The Antibacterial Potential of Brazilian Red Propolis against the Formation and Eradication of Biofilm of Helicobacter pylori
Abstract
1. Introduction
2. Results
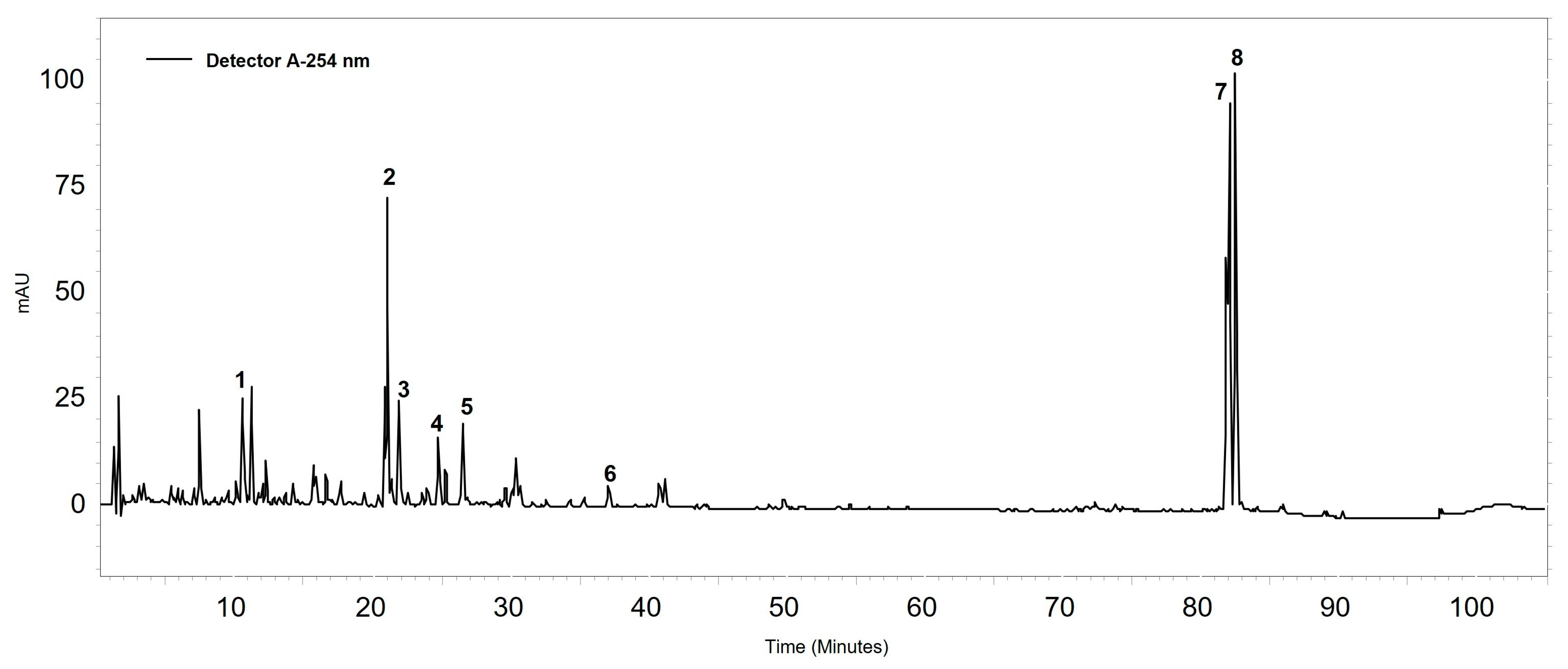
2.1. Chemical Characterization
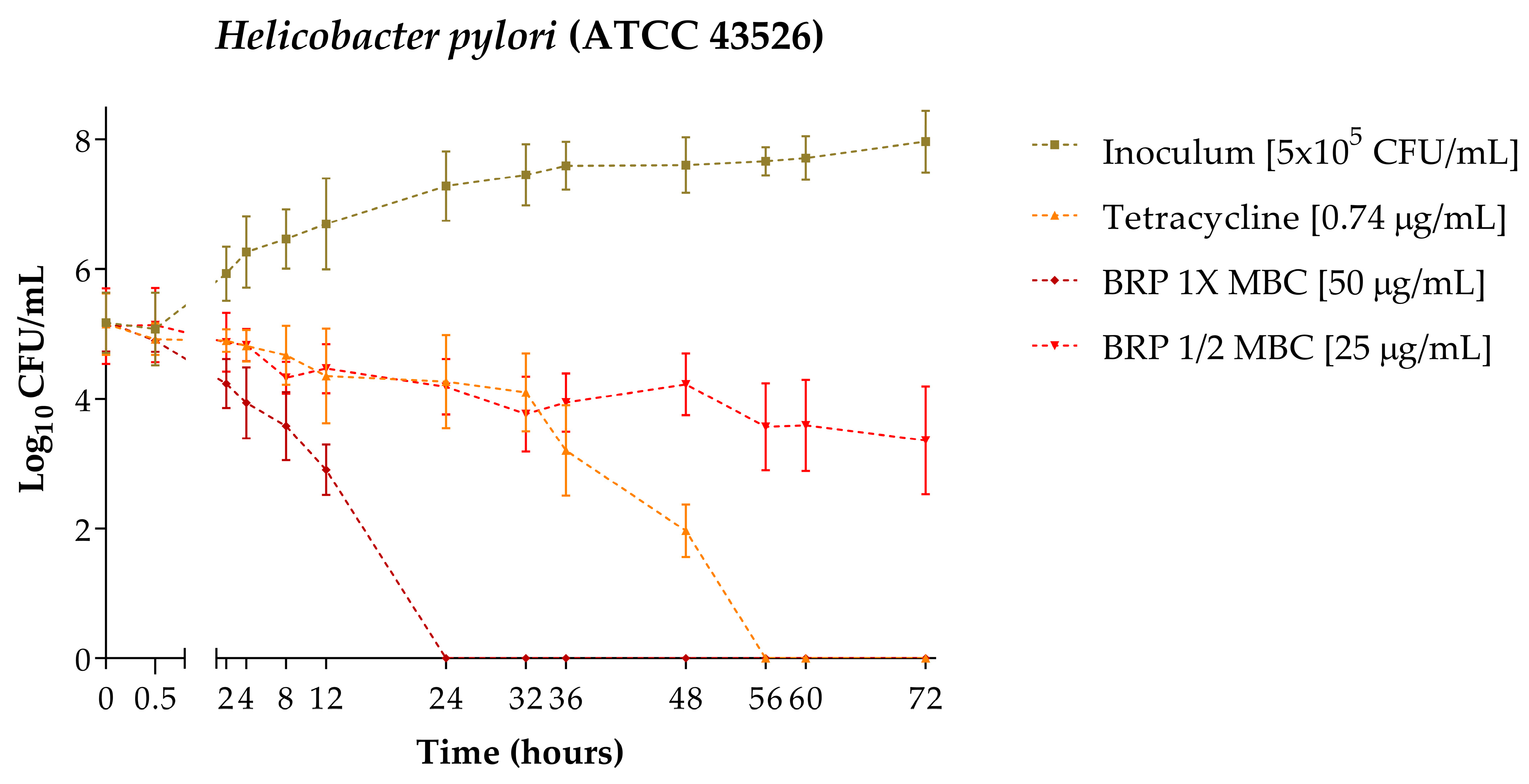
2.2. Time–Kill
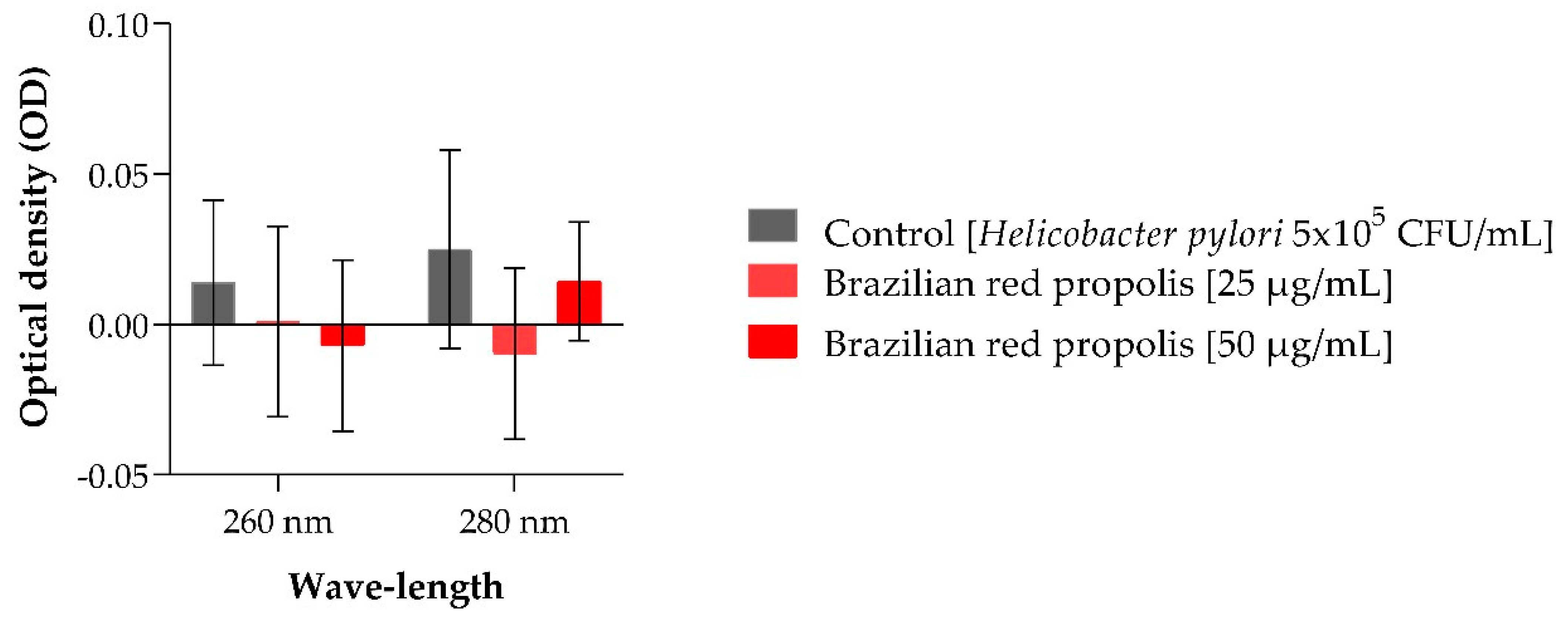
2.3. Nucleotide Leakage
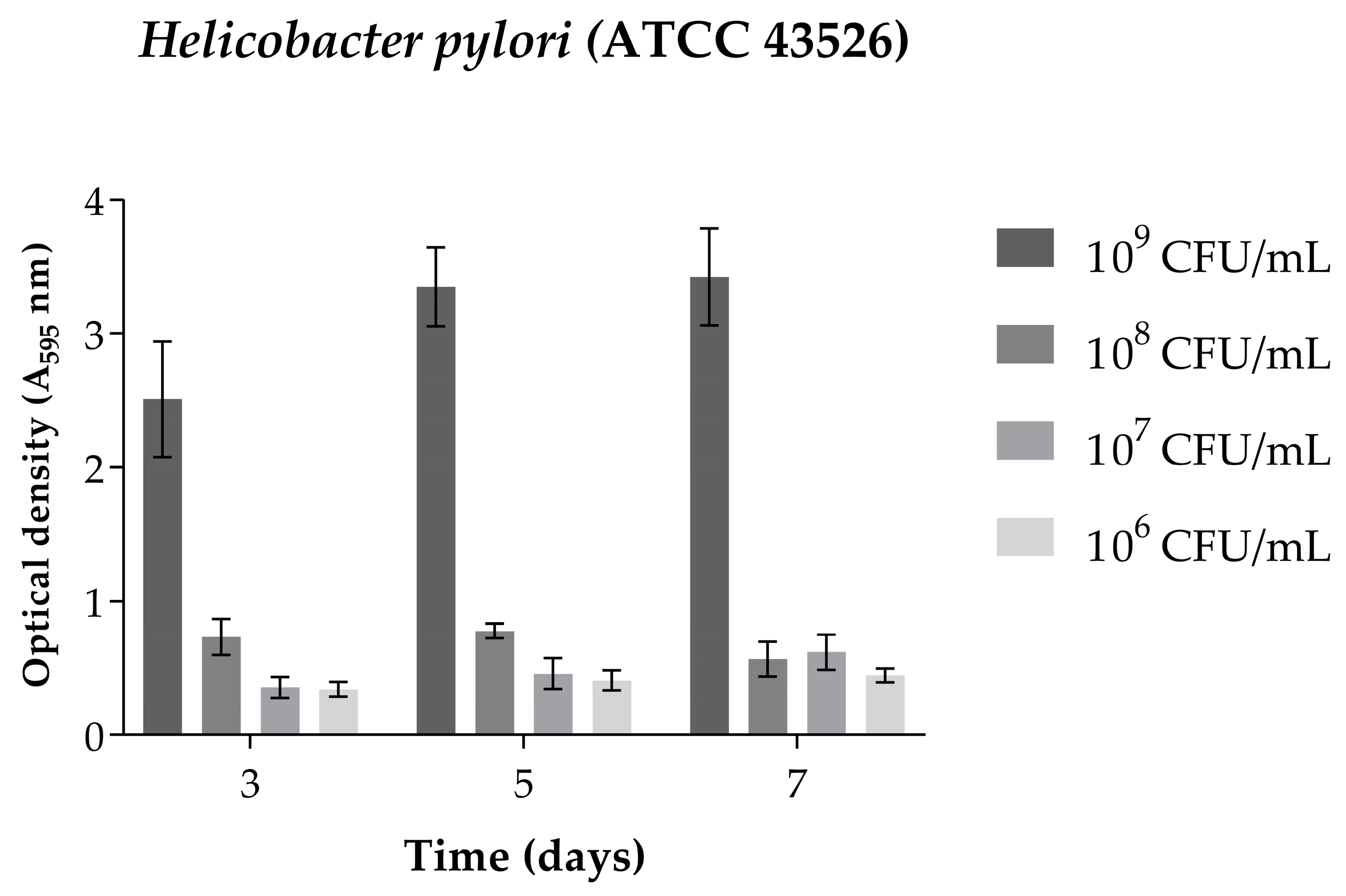
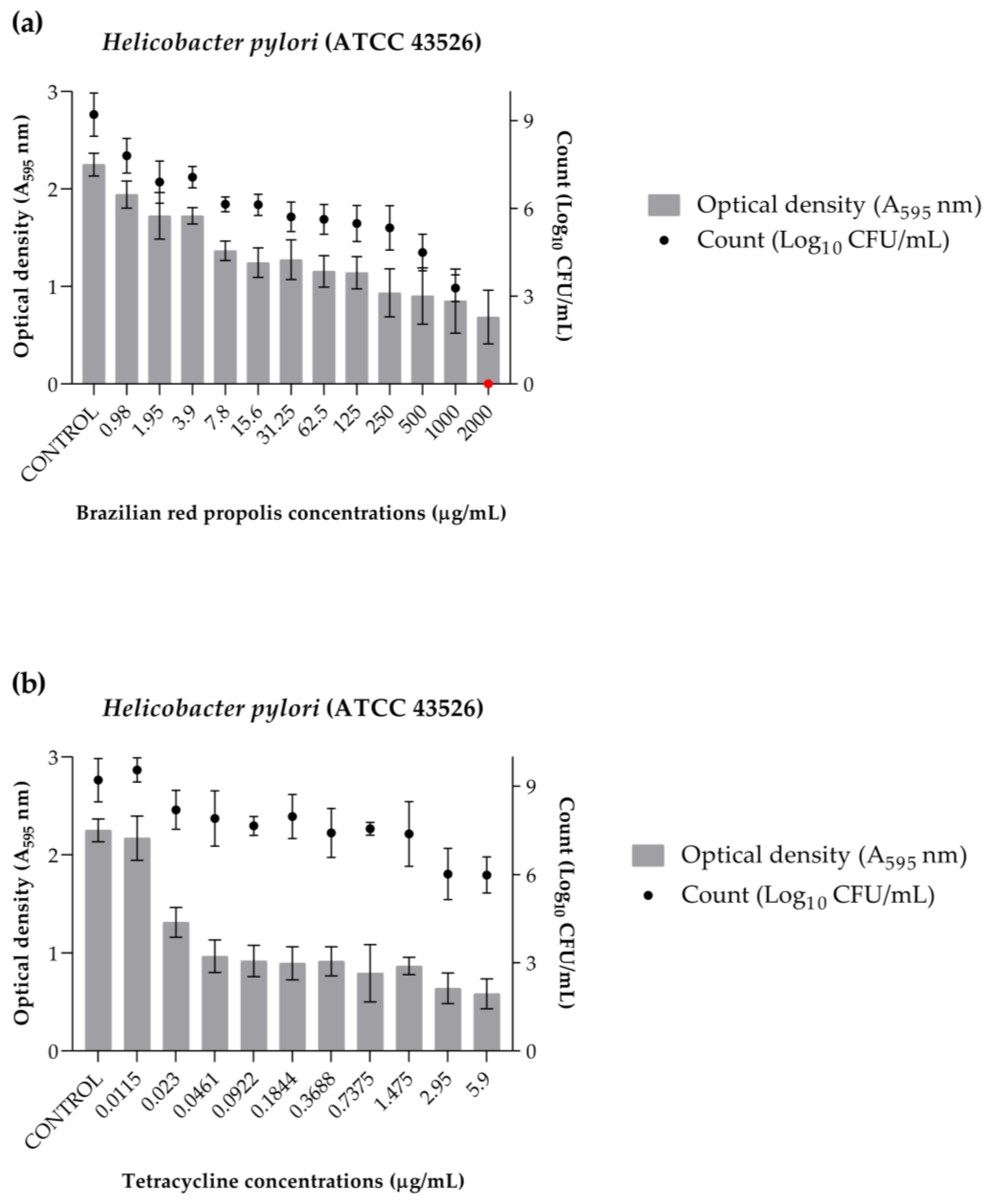
2.4. Biofilm Formation
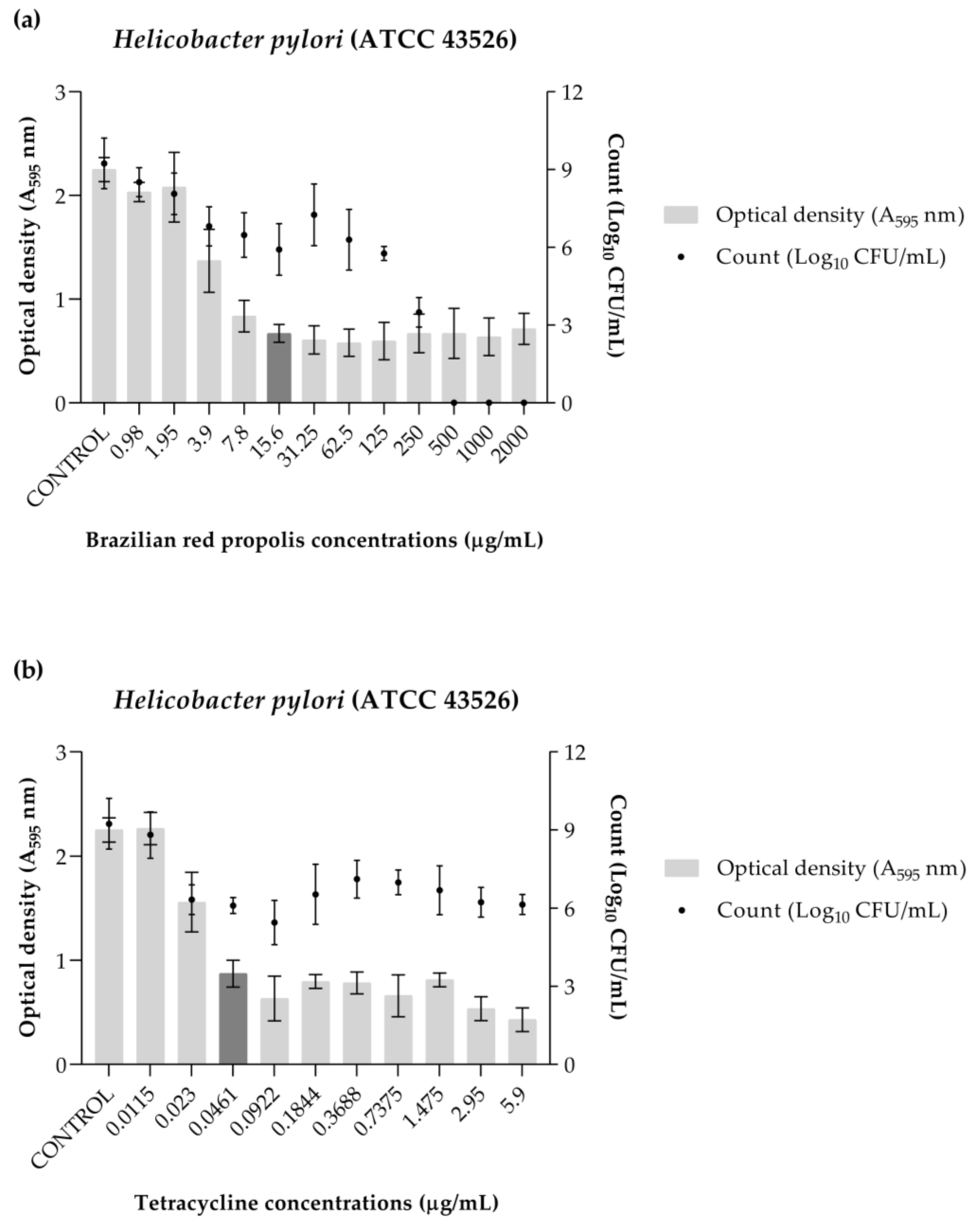
2.5. Inhibition of Biofilm Formation
2.6. Biofilm Eradication
3. Discussion
4. Materials and Methods
4.1. Chemical Characterization
4.1.1. Acquisition and Extraction of Red Propolis
4.1.2. Extract Analysis by HPLC-DAD
4.2. Anti-H. pylori Activity
4.2.1. Bacteria Used in the Assays
4.2.2. Time–Kill
4.2.3. Nucleotide Leakage
4.2.4. Standardization of Biofilm Formation
4.2.5. Inhibition of Biofilm Formation
4.2.6. Biofilm Eradication
4.2.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chmiela, M.; Kupcinskas, J. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2019, 24 (Suppl. 1), e12638. [Google Scholar] [CrossRef]
- Al-Fakhrany, O.M.; Elekhnawy, E. Helicobacter pylori in the post-antibiotics era: From virulence factors to new drug targets and therapeutic agents. Arch. Microbiol. 2023, 205, 301. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Wenker, T.N.; El-Serag, H.B. Global burden of gastric cancer: Epidemiological trends, risk factors, screening and prevention. Nat. Rev. Clin. Oncol. 2023, 20, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Blaser, M.J. Helicobacter pylori in health and disease. Gastroenterology 2009, 136, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Sharndama, H.C.; Mba, I.E. Helicobacter pylori: An up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 2022, 53, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Buzas, G.M.; Birinyi, P. Newer, Older, and Alternative Agents for the Eradication of Helicobacter pylori Infection: A Narrative Review. Antibiotics 2023, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef]
- Krzyzek, P.; Grande, R.; Migdal, P.; Paluch, E.; Gosciniak, G. Biofilm Formation as a Complex Result of Virulence and Adaptive Responses of Helicobacter pylori. Pathogens 2020, 9, 1062. [Google Scholar] [CrossRef]
- Elshenawi, Y.; Hu, S.; Hathroubi, S. Biofilm of Helicobacter pylori: Life Cycle, Features, and Treatment Options. Antibiotics 2023, 12, 1260. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lachance, H.; Wetzel, S.; Kumar, K.; Waldmann, H. Charting, navigating, and populating natural product chemical space for drug discovery. J. Med. Chem. 2012, 55, 5989–6001. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horcinova-Sedlackova, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.L.; Campesatto, E.A.; Assis, M.L.; Barbosa, A.P.; Grillo, L.A.; Dornelas, C.B. Comparative study of topical green and red propolis in the repair of wounds induced in rats. Rev. Colégio Bras. Cir. 2012, 39, 515–520. [Google Scholar] [CrossRef]
- de Freitas, M.C.D.; de Miranda, M.B.; de Oliveira, D.T.; Vieira-Filho, S.A.; Caligiorne, R.B.; de Figueiredo, S.M. Biological Activities of Red Propolis: A Review. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 11, 3–12. [Google Scholar] [CrossRef]
- Moise, A.R.; Bobis, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, Main Plant Sources for Bioactive Properties in Green and Red Brazilian Propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef]
- Aldana-Mejia, J.A.; Ccana-Ccapatinta, G.V.; Ribeiro, V.P.; Arruda, C.; Veneziani, R.C.S.; Ambrosio, S.R.; Bastos, J.K. A validated HPLC-UV method for the analysis of phenolic compounds in Brazilian red propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 2021, 198, 114029. [Google Scholar] [CrossRef] [PubMed]
- Alencar, S.M.; Oldoni, T.L.; Castro, M.L.; Cabral, I.S.; Costa-Neto, C.M.; Cury, J.A.; Rosalen, P.L.; Ikegaki, M. Chemical composition and biological activity of a new type of Brazilian propolis: Red propolis. J. Ethnopharmacol. 2007, 113, 278–283. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Marsola, A.; Ikegaki, M.; Alencar, S.M.; Rosalen, P.L. The effect of seasons on Brazilian red propolis and its botanical source: Chemical composition and antibacterial activity. Nat. Prod. Res. 2016, 31, 1318–1324. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Mejia, J.A.A.; Tanimoto, M.H.; Groppo, M.; Carvalho, J.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef]
- Mendonça, M.A.A.; Ribeiro, A.R.S.; Lima, A.K.; Bezerra, G.B.; Pinheiro, M.S.; Albuquerque-Junior, R.L.C.; Gomes, M.Z.; Padilha, F.F.; Thomazzi, S.M.; Novellino, E.; et al. Red Propolis and Its Dyslipidemic Regulator Formononetin: Evaluation of Antioxidant Activity and Gastroprotective Effects in Rat Model of Gastric Ulcer. Nutrients 2020, 12, 2951. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.B.; Leandro, L.F.; Rosa, R.B.; Silva, M.V.; Teixeira, S.C.; Servato, J.P.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Aldana-Mejia, J.A.; Bastos, J.K.; et al. Brazilian Red Propolis Presents Promising Anti-H. pylori Activity in In Vitro and In Vivo Assays with the Ability to Modulate the Immune Response. Molecules 2022, 27, 7310. [Google Scholar] [CrossRef]
- Baltas, N.; Karaoglu, S.A.; Tarakci, C.; Kolayli, S. Effect of propolis in gastric disorders: Inhibition studies on the growth of Helicobacter pylori and production of its urease. J. Enzym. Inhib. Med. Chem. 2016, 31, 46–50. [Google Scholar] [CrossRef]
- Boyanova, L.; Derejian, S.; Koumanova, R.; Katsarov, N.; Gergova, G.; Mitov, I.; Nikolov, R.; Krastev, Z. Inhibition of Helicobacter pylori growth in vitro by Bulgarian propolis: Preliminary report. J. Med. Microbiol. 2003, 52, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.; Gonzalez, M.; Fernandez, H.; Wilson, M.; Manquian, N.; Otth, C.; Otth, L. In vitro antibacterial activity of Chilean propolis against Helicobacter pylori. Rev. Chilena Infectol. 2015, 32, 530–535. [Google Scholar] [CrossRef][Green Version]
- Widelski, J.; Okinczyc, P.; Paluch, E.; Mroczek, T.; Szperlik, J.; Zuk, M.; Sroka, Z.; Sakipova, Z.; Chinou, I.; Skalicka-Wozniak, K.; et al. The Antimicrobial Properties of Poplar and Aspen-Poplar Propolises and Their Active Components against Selected Microorganisms, including Helicobacter pylori. Pathogens 2022, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, Y.; Li, N.; Yu, J.; Cheng, H.; Li, J.; Zhang, X.Z. Anti-Helicobacter pylori activities of Chenopodium ambrosioides L. in vitro and in vivo. World J. Gastroenterol. 2015, 21, 4178–4183. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Stoodley, P.; Hall-Stoodley, L.; Costerton, J.W. Bacterial biofilms: A diagnostic and therapeutic challenge. Expert Rev. Anti Infect. Ther. 2003, 1, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.A.; Pridgen, E.M.; Manasherob, R.; Suh, G.; Blackwell, H.E.; Barron, A.E.; Bollyky, P.L.; Goodman, S.B.; Amanatullah, D.F. Periprosthetic bacterial biofilm and quorum sensing. J. Orthop. Res. 2018, 36, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.J.; Melander, C. Controlling bacterial biofilms. Chembiochem 2009, 10, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Sa Assis, M.A.; de Paula Ramos, L.; Abu Hasna, A.; de Queiroz, T.S.; Pereira, T.C.; Nagai de Lima, P.M.; Berretta, A.A.; Marcucci, M.C.; Talge Carvalho, C.A.; de Oliveira, L.D. Antimicrobial and Antibiofilm Effect of Brazilian Green Propolis Aqueous Extract against Dental Anaerobic Bacteria. Molecules 2022, 27, 8128. [Google Scholar] [CrossRef] [PubMed]
- Meccatti, V.M.; Martins, K.M.C.; Ramos, L.P.; Pereira, T.C.; de Menezes, R.T.; Marcucci, M.C.; Abu Hasna, A.; de Oliveira, L.D. Synergistic Antibiofilm Action of Cinnamomum verum and Brazilian Green Propolis Hydroethanolic Extracts against Multidrug-Resistant Strains of Acinetobacter baumannii and Pseudomonas aeruginosa and Their Biocompatibility on Human Keratinocytes. Molecules 2023, 28, 6904. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.X.; Campagna, A.N.; Bobek, L.A. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J. Antimicrob. Chemother. 2006, 57, 1100–1109. [Google Scholar] [CrossRef]
- Olson, M.E.; Ceri, H.; Morck, D.W.; Buret, A.G.; Read, R.R. Biofilm bacteria: Formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 2002, 66, 86–92. [Google Scholar]
- Fukai, T.; Marumo, A.; Kaitou, K.; Kanda, T.; Terada, S.; Nomura, T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002, 71, 1449–1463. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Alencar, S.M.; Koo, H.; Ikegaki, M.; Silva, G.V.; Napimoga, M.H.; Rosalen, P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef]
- Inui, S.; Hatano, A.; Yoshino, M.; Hosoya, T.; Shimamura, Y.; Masuda, S.; Ahn, M.R.; Tazawa, S.; Araki, Y.; Kumazawa, S. Identification of the phenolic compounds contributing to antibacterial activity in ethanol extracts of Brazilian red propolis. Nat. Prod. Res. 2014, 28, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Boeing, T.; Mejia, J.A.A.; Ccana-Ccapatinta, G.V.; Mariott, M.; Melo Vilhena de Andrade Fonseca Da Silva, R.C.; de Souza, P.; Mariano, L.N.B.; Oliveira, G.R.; da Rocha, I.M.; da Costa, G.A.; et al. The gastroprotective effect of red propolis extract from Northeastern Brazil and the role of its isolated compounds. J. Ethnopharmacol. 2021, 267, 113623. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.G.; Schmidt, E.M.; Eberlin, M.N.; Sawaya, A.C. Phytochemical markers of different types of red propolis. Food Chem. 2013, 146, 174–180. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rastrelli, L. Cuban and Brazilian red propolis: Botanical origin and comparative analysis by high-performance liquid chromatography-photodiode array detection/electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef]
- Regueira, M.S.N.; Tintino, S.R.; da Silva, A.R.P.; Costa, M.D.S.; Boligon, A.A.; Matias, E.F.F.; de Queiroz Balbino, V.; Menezes, I.R.A.; Melo Coutinho, H.D. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food Chem. Toxicol. 2017, 107, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Nysten, J.; Sofras, D.; Van Dijck, P. One species, many faces: The underappreciated importance of strain diversity. PLoS Pathog. 2024, 20, e1011931. [Google Scholar] [CrossRef]
- Sposito, L.; Oda, F.B.; Vieira, J.H.; Carvalho, F.A.; Dos Santos Ramos, M.A.; de Castro, R.C.; Crevelin, E.J.; Crotti, A.E.M.; Santos, A.G.; da Silva, P.B.; et al. In vitro and in vivo anti-Helicobacter pylori activity of Casearia sylvestris leaf derivatives. J. Ethnopharmacol. 2019, 233, 1–12. [Google Scholar] [CrossRef]
- Sandberg, M.; Maattanen, A.; Peltonen, J.; Vuorela, P.M.; Fallarero, A. Automating a 96-well microtitre plate model for Staphylococcus aureus biofilms: An approach to screening of natural antimicrobial compounds. Int. J. Antimicrob. Agents 2008, 32, 233–240. [Google Scholar] [CrossRef]
- Polonio, R.E.; Mermel, L.A.; Paquette, G.E.; Sperry, J.F. Eradication of biofilm-forming Staphylococcus epidermidis (RP62A) by a combination of sodium salicylate and vancomycin. Antimicrob. Agents Chemother. 2001, 45, 3262–3266. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, M.B.; Tanimoto, M.H.; Ambrosio, M.A.L.V.; Veneziani, R.C.S.; Bastos, J.K.; Sabino-Silva, R.; Martins, C.H.G. The Antibacterial Potential of Brazilian Red Propolis against the Formation and Eradication of Biofilm of Helicobacter pylori. Antibiotics 2024, 13, 719. https://doi.org/10.3390/antibiotics13080719
Santiago MB, Tanimoto MH, Ambrosio MALV, Veneziani RCS, Bastos JK, Sabino-Silva R, Martins CHG. The Antibacterial Potential of Brazilian Red Propolis against the Formation and Eradication of Biofilm of Helicobacter pylori. Antibiotics. 2024; 13(8):719. https://doi.org/10.3390/antibiotics13080719
Chicago/Turabian StyleSantiago, Mariana B., Matheus H. Tanimoto, Maria Anita L. V. Ambrosio, Rodrigo Cassio S. Veneziani, Jairo K. Bastos, Robinson Sabino-Silva, and Carlos Henrique G. Martins. 2024. "The Antibacterial Potential of Brazilian Red Propolis against the Formation and Eradication of Biofilm of Helicobacter pylori" Antibiotics 13, no. 8: 719. https://doi.org/10.3390/antibiotics13080719
APA StyleSantiago, M. B., Tanimoto, M. H., Ambrosio, M. A. L. V., Veneziani, R. C. S., Bastos, J. K., Sabino-Silva, R., & Martins, C. H. G. (2024). The Antibacterial Potential of Brazilian Red Propolis against the Formation and Eradication of Biofilm of Helicobacter pylori. Antibiotics, 13(8), 719. https://doi.org/10.3390/antibiotics13080719





