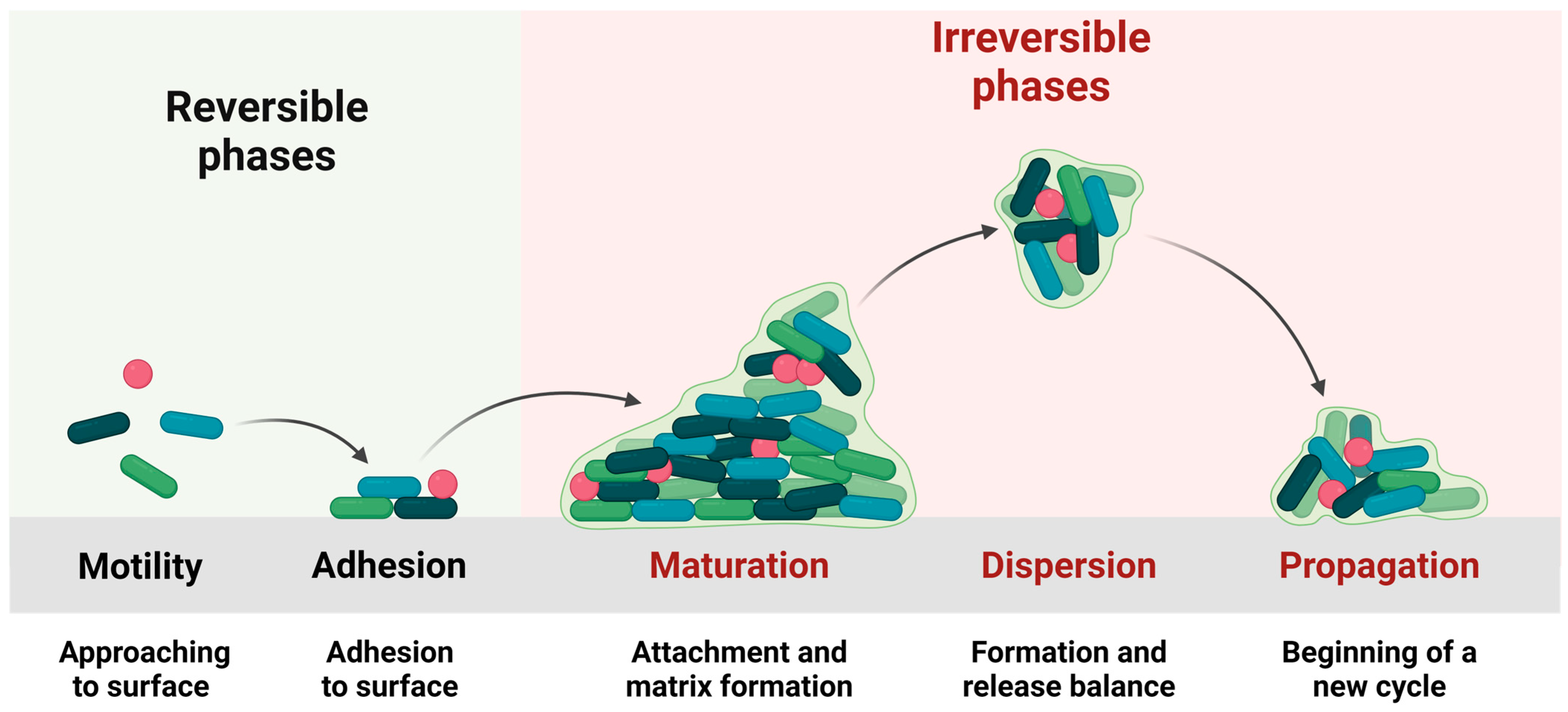
Biofilm Formation and the Interactions between Biofilm Components and Antimicrobial Agents
2. Recent Advances in Nanometal Research
2.1. Types of Metallic Nanoparticles and Their Efficacy
2.1.1. Silver Nanoparticles
2.1.2. Gold Nanoparticles
2.1.3. Copper Nanoparticles
2.1.4. Zinc Nanoparticles
2.1.5. Other Metallic Nanoparticles
2.2. Combinations of Metal Nanoparticles with Other Agents
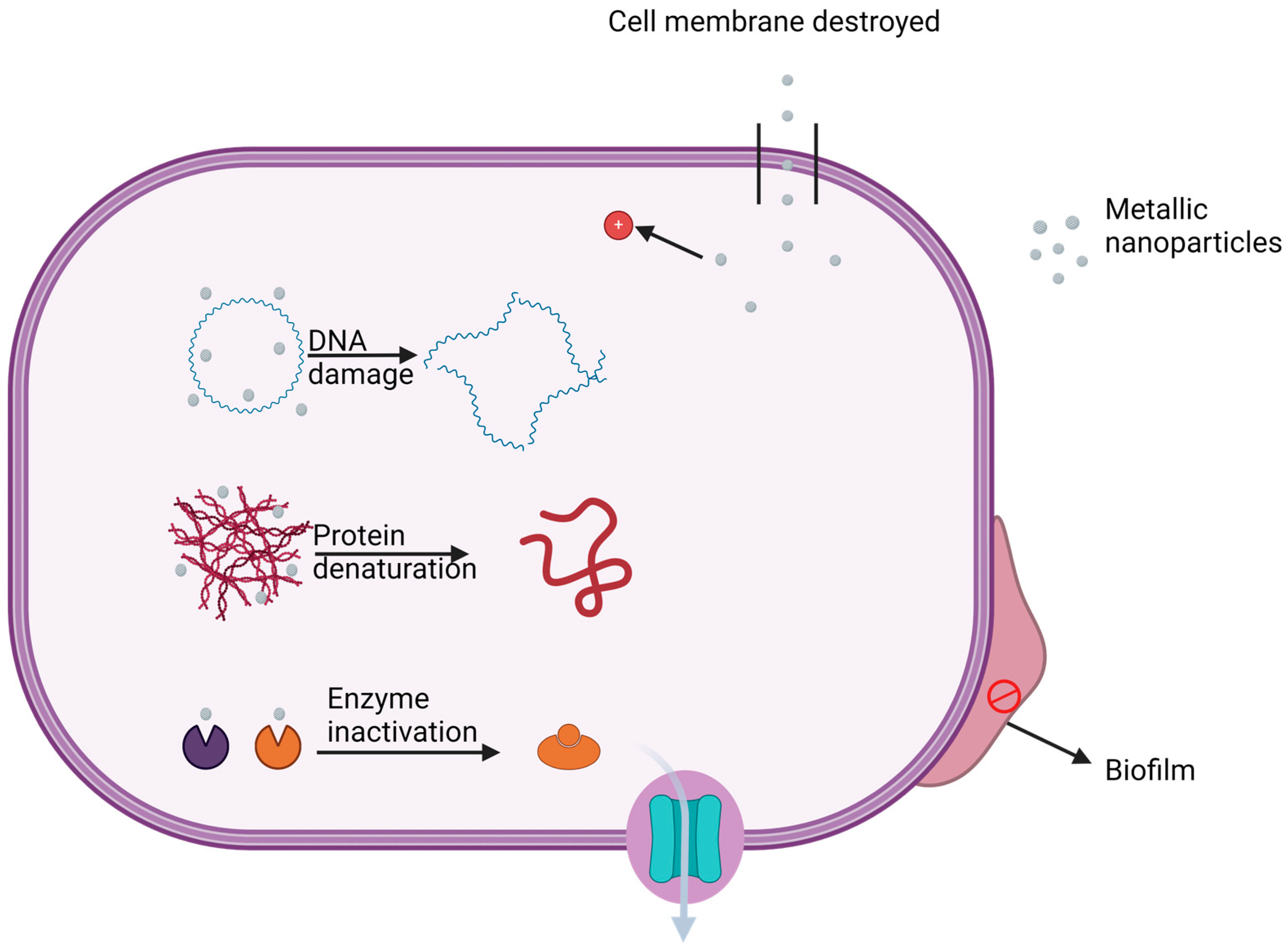
2.3. Biofilm Inhibition by Metallic Nanoparticles
2.4. Methods for the Synthesis of Silver Nanoparticles
Methods for Synthesis of AgNPs
- A.
- Chemical methods
- B.
- Physical methods
- C.
- Biological methods including green synthesis
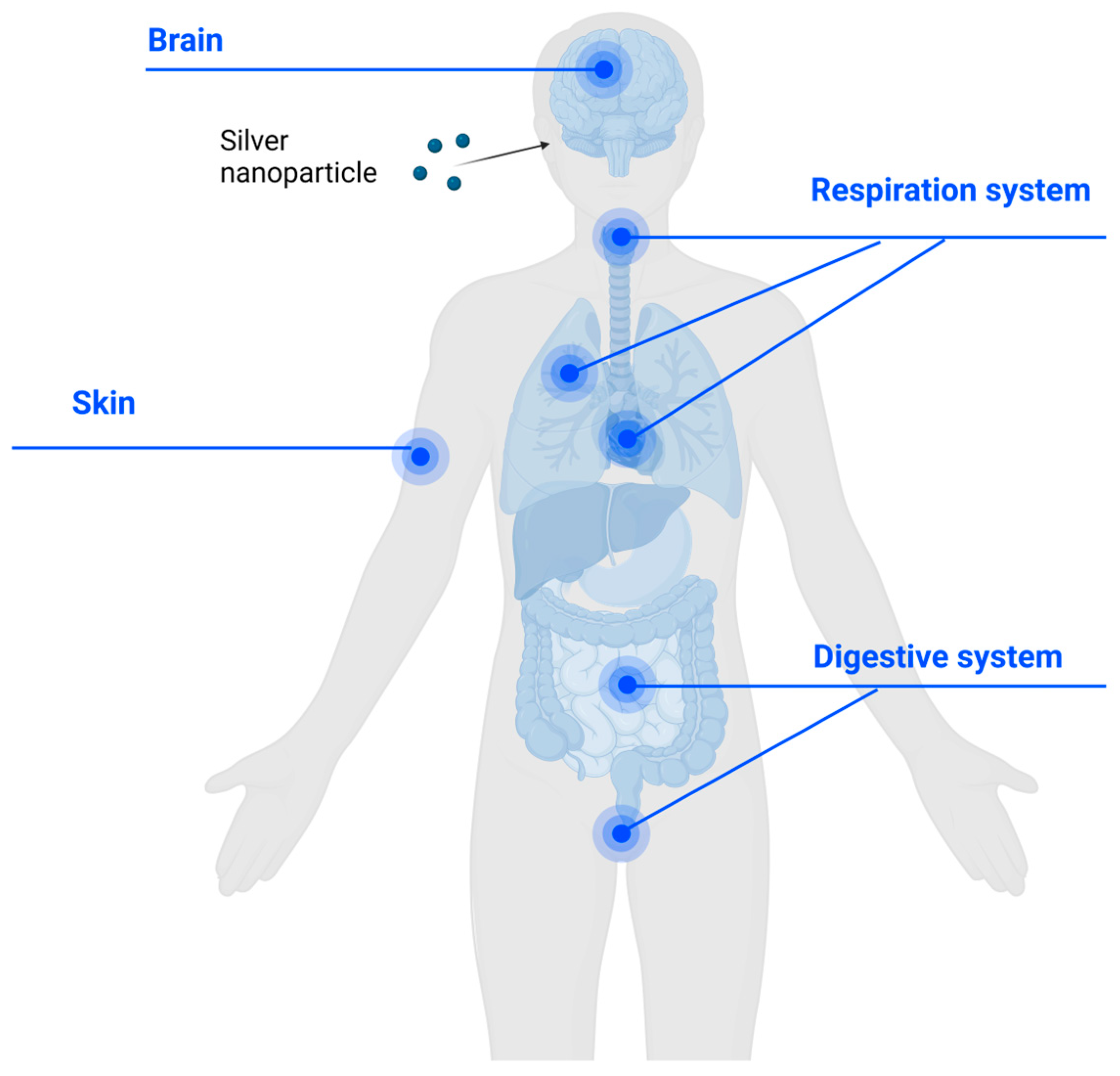
2.5. Biosafety of AgNPs When Applied onto the Human Body
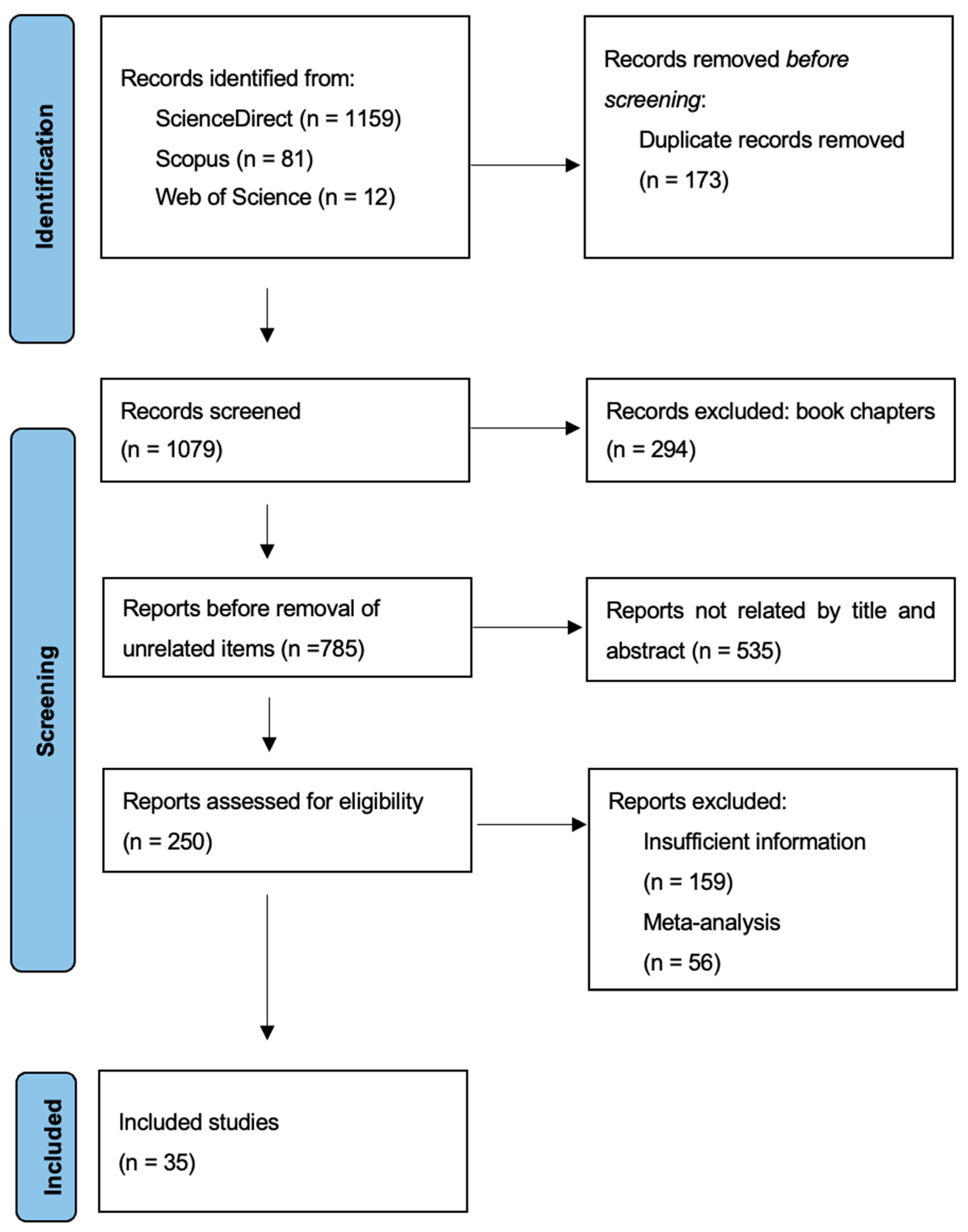
2.6. Analysis of the Literature from 2004 to 2024
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Del Pozo, J.; Rouse, M.; Patel, R. Bioelectric effect and bacterial biofilms. A systematic review. Int. J. Artif. Organs 2008, 31, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V. Quorum sensing in biofilms—How to destroy the bacterial citadels or their cohesion/power? Anaerobe 2011, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Madalina Mihai, M.; Maria Holban, A.; Giurcaneanu, C.; Gabriela Popa, L.; Mihaela Oanea, R.; Lazar, V.; Carmen Chifiriuc, M.; Popa, M.; Ioan Popa, M. Microbial biofilms: Impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr. Top. Med. Chem. 2015, 15, 1552–1576. [Google Scholar] [CrossRef] [PubMed]
- Rajput, N. Methods of preparation of nanoparticles-a review. Int. J. Adv. Eng. Technol. 2015, 7, 1806. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Mukherjee, D.; Sil, M.; Goswami, A.; Lahiri, D.; Nag, M. Effectiveness of metal and metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. J. Basic Microbiol. 2023, 63, 971–985. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Alotaibi, G.F.; Bukhari, M.A. Factors influencing bacterial biofilm formation and development. Am. J. Biomed. Sci. Res. 2021, 12, 617–626. [Google Scholar] [CrossRef]
- Hermansson, M. The DLVO theory in microbial adhesion. Colloids Surf. B Biointerfaces 1999, 14, 105–119. [Google Scholar] [CrossRef]
- Kumar, L.; Bisen, M.; Harjai, K.; Chhibber, S.; Azizov, S.; Lalhlenmawia, H.; Kumar, D. Advances in Nanotechnology for Biofilm Inhibition. ACS Omega 2023, 8, 21391–21409. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef]
- Madsen, J.S.; Røder, H.L.; Russel, J.; Sørensen, H.; Burmølle, M.; Sørensen, S.J. Coexistence facilitates interspecific biofilm formation in complex microbial communities. Environ. Microbiol. 2016, 18, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Carmo, P.H.F.d.; Garcia, M.T.; Figueiredo-Godoi, L.M.A.; Lage, A.C.P.; Silva, N.S.d.; Junqueira, J.C. Metal Nanoparticles to Combat Candida albicans Infections: An Update. Microorganisms 2023, 11, 138. [Google Scholar] [CrossRef]
- Omietimi, H.B.; Afolalu, S.A.; Kayode, J.F.; Monye, S.I.; Lawal, S.L.; Emetere, M.E. An overview of nanotechnology and its application. In Proceedings of the E3S Web of Conferences, Tamilnadu, India, 22–23 November 2023; p. 01079. [Google Scholar]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Xu, F.F.; Imlay, J.A. Silver (I), mercury (II), cadmium (II), and zinc (II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Mital, M.; Ziora, Z. Biological applications of Ru (II) polypyridyl complexes. Coord. Chem. Rev. 2018, 375, 434–458. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Li, Y.; Zhao, L.; Liang, S.; Guo, D.; Hu, J.; Wang, D. Synergy between polyvinylpyrrolidone-coated silver nanoparticles and azole antifungal against drug-resistant Candida albicans. J. Nanosci. Nanotechnol. 2016, 16, 2325–2335. [Google Scholar] [CrossRef]
- Hussain, M.A.; Ahmed, D.; Anwar, A.; Perveen, S.; Ahmed, S.; Anis, I.; Shah, M.R.; Khan, N.A. Combination therapy of clinically approved antifungal drugs is enhanced by conjugation with silver nanoparticles. Int. Microbiol. 2019, 22, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, K.; Mehta, S.; Chavan, R.; Chitalia, V.; Deogharkar, D.; Deshpande, S. Efficiency of biosynthesized silver and zinc nanoparticles against multi-drug resistant pathogens. Front. Microbiol. 2018, 9, 2207. [Google Scholar] [CrossRef]
- Nasiri, K.; Masoumi, S.M.; Amini, S.; Goudarzi, M.; Tafreshi, S.M.; Bagheri, A.; Yasamineh, S.; Alwan, M.; Arellano, M.T.C.; Gholizadeh, O. Recent advances in metal nanoparticles to treat periodontitis. J. Nanobiotechnol. 2023, 21, 283. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Akram, F.E.; El-Tayeb, T.; Abou-Aisha, K.; El-Azizi, M. A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). Ann. Clin. Microbiol. Antimicrob. 2016, 15, 48. [Google Scholar] [CrossRef]
- Nour El Din, S.; El-Tayeb, T.A.; Abou-Aisha, K.; El-Azizi, M. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int. J. Nanomed. 2016, 11, 1749–1758. [Google Scholar]
- Graves, J.L., Jr.; Thomas, M.; Ewunkem, J.A. Antimicrobial nanomaterials: Why evolution matters. Nanomaterials 2017, 7, 283. [Google Scholar] [CrossRef]
- Padilla-Cruz, A.; Garza-Cervantes, J.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J. Synthesis and design of Ag–Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef] [PubMed]
- Martínez Espinosa, J.C.; Carrera Cerritos, R.; Ramírez Morales, M.A.; Sánchez Guerrero, K.P.; Silva Contreras, R.A.; Macías, J.H. Characterization of silver nanoparticles obtained by a green route and their evaluation in the bacterium of Pseudomonas aeruginosa. Crystals 2020, 10, 395. [Google Scholar] [CrossRef]
- da Cunha, K.F.; Oliveira Garcia, M.; Allend, S.O.; de Albernaz, D.F.T.; Panagio, L.A.; Neto, A.; Larré Oliveira, T.; Hartwig, D.D. Biogenic silver nanoparticles: In vitro activity against Staphylococcus aureus methicillin-resistant (MRSA) and multidrug-resistant coagulase-negative Staphylococcus (CoNS). Braz. J. Microbiol. 2023, 54, 2641–2650. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Mohamed, B.S.; Zayed, M.; El-Sabbagh, S.M. Antibacterial, antibiofilm, and anticancer activity of silver-nanoparticles synthesized from the cell-filtrate of Streptomyces enissocaesilis. BMC Biotechnol. 2024, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Aisida, S.O.; Ugwu, K.; Nwanya, A.C.; Bashir, A.; Nwankwo, N.U.; Ahmed, I.; Ezema, F.I. Biosynthesis of silver oxide nanoparticles using leave extract of Telfairia Occidentalis and its antibacterial activity. Mater. Today Proc. 2021, 36, 208–213. [Google Scholar] [CrossRef]
- D’Lima, L.; Phadke, M.; Ashok, V.D. Biogenic silver and silver oxide hybrid nanoparticles: A potential antimicrobial against multi drug-resistant Pseudomonas aeruginosa. New J. Chem. 2020, 44, 4935–4941. [Google Scholar] [CrossRef]
- Lungu, M.; Gavriliu, Ş.; Enescu, E.; Ion, I.; Brătulescu, A.; Mihăescu, G.; Măruţescu, L.; Chifiriuc, M.C. Silver–titanium dioxide nanocomposites as effective antimicrobial and antibiofilm agents. J. Nanoparticle Res. 2014, 16, 2203. [Google Scholar] [CrossRef]
- Kamli, M.R.; Alzahrani, E.A.; Albukhari, S.M.; Ahmad, A.; Sabir, J.S.; Malik, M.A. Combination effect of novel bimetallic Ag-Ni nanoparticles with fluconazole against Candida albicans. J. Fungi 2022, 8, 733. [Google Scholar] [CrossRef]
- Albayrak, S.; Farajzadeh, N.; Yasemin yenilmez, H.; Özdemir, S.; Gonca, S.; Altuntaş Bayır, Z. Fluorinated Phthalocyanine/Silver Nanoconjugates for Multifunctional Biological Applications. Chem. Biodivers. 2023, 20, e202300389. [Google Scholar] [CrossRef]
- El-Behery, R.R.; El-Sayed, E.-S.R.; El-Sayyad, G.S. Gamma rays-assisted bacterial synthesis of bimetallic silver-selenium nanoparticles: Powerful antimicrobial, antibiofilm, antioxidant, and photocatalytic activities. BMC Microbiol. 2023, 23, 224. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.-Y.; Rohanizadeh, R.; Young, P.M.; Traini, D.; Cavaliere, R.; Whitchurch, C.B.; Lee, W.-H. Combination of Silver Nanoparticles and Curcumin Nanoparticles for Enhanced Anti-biofilm Activities. J. Agric. Food Chem. 2016, 64, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.; Doss, A.; Selvi, G.S.A.; Rani, T.P.K.P. Antibiofilm, antibacterial and antioxidant activity of biofabricated bimetallic (Ag-ZnO) nanoparticles from Elephantopus scaber L. Biomass Convers. Biorefin. 2023, 1–11. [Google Scholar] [CrossRef]
- Al-Dulaimi, N.M.H.D.; Mohammed, M.J.; Shartooh, S.M. The role of biofilm to isolate the Serratia marcescens in its resistance to antibiotics and study the effect of gold nanoparticles in inhibiting this resistance. Ann. Rom. Soc. Cell Biol. 2021, 1193–1200. [Google Scholar]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Vakyly, S.; Sedighian, H.; Jahromi, Z.; Jahangiri, A.; Halabian, R.; Rezaei, A.; Keshmiri, F. A sensitive and selective electrochemical sensor based on gold nanoparticle/multi-walled carbon nanotubes for detection of Staphylococcus aureus Alpha-toxin. Appl. Phys. A 2022, 128, 680. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibiofilm properties. Colloids Surf. B Biointerfaces 2017, 160, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.L.; Kariyawasam, C.S.; Hejny, M.A.; Torgall, V.B.; Werfel, T.A.; Fitzkee, N.C. Protein-Functionalized Gold Nanospheres with Tunable Photothermal Efficiency for the Near-Infrared Photothermal Ablation of Biofilms. ACS Appl. Mater. Interfaces 2024, 16, 4321–4332. [Google Scholar] [CrossRef]
- Naseer, M.; Ramadan, R.; Xing, J.; Samak, N.A. Facile green synthesis of copper oxide nanoparticles for the eradication of multidrug resistant Klebsiella pneumonia and Helicobacter pylori biofilms. Int. Biodeterior. Biodegrad. 2021, 159, 105201. [Google Scholar] [CrossRef]
- Gupta, A.; Maruthapandi, M.; Das, P.; Saravanan, A.; Jacobi, G.; Natan, M.; Banin, E.; Luong, J.H.; Gedanken, A. Cuprous Oxide Nanoparticles Decorated Fabric Materials with Anti-Biofilm Properties. ACS Appl. Bio-Mater. 2022, 5, 4310–4320. [Google Scholar] [CrossRef]
- Munusamy, T.; Shanmugam, R. Green Synthesis of Copper Oxide Nanoparticles Synthesized by Terminalia chebula Dried Fruit Extract: Characterization and Antibacterial Action. Cureus 2023, 15, e50142. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.H.; Zubair, M.; Aslam, B.; Ashraf, A.; Siddique, M.H.; Hayat, S.; Cruz, J.N.; Muzammil, S.; Khurshid, M.; Sarfraz, M.F.; et al. Comparative analysis of phyto-fabricated chitosan, copper oxide, and chitosan-based CuO nanoparticles: Antibacterial potential against Acinetobacter baumannii isolates and anticancer activity against HepG2 cell lines. Front. Microbiol. 2023, 14, 1188743. [Google Scholar] [CrossRef]
- Zhao, H.; Maruthupandy, M.; Al-mekhlafi, F.A.; Chackaravarthi, G.; Ramachandran, G.; Chelliah, C.K. Biological synthesis of copper oxide nanoparticles using marine endophytic actinomycetes and evaluation of biofilm producing bacteria and A549 lung cancer cells. J. King Saud Univ. Sci. 2022, 34, 101866. [Google Scholar] [CrossRef]
- Khan, M.F.; Husain, F.M.; Zia, Q.; Ahmad, E.; Jamal, A.; Alaidarous, M.; Banawas, S.; Alam, M.M.; Alshehri, B.A.; Jameel, M.; et al. Anti-quorum sensing and anti-biofilm activity of zinc oxide nanospikes. ACS Omega 2020, 5, 32203–32215. [Google Scholar] [CrossRef]
- Husain, F.M.; Hasan, I.; Qais, F.A.; Khan, R.A.; Alam, P.; Alsalme, A. Fabrication of zinc oxide-xanthan gum nanocomposite via green route: Attenuation of quorum sensing regulated virulence functions and mitigation of biofilm in gram-negative bacterial pathogens. Coatings 2020, 10, 1190. [Google Scholar] [CrossRef]
- McGuffie, M.J.; Hong, J.; Bahng, J.H.; Glynos, E.; Green, P.F.; Kotov, N.A.; Younger, J.G.; VanEpps, J.S. Zinc oxide nanoparticle suspensions and layer-by-layer coatings inhibit staphylococcal growth. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Qais, F.A.; Ahmad, I.; Hakeem, M.J.; Baig, M.H.; Masood Khan, J.; Al-Shabib, N.A. Biosynthesized zinc oxide nanoparticles disrupt established biofilms of pathogenic bacteria. Appl. Sci. 2022, 12, 710. [Google Scholar] [CrossRef]
- Shalom, Y.; Perelshtein, I.; Perkas, N.; Gedanken, A.; Banin, E. Catheters coated with Zn-doped CuO nanoparticles delay the onset of catheter-associated urinary tract infections. Nano Res. 2017, 10, 520–533. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Nath, R.; Tewari, S. Curcumin-ZnO nanocomposite mediated inhibition of Pseudomonas aeruginosa biofilm and its mechanism of action. J. Drug Deliv. Sci. Technol. 2023, 81, 104301. [Google Scholar] [CrossRef]
- Vidic, J.; Stankic, S.; Haque, F.; Ciric, D.; Le Goffic, R.; Vidy, A.; Jupille, J.; Delmas, B. Selective antibacterial effects of mixed ZnMgO nanoparticles. J. Nanoparticle Res. 2013, 15, 1595. [Google Scholar] [CrossRef]
- Alzahrani, K.E.; Niazy, A.A.; Alswieleh, A.M.; Wahab, R.; El-Toni, A.M.; Alghamdi, H.S. Antibacterial activity of trimetal (CuZnFe) oxide nanoparticles. Int. J. Nanomed. 2018, 77–87. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef]
- Gingasu, D.; Culita, D.C.; Calderon Moreno, J.M.; Marinescu, G.; Bartha, C.; Oprea, O.; Preda, S.; Chifiriuc, M.C.; Popa, M. Synthesis of CoFe2O4 through Wet Ferritization Method Using an Aqueous Extract of Eucalyptus Leaves. Coatings 2023, 13, 1250. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [PubMed]
- Rothpan, M.; Chandra Teja Dadi, N.; McKay, G.; Tanzer, M.; Nguyen, D.; Hart, A.; Tabrizian, M. Titanium-Dioxide-Nanoparticle-Embedded Polyelectrolyte Multilayer as an Osteoconductive and Antimicrobial Surface Coating. Materials 2023, 16, 7026. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Abbastabar, M.; Nosratabadi, M.; Ebrahimzadeh, M.A. High antimicrobial, cytotoxicity, and catalytic activities of biosynthesized selenium nanoparticles using Crocus caspius extract. Arab. J. Chem. 2023, 16, 104705. [Google Scholar] [CrossRef]
- Hayat, S.; Muzammil, S.; Rasool, M.H.; Nisar, Z.; Hussain, S.Z.; Sabri, A.N.; Jamil, S. In vitro antibiofilm and anti-adhesion effects of magnesium oxide nanoparticles against antibiotic resistant bacteria. Microbiol. Immunol. 2018, 62, 211–220. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 335574. [Google Scholar] [CrossRef]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Ren, E.; Zhang, C.; Li, D.; Pang, X.; Liu, G. Leveraging metal oxide nanoparticles for bacteria tracing and eradicating. View 2020, 1, 20200052. [Google Scholar] [CrossRef]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Schillaci, D. Bacterial metal nanoparticles to develop new weapons against bacterial biofilms and infections. Appl. Microbiol. Biotechnol. 2021, 105, 5357–5366. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Jain, M.; Pandey, A.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.; Shetti, N.P.; Aminabhavi, T.M. Leveraging the potential of silver nanoparticles-based materials towards sustainable water treatment. J. Environ. Manag. 2022, 319, 115675. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 336–343. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Chen, Z.; Balankura, T.; Fichthorn, K.A.; Rioux, R.M. Revisiting the polyol synthesis of silver nanostructures: Role of chloride in nanocube formation. ACS Nano 2019, 13, 1849–1860. [Google Scholar] [CrossRef]
- Ishak, N.M.; Kamarudin, S.; Timmiati, S. Green synthesis of metal and metal oxide nanoparticles via plant extracts: An overview. Mater. Res. Express 2019, 6, 112004. [Google Scholar] [CrossRef]
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Kibis, L.; Stadnichenko, A.; Pajetnov, E.; Koscheev, S.; Zaykovskii, V.; Boronin, A. The investigation of oxidized silver nanoparticles prepared by thermal evaporation and radio-frequency sputtering of metallic silver under oxygen. Appl. Surf. Sci. 2010, 257, 404–413. [Google Scholar] [CrossRef]
- Kylián, O.; Kuzminova, A.; Štefaníková, R.; Hanuš, J.; Solař, P.; Kúš, P.; Cieslar, M.; Choukourov, A.; Biederman, H. Silver/plasma polymer strawberry-like nanoparticles produced by gas-phase synthesis. Mater. Lett. 2019, 253, 238–241. [Google Scholar] [CrossRef]
- Kang, W.J.; Cheng, C.Q.; Li, Z.; Feng, Y.; Shen, G.R.; Du, X.W. Ultrafine Ag nanoparticles as active catalyst for electrocatalytic hydrogen production. ChemCatChem 2019, 11, 5976–5981. [Google Scholar] [CrossRef]
- Scuderi, M.; Esposito, M.; Todisco, F.; Simeone, D.; Tarantini, I.; De Marco, L.; De Giorgi, M.; Nicotra, G.; Carbone, L.; Sanvitto, D.; et al. Nanoscale study of the tarnishing process in electron beam lithography-fabricated silver nanoparticles for plasmonic applications. J. Phys. Chem. C 2016, 120, 24314–24323. [Google Scholar] [CrossRef]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Puiatti, G.A.; de Carvalho, J.P.; de Matos, A.T.; Lopes, R.P. Green synthesis of Fe0 nanoparticles using Eucalyptus grandis leaf extract: Characterization and application for dye degradation by a (Photo) Fenton-like process. J. Environ. Manag. 2022, 311, 114828. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Li, H.; Zhao, Y.; Zhang, X.; Wang, X. Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J. Environ. Manag. 2020, 275, 111262. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Lansdown, A.B. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef]
- Möhler, J.S.; Sim, W.; Blaskovich, M.A.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol. Adv. 2018, 36, 1391–1411. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Mucosal immunity in the female genital tract. J. Reprod. Immunol. 1997, 36, 23–50. [Google Scholar] [CrossRef]
- Javurek, A.B.; Suresh, D.; Spollen, W.G.; Hart, M.L.; Hansen, S.A.; Ellersieck, M.R.; Bivens, N.J.; Givan, S.A.; Upendran, A.; Kannan, R.; et al. Gut dysbiosis and neurobehavioral alterations in rats exposed to silver nanoparticles. Sci. Rep. 2017, 7, 2822. [Google Scholar] [CrossRef]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 115–126. [Google Scholar] [CrossRef] [PubMed]

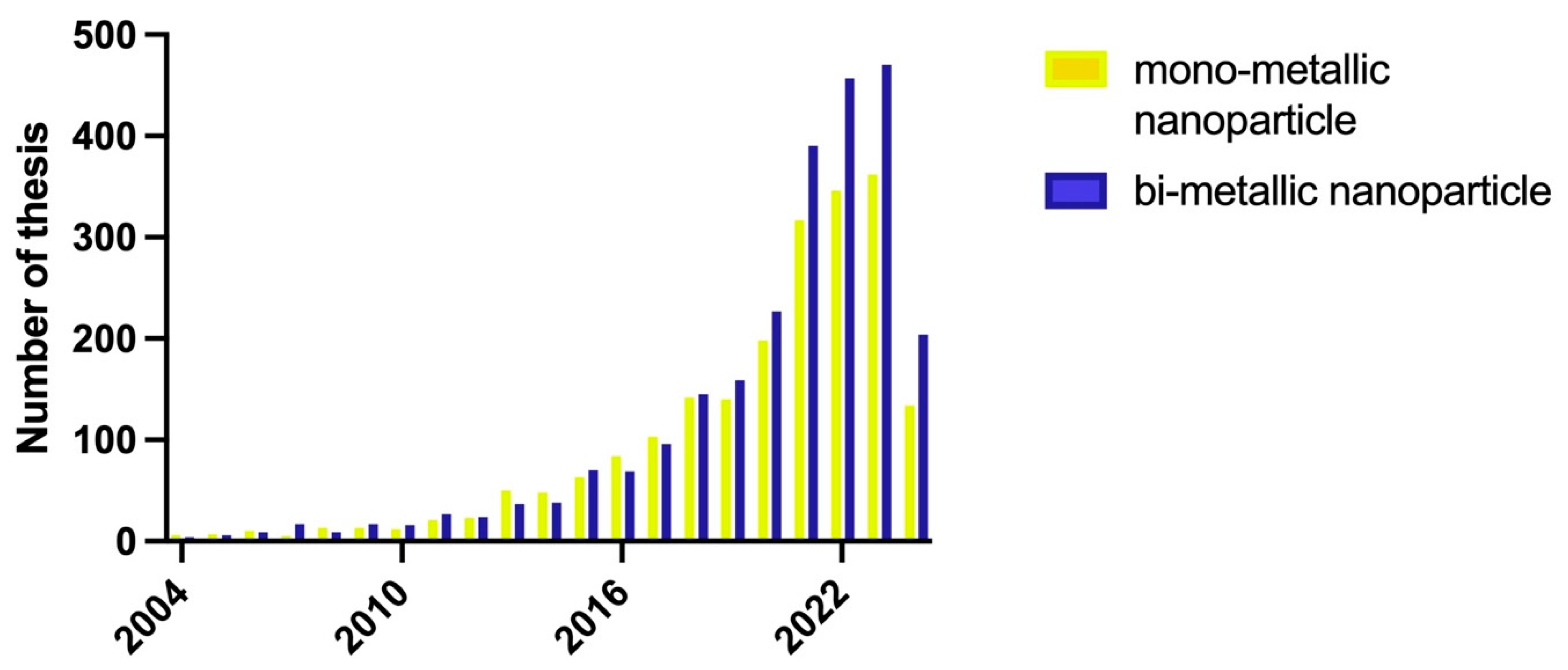
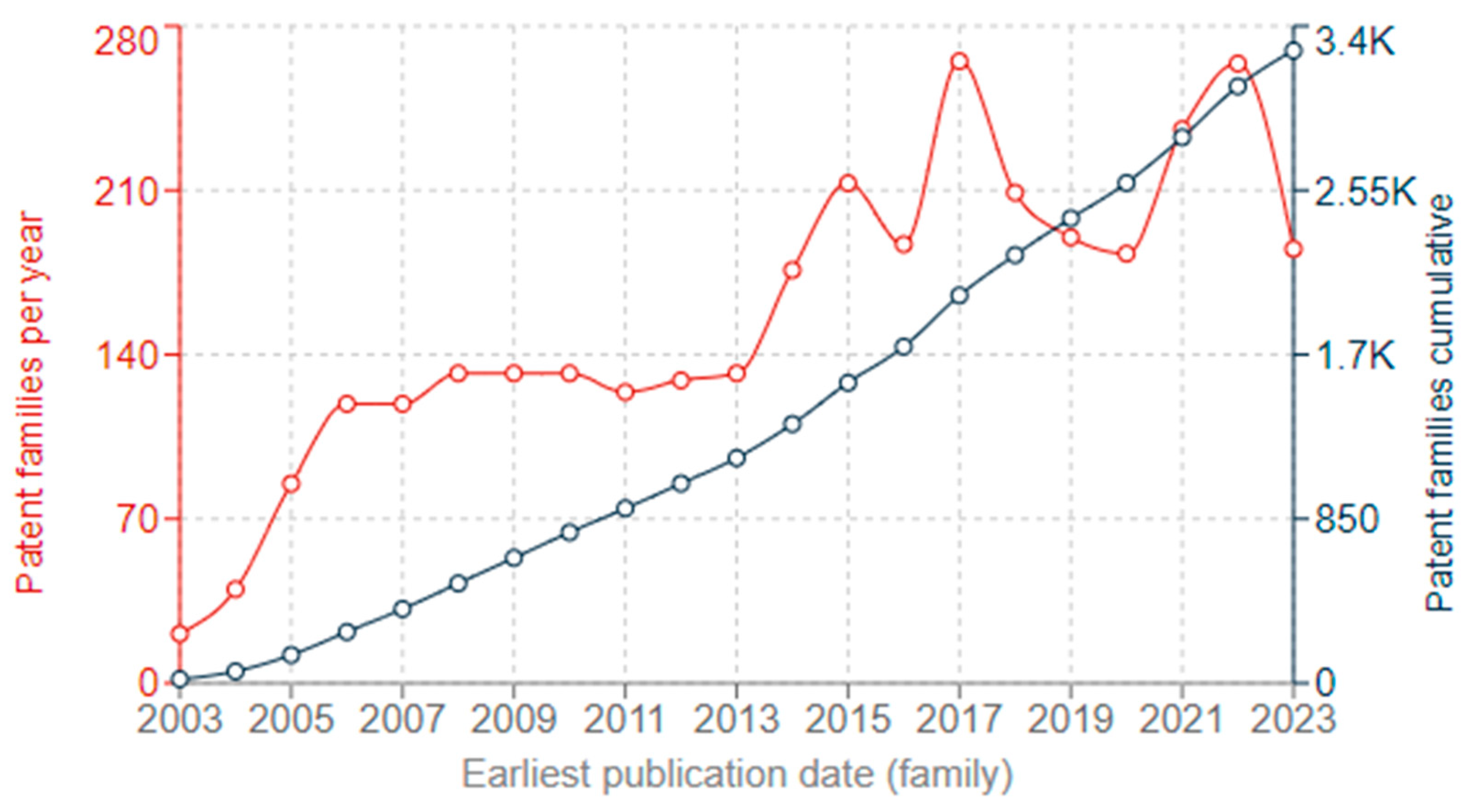
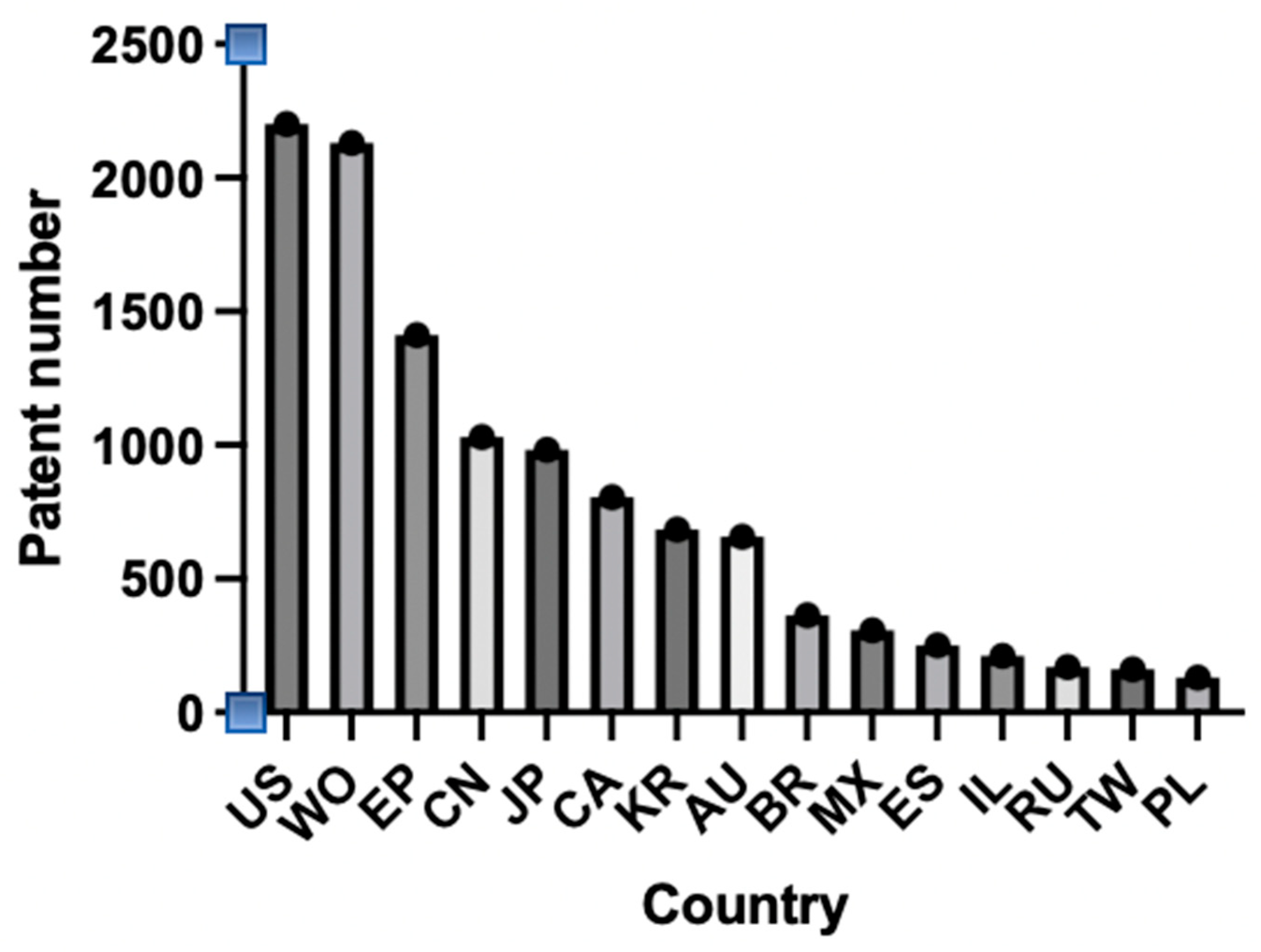
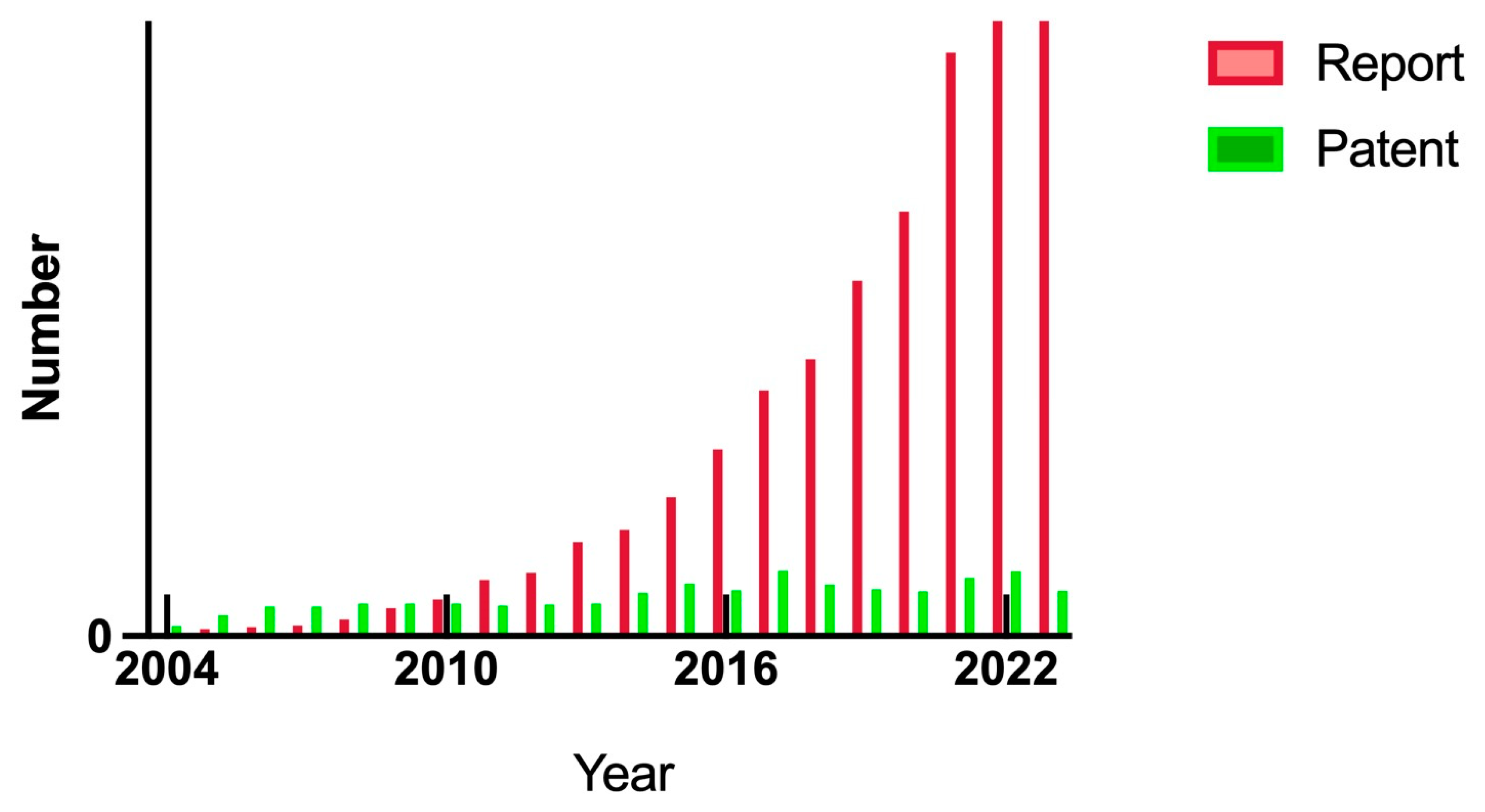
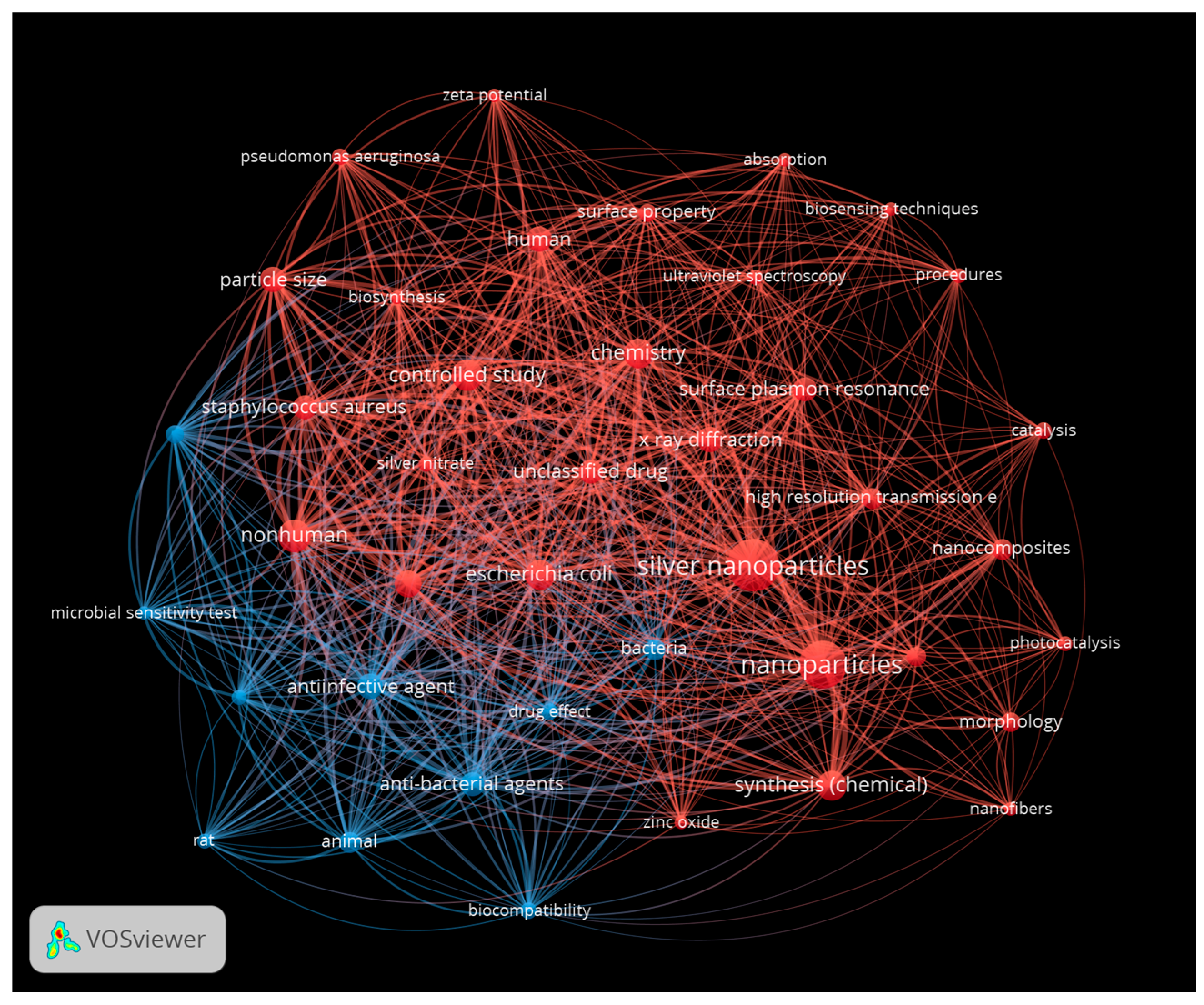
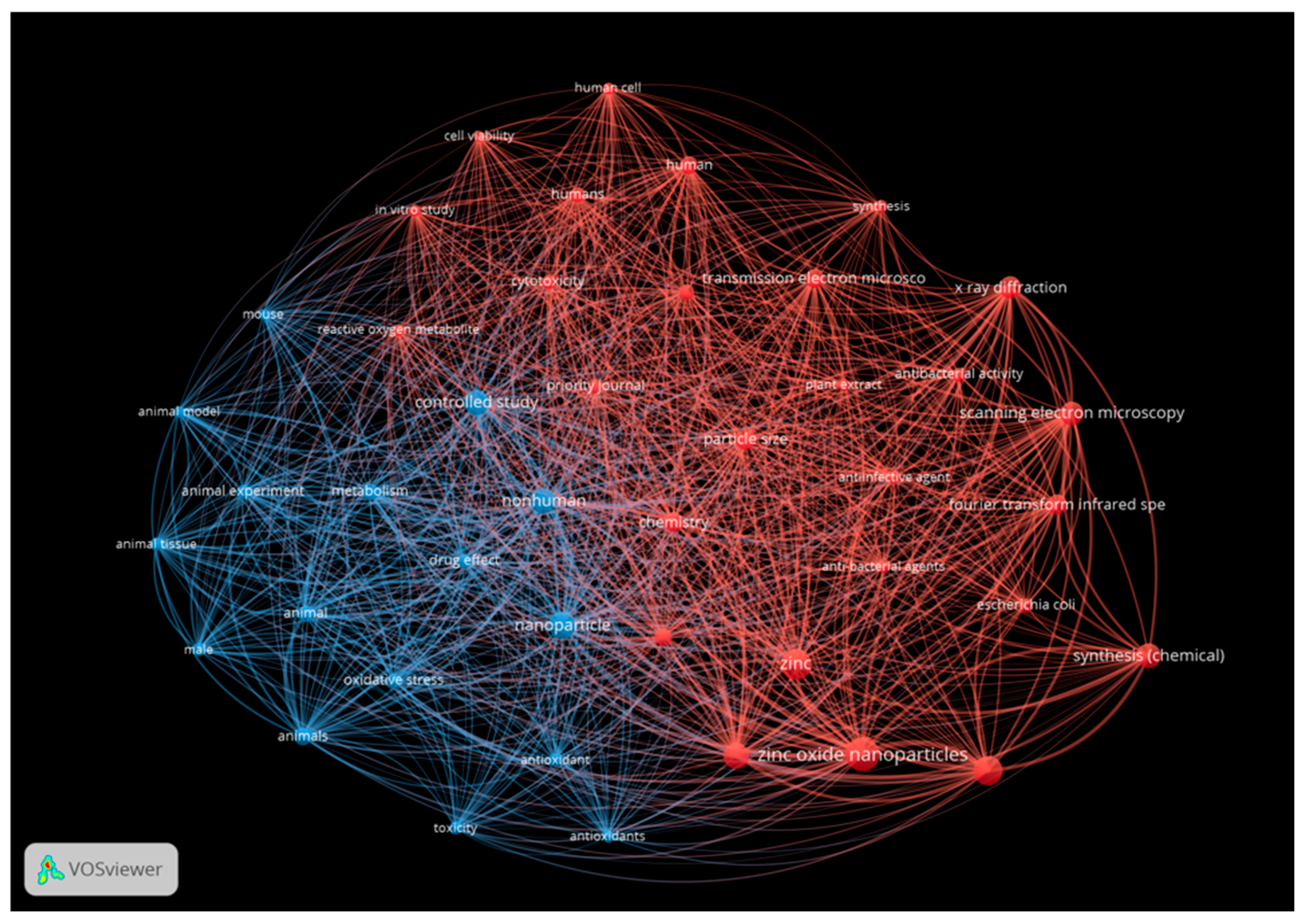
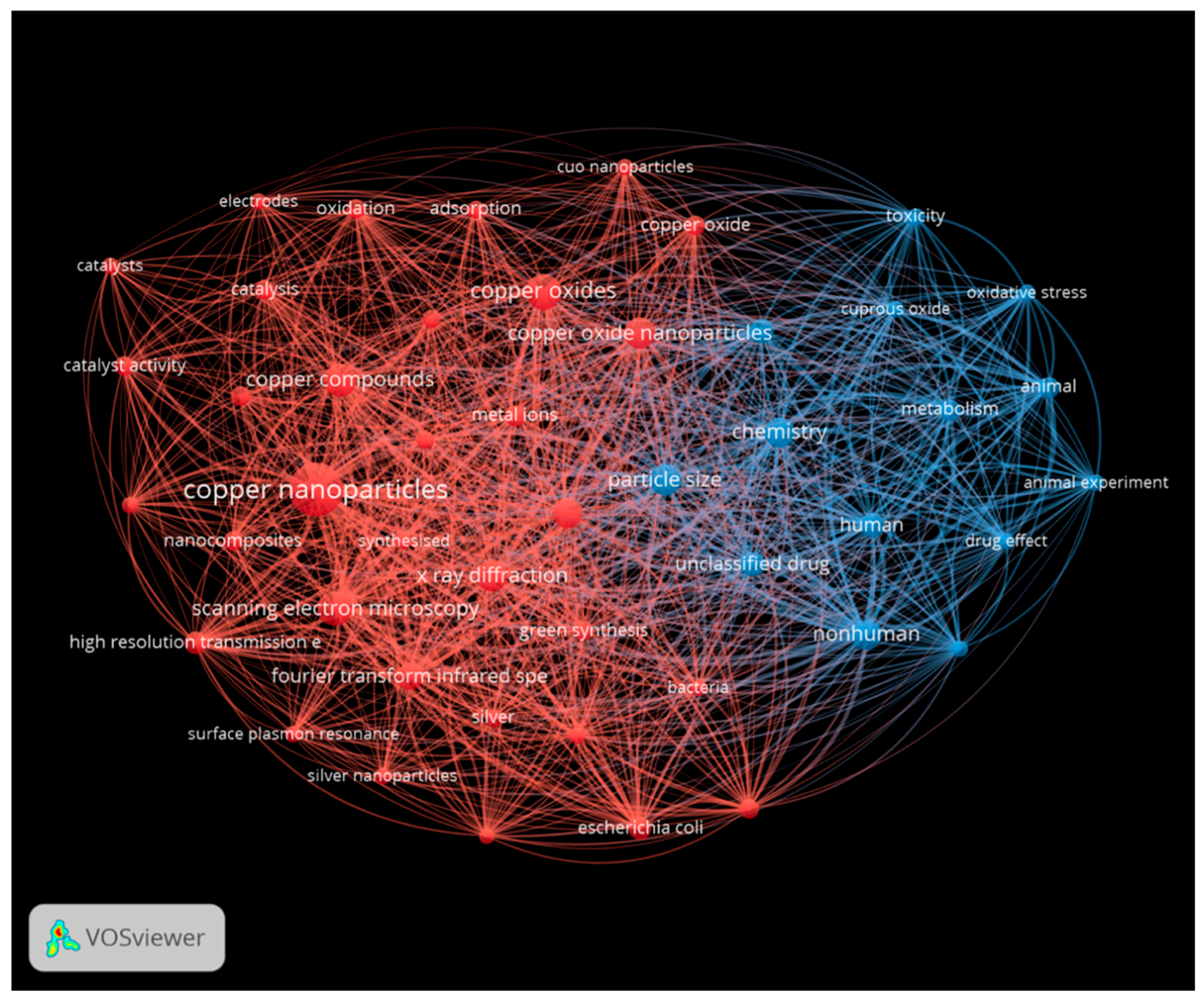
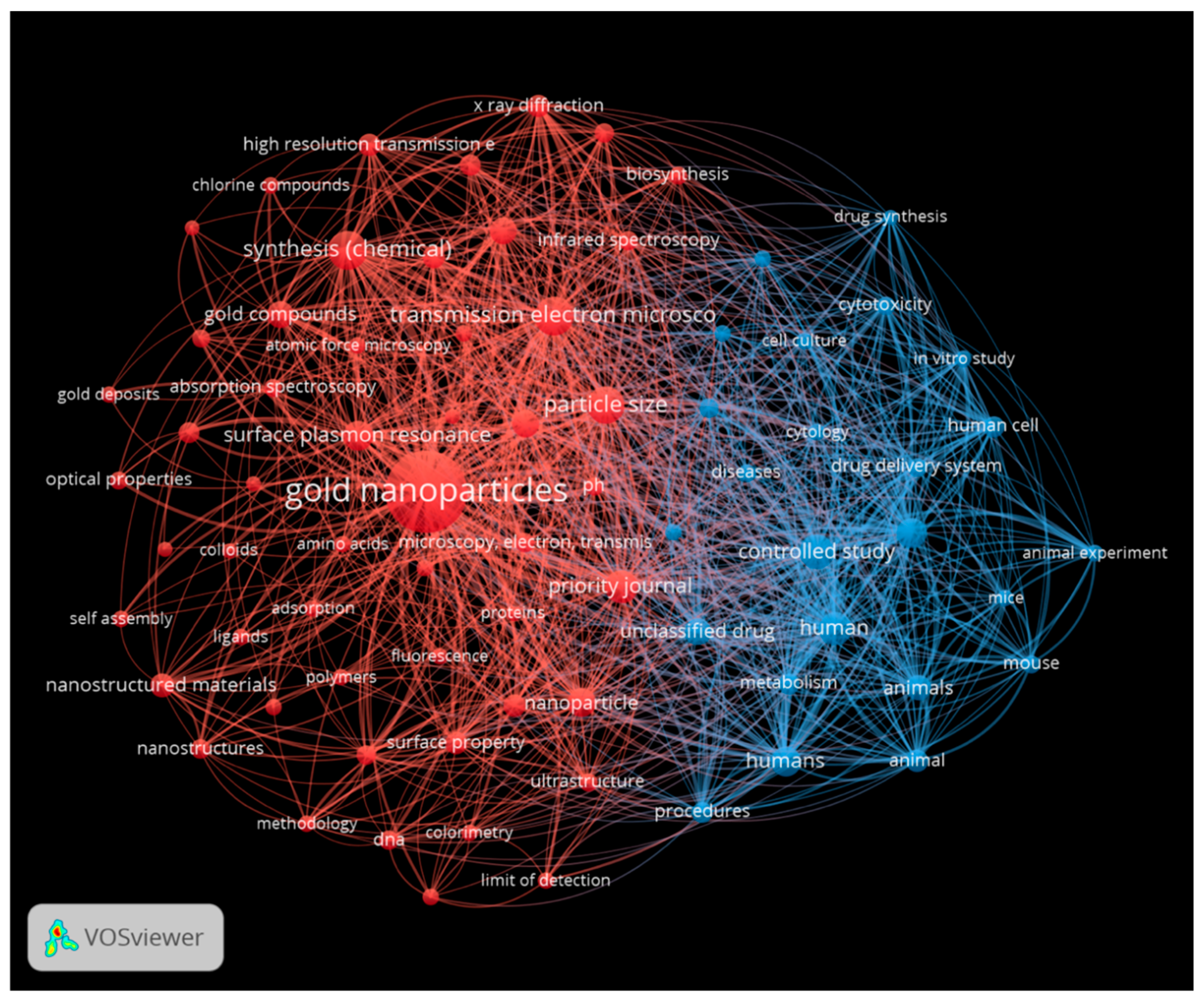
| Metallic Nanoparticles (Reference) | Size (nm) and Shape | Synthesis Resource | MIC (μg/mL) Reduction Ratio Inhibition Zone (mm) | Microorganism | Comments | |
|---|---|---|---|---|---|---|
| Silver | Silver (AgNPs) [33] | 50 Hexagonal | AgNO3 + leaves of Geranium | 0.18 - 8.5 | P. aeruginosa ATCC-27853 | Antibacterial actions |
| Silver (AgNPs) [34] | 100 Spherical | AgNO3 + malt extract | 3.75–15 60–100% - | S. aureus ATCC 25904 | For standard strains and MDR isolates and reduced and eradicated biofilms. Anti-biofilm actions. | |
| Silver (AgNPs) [35] | 1.2–62 Spherical | AgNO3 + L. acapulcensis extract | 0.06 99.9% 16 | S. aureus ATCC 49476 | Significant antimicrobial effects on S. aureus and P. aeruginosa | |
| 0.06 99.9% 15 | P. aeruginosa ATCC 27853 | |||||
| Silver (AgNPs) [36] | 32.2 Spherical | AgNO3 + starch broth medium | - 10.7% 34.6% 39.08% 34.75% - | P. aeruginosa ATCC 9027 | Antibacterial and anti-biofilm actions | |
| S. typhi ATCC 12023 | ||||||
| S. aureus ATCC 6598 | ||||||
| E. coli ATCC 8739 | ||||||
| Silver oxide | Silver oxide (AgONPs) [37] | 10–25 Spherical | Fresh Telfairia occidentalis leaves + AgNO3 | 20 - 15 | K. pneumoniae isolated from humans | Strong antibacterial activity |
| Silver oxide (AgONPs) [38] | 10–50 Spherical | Actinomycetes spp. + AgNO3 | 500 - 15 | MDR P. aeruginosa | Antibacterial activity | |
| Silver-containing nanoparticles | Ag–TiO2 NPs [39] | 31.3 ± 0.5 or 23.4 ± 0.4 - | TiO2 nanopowder | 150 - - | B. subtilis IC 12488 | Antibacterial and anti-biofilm activity |
| 150 - | S. aureus IC 13204 | |||||
| AgNO3 | 1200 - | E. coli IC 13529 | ||||
| 1200 - | K. pneumoniae IC 13420 | |||||
| PSSA-co-MA | 1200 - | P. aeruginosa IC 13202 | ||||
| Ag-Fe NPs [32] | 13 - | G. jasminoides | 65 - - | C. albicans | Growth inhibition | |
| AgNO3 | ||||||
| Fe(NO3)3 | ||||||
| Ag-Ni nanoparticles [40] | 31.84–47.85 - | AgNO3 | 1.56 - - | C. albicans SC 5314 | Growth inhibition and anti-biofilm activity | |
| Ni(NO3)2·6H2O | Inhibition of morphogenesis | |||||
| Salvia officinalis | Reduced efflux pump genes | |||||
| ROS production | ||||||
| Fluorinated Phthalocyanine–silver NPs [41] | 15–20 - | Silver nanoparticles | 8 95–100% - | P. aeruginosa ATCC 27853 | Antioxidant effects | |
| Phthalonitrile | 8 99–100% - | E. coli ATCC 25922 | Antimicrobial and anti-biofilm actions | |||
| Phthalocyanines | Antimicrobial photodynamic therapy activities | |||||
| Ag-Se nanoparticles [42] | 66.5 - | AgNO3 | 62.5 90.7% - | C. albicans ATCC 10231 | Antifungal | |
| Sodium selenite | 62.5 90.6% - | Antibacterial activity | ||||
| E. coli ATCC 11229 | Anti-biofilm actions | |||||
| 125 86.2% - | P. aeruginosa ATCC 6538 | Free radical scavenging | ||||
| Silver–curcumin NPs [43] | 30 - | AgNO3 | 3 - - | P. aeruginosa PA01 | Antibacterial and anti-biofilm actions | |
| Gallic acid | ||||||
| Curcumin | 50 - - | S. aureus ATCC 25923 | ||||
| PVP | ||||||
| Ag-ZnO NPs [44] | 28 - | E. scaber leaves | 0.125 62.5% - | S. aureus | Antibacterial actions | |
| Zinc nitrate | B. subtilis | Anti-biofilm actions | ||||
| AgNO3 | Antioxidant | |||||
| Gold | Gold (AuNPs) [45] | 5–50 Spherical | Trisodium citrate HAuCl4·4H2O | 18.71 80.4% 6.3 | S. marcescens Clinical collection | Antibacterial and anti-biofilm activity, through production of ROS |
| Gold(AuNPs) [46] | 20–100 Hexagonal | Marine alga G. elongate HAuCl4 aqueous solution | - - 13–17 | K. pneumoniae ATCC 27738 | Antibacterial activity | |
| Gold(AuNPs) [47] | 18 Spherical | HAuCl4·4H2O Trisodium citrate solution | - - - | S. aureus collected from volunteer | / | |
| Gold(AuNPs) [48] | 11 ± 3 Spherical | Cinnamaldehyde HAuCl4·4H2O | - 93% - | C. albicans DAY185 | Anti-biofilm actions | |
| Gold(AuNPs) [49] | 43 Spherical | Chloroauric acid Citrate | 120 - - | E. coli ATCC25992 | Antibacterial and anti-biofilm actions | |
| S. epidermidis 1301 | ||||||
| 1 - 15.5 | C. krusei not determined | |||||
| 1 - 20.5 | C. glabrata not determined | |||||
| Copper | Copper (CuNPs) [50] | 28.3 (C), 43.8 (M) Spherical | Cassia fistula (C) and Melia azedarach (M) leaves + cupric nitrate trihydrate | 1000 99.8%,92.5% 15.13 | K. pneumonia from collection center | Antibacterial and anti-biofilm actions toward both species |
| 1000 100%,99.5% 2.6 | H. pylori from collection center | |||||
| Copper oxide | Copper oxide (CuONPs) [51] | 80–300 Spherical | Cu2O microparticles + ethanol | - 98% - | MDR E. coli | Antibacterial and anti-biofilm actions |
| - 99.2% - | MDR S. aureus | |||||
| Copper oxide (CuONPs) [52] | 10–12 Spherical | Copper sulfate T. chebula extract | 1000 – 15.67 | E. coli not determined | Antibacterial and anti-biofilm actions | |
| 1000 - 16 | S. aureus not determined | |||||
| 750 - 17 | P. aeruginosa not determined | |||||
| Copper oxide(CuONPs) [53] | 28–33 Spherical | Copper sulfate solution Leaf extract | 125 - 12–13 | A. baumannii MH 605335 | Antibacterial and anti-biofilm actions | |
| Copper oxide(CuONPs) [54] | 20 Spherical | Copper sulfate (CuSO4∙5H2O) Cell free supernatant | 1000 96% - | E. coli from mat | Anti-biofilm actions | |
| Zinc oxide | Zinc oxide (ZnONPs) [55] | 40–130 Spikes | Zinc acetate Dihydrate DDW CTAB | 800 80–85% - | P. aeruginosa PAO 1 | Anti-biofilm actions and inhibition of QS for resistant P. aeruginosa |
| 500 80% - | C. violaceum CVO26 | |||||
| Zinc oxide–Xanthan nanocomposite [56] | 16 Rod | Zn(NO3)2∙6H2O + xanthan gum + NaOH | 256 70% - | C. violaceum ATCC 12472 | Anti-biofilm actions | |
| 256 75% - | S. marcescens ATCC 13880 | |||||
| Zinc oxide (ZnONPs) [57] | 4.4 Spherical hexagonal | Zn(Ac)2∙2H2O Anhydrous methanol KOH | 500 98.5% - | S. aureus SH 1000 | Antibacterial and anti-biofilm actions | |
| 500 - - | E. coli UT 189 | |||||
| Zinc oxide (ZnONPs) [58] | 24.62 Spherical spheroidal | Plumbago zeylanica L. Zinc acetate | - 52.69% - | E. coli ATCC 25922 | Inhibition of biofilm growth and anti-biofilm actions | |
| - 59.79% - | S. aureus MTCC 3160 | |||||
| - 67.22% - | P. aeruginosa PAO 1 | |||||
| Zinc-containing nanoparticles | Zn-doped CuO NPs [59] | - - | Copper zinc acetates | - 91% - | E. coli ATCC 25922 | Inhibition of biofilm formation and anti-biofilm actions |
| Aqueous ammonium hydroxide | - 92% - | S. aureus ATCC 29213 | ||||
| Ethanol | - 95% - | P. mirabilis not determined | ||||
| Curcumin-ZnO NPs [60] | 110.51 - | Zinc nitrate hexahydrate | 62.5 45–90% - | P. aeruginosa PAO 1 | Anti-biofilm actions | |
| 2-Thiobarbituric acid | ||||||
| ZnMgO NPs [61] | 10 - | MgO | - 61% - | E. coli BL21 DE3 | Antibacterial actions | |
| ZnO | - 25% - | B. subtilis 168 | ||||
| ZnCuFe NPs [62] | 42 - | Zinc acetate dihydrate | 150 85% - | E. coli from chronic infection | Antibacterial and anti-biofilm activity | |
| Copper acetate hydrate | ||||||
| Iron nitrate nonahydrate | 150 55% - | E. faecalis from chronic infection | ||||
| n-propyl amine | ||||||
| ZnO-Au hybrid NPs [63] | 30 - | ZnO | - 90% - | S. aureus not determined | Antibacterial effects | |
| AuCl4− | E. coli not determined | |||||
| Cobalt-containing nanoparticles | CoFe2O4 NPs [64] | 10 - | Iron nitrate | 5000 - - | C. albicans ATCC 10231 | Antimicrobial effects and anti-biofilm actions |
| Cobalt nitrate | 5000 - - | P. aeruginosa ATCC 27853 | ||||
| Eucalyptus plant extract | 5000 - - | E. coli ATCC 25922 | ||||
| Iron oxide | Iron oxide NPs [65] | 10–11 Spherical | Ferrous chloride tetrahydrate (FeCl2∙4H2O) | 50 - - | E. coli not determined | Antimicrobial effects and anti-biofilm actions |
| Ferric chloride hexahydrate (FeCl3∙6H2O) | ||||||
| Titanium Dioxide | TiO2 NPs [66] | 39.2 Rods | Titanium rods, Chitosan, Alginic acid sodium salt | 400 - - | S. aureus DNC274 ATCC 29213 | Antibacterial and anti-biofilm actions |
| Selenium | Se NPs [67] | 23.47 Red spherical | Sodium selenite DPPH Sodium borohydride | 25 - - | C. albicans IFRC 1873 | Antifungal activity against tested fungi strains |
| F. proliferatum IFRC 1871 | ||||||
| F. equiseti IFRC 1872 | ||||||
| T. mentagrophytes FR5_22130 | ||||||
| A. fumigatus IFRC 1649 | ||||||
| Magnesium oxide | MgO NPs [68] | 50–70 spherical | NaOH, NaNO3, MgCl2 | 250 82.9% 28 ± 0.33 mm (2000 μg/mL) 21 ± 0.288 mm (1000 μg/mL) 17.5 ± 0.288 mm (500 μg/mL) | E. coli KT273995 | Antibacterial activity and anti-biofilm actions |
| 125 82.9% 35.5 ± 0.33 mm (2000 μg/mL) 35.5 ± 0.288 mm (1000 μg/mL) 30.5 ± 1.90 mm (500 μg/mL) | Klebsiella pneumoniae KT273996 | |||||
| 500 82.9% 25.5 ± 0.268 mm (2000 μg/mL) 23.5 ± 1.32 mm (1000 μg/mL) 20.5 ± 2.18 mm (500 μg/mL) | Staphylococcus aureus KT250728 | |||||
| [69] | 50–100 irregular but spherical particle-like shapes | Purchased from Sigma Aldrich Chemical Co. (Saint Louis, MO, USA) | 200 93.40 to 95.60% 14.30 mm | R. solanacearum from infected tobacco | Bacteriostatic at low concentrations and anti-biofilm activity | |
| [70] | 4 square and polyhedral shape | Mg(CH3COO)2, Aerogel AP-MgO | 625 99.9% - | E. coli not determined | Antibacterial effects and anti-biofilm activity | |
| 625 95% - | S. aureus not determined | |||||
| Nickle oxide | NiO NPs [71] | 14 ± 5.8 polymorphic | Eucalyptus leaf extract (ELE), Ni(NO3)2·6H2O | 800 - 15 | Methicillin sensitive S. aureus-06 | Antibacterial and anti-biofilm activity against the tested strains |
| 800 - 13 | Methicillin sensitive S. aureus-02 | |||||
| 800 - 15 | P. aeruginosa-48 | |||||
| 800 - 14 | P. aeruginosa-64 | |||||
| 1600 - 17 | E. coli-60 | |||||
| 800 - 17 | E. coli-52 | |||||
| 800 - 15 | Methicillin-resistant S. aureus-10 | |||||
| 800 - 14 | Methicillin-resistant S. aureus-31 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, Z.; Khan, S.A.; Walsh, L.J.; Seneviratne, C.J.; Ziora, Z.M. Characteristics of Metallic Nanoparticles (Especially Silver Nanoparticles) as Anti-Biofilm Agents. Antibiotics 2024, 13, 819. https://doi.org/10.3390/antibiotics13090819
Li H, Yang Z, Khan SA, Walsh LJ, Seneviratne CJ, Ziora ZM. Characteristics of Metallic Nanoparticles (Especially Silver Nanoparticles) as Anti-Biofilm Agents. Antibiotics. 2024; 13(9):819. https://doi.org/10.3390/antibiotics13090819
Chicago/Turabian StyleLi, Hongze, Zhihe Yang, Sadaf Aiman Khan, Laurence J. Walsh, Chaminda Jayampath Seneviratne, and Zyta M. Ziora. 2024. "Characteristics of Metallic Nanoparticles (Especially Silver Nanoparticles) as Anti-Biofilm Agents" Antibiotics 13, no. 9: 819. https://doi.org/10.3390/antibiotics13090819
APA StyleLi, H., Yang, Z., Khan, S. A., Walsh, L. J., Seneviratne, C. J., & Ziora, Z. M. (2024). Characteristics of Metallic Nanoparticles (Especially Silver Nanoparticles) as Anti-Biofilm Agents. Antibiotics, 13(9), 819. https://doi.org/10.3390/antibiotics13090819










