Antimicrobial and Hemostatic Diatom Biosilica Composite Sponge
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of the Antimicrobial Activity of Antibiotics before and after Soaking in Diatom Biosilica
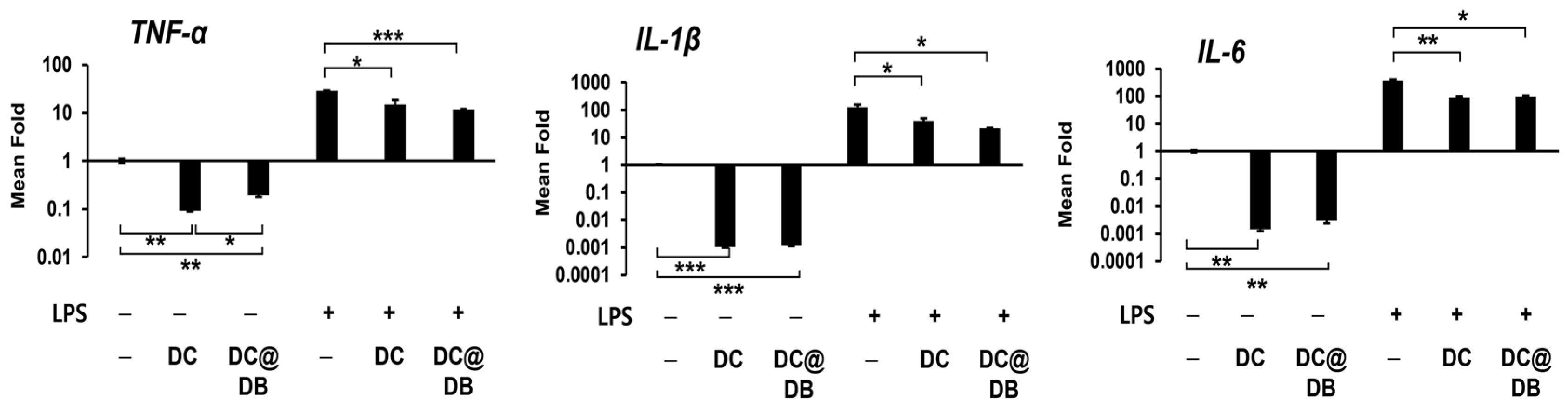
2.2. Anti-Inflammatory Effect of DC and DC@DB on Lipopolysaccharide (LPS)-Induced Inflammation
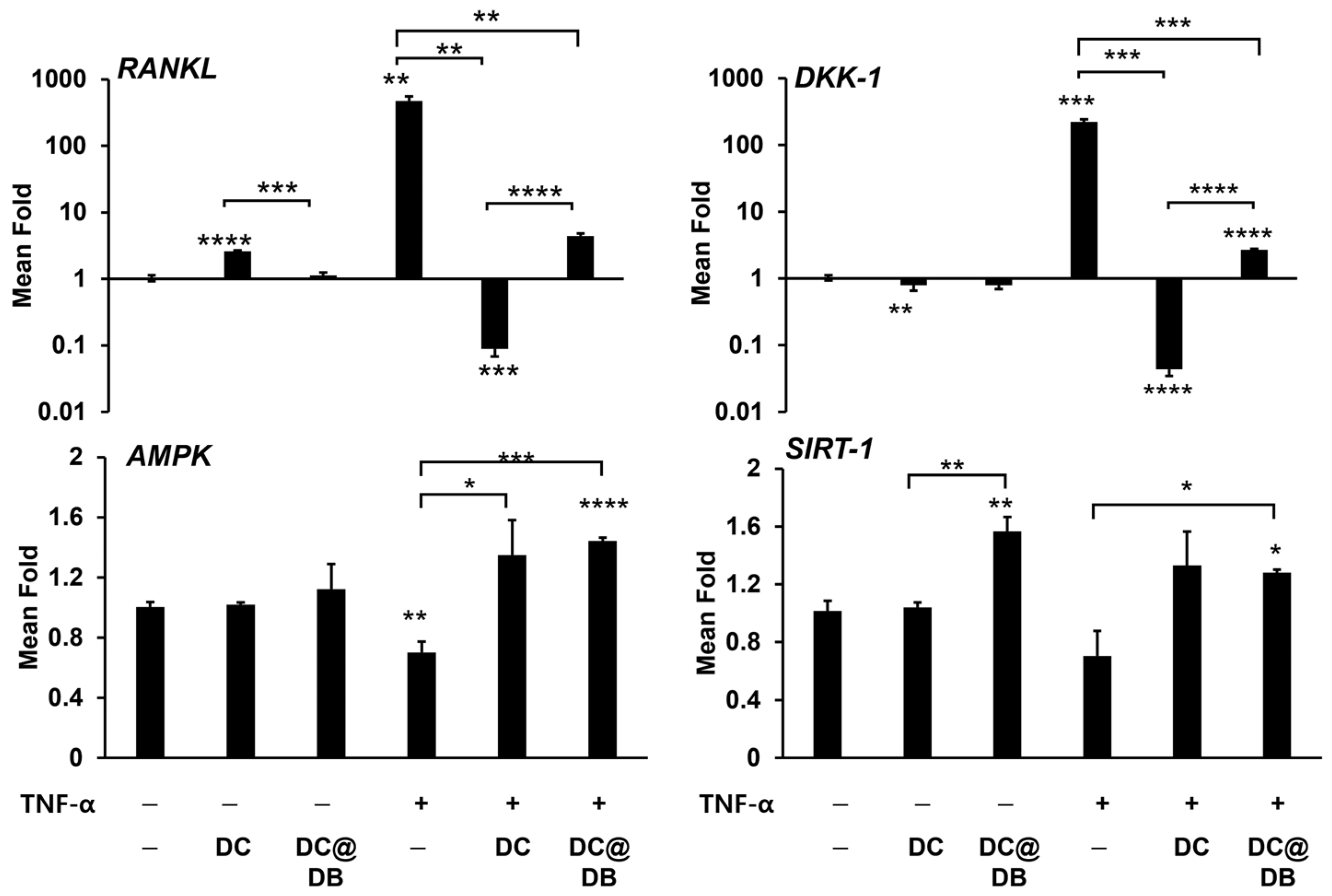
2.3. Effects of DC and DC@DB on TNF-α Exposed Osteoblasts
2.4. Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy (ATR−FTIR) of Gelatin-DB-DC Composites
2.5. Hemolysis and Hemostatic Ability
2.6. Comparison of Antibiotic Release Patterns and Antimicrobial Activity of Sponge Composites
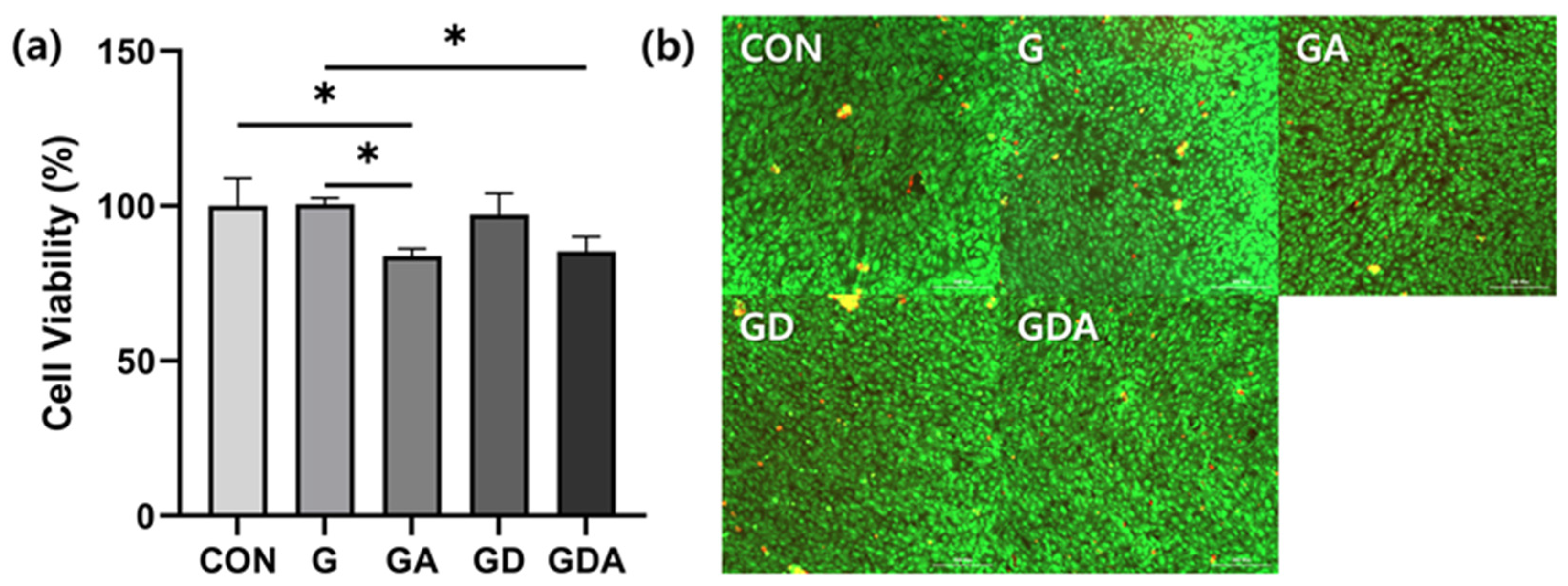
2.7. Biocompatibility of Sponge Composites
3. Materials and Methods
3.1. Materials
3.2. Bacterial Strains
3.3. Preparation of Diatom Biosilica
3.4. Minimum Inhibitory Concentration (MIC) of Antibiotics
3.5. Measurement of Loading Efficiency of Antibiotics In Silica Particles (SP) or Diatom Biosilica (DB)
3.6. Fabrication of Gelatin-DB-Antibiotics Composite Sponge (GDA)
3.7. Cytotoxicity
3.8. Hemocompatibility
3.9. Hemostatic Assay
3.10. Comparison of Antibiotic Release Patterns and Antimicrobial Activity of Sponge Composites
3.11. qRT-PCR for mRNA Expression
3.12. ATR−FTIR
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.-Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature engineered diatom biosilica as drug delivery systems. J. Control Release 2018, 281, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, R.; Iqbal, K.; Tyagi, J.; Joshi, N.C.; Shukla, S.; Shukla, K.; Varma, A.; Mishra, A. Diatoms in Biomedicines and Nanomedicines. In Insights into the World of Diatoms: From Essentials to Applications; Springer: Singapore, 2023; pp. 195–210. [Google Scholar]
- Kurkuri, M.; Losic, D.; Uthappa, U.T.; Jung, H.-Y. Advanced Porous Biomaterials for Drug Delivery Applications; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Hussein, H.A.; Nazir, M.S.; Azra, N.; Qamar, Z.; Seeni, A.; Tengku Din, T.; Abdullah, M.A. Novel Drug and Gene Delivery System and Imaging Agent Based on Marine Diatom Biosilica Nanoparticles. Mar. Drugs 2022, 20, 480. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, D.H.; Youn, S.; Pack, S.P. Biomimetic Diatom Biosilica and Its Potential for Biomedical Applications and Prospects: A Review. Int. J. Mol. Sci. 2024, 25, 2023. [Google Scholar] [CrossRef]
- Dhanker, R.; Singh, P.; Sharma, D.; Tyagi, P.; Kumar, M.; Singh, R.; Prakash, S. Diatom Silica a Potential Tool as Biosensors and for Biomedical Field. In Insights into the World of Diatoms: From Essentials to Applications; Springer: Singapore, 2023; pp. 175–193. [Google Scholar]
- Narkhedkar, V.; Bramhanwade, K. Recent Advances in Biomedicine: Diatomaceous Applications. In Insights into the World of Diatoms: From Essentials to Applications; Springer: Singapore, 2023; pp. 211–224. [Google Scholar]
- Vasani, R.B.; Losic, D.; Cavallaro, A.; Voelcker, N.H. Fabrication of stimulus-responsive diatom biosilica microcapsules for antibiotic drug delivery. J. Mater. Chem. B 2015, 3, 4325–4329. [Google Scholar] [CrossRef]
- Liu, H.; Qiao, Z.; Jang, Y.O.; Kim, M.G.; Zou, Q.; Lee, H.J.; Koo, B.; Kim, S.H.; Yun, K.; Kim, H.S.; et al. Diatomaceous earth/zinc oxide micro-composite assisted antibiotics in fungal therapy. Nano Converg. 2021, 8, 32. [Google Scholar] [CrossRef]
- Lodi, G.; Azzi, L.; Varoni, E.M.; Pentenero, M.; Del Fabbro, M.; Carrassi, A.; Sardella, A.; Manfredi, M. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst. Rev. 2021, 2, CD003811. [Google Scholar] [PubMed]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef]
- Toure, B.; Faye, B.; Kane, A.W.; Lo, C.M.; Niang, B.; Boucher, Y. Analysis of reasons for extraction of endodontically treated teeth: A prospective study. J. Endod. 2011, 37, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Dodson, T.B.; Susarla, S.M. Impacted wisdom teeth. BMJ Clin. Evid. 2014, 2014, 1302. [Google Scholar]
- Mahardawi, B.; Jiaranuchart, S.; Rochanavibhata, S.; Arunjaroensuk, S.; Mattheos, N.; Pimkhaokham, A. The role of hemostatic agents after tooth extractions: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2023, 154, 742–752.e1. [Google Scholar] [CrossRef]
- Suleiman, A.M. Influence of Surgicel gauze on the incidence of dry socket after wisdom tooth extraction. East. Mediterr. Health J. 2006, 12, 440–445. [Google Scholar] [PubMed]
- Radhakrishna, S.; Shukla, V.; Shetty, S.K. Is chitosan dental dressing better than cotton gauze in achieving hemostasis in patients on antithrombotics? J. Oral. Maxillofac. Surg. 2023, 81, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jeong, D.; Ki, M.-R.; Pack, S.P.; Choi, Y.S. Tyrosinase-mediated rapid and permanent chitosan/gelatin and chitosan/gelatin/nanohydroxyapatite hydrogel. Korean J. Chem. Eng. 2021, 38, 98–103. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-based biomaterials and gelatin as an additive for chronic wound repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.A.; Shin, M.; Juang, L.J.; Kastrup, C.J.; Go, G.M.; Lee, H. Diatom Frustule Silica Exhibits Superhydrophilicity and Superhemophilicity. ACS Nano 2020, 14, 4755–4766. [Google Scholar] [CrossRef] [PubMed]
- Daneshi, M.; Rashidpanah, J.; Narouei, F. An Overview of Hemostasis. In Congenital Bleeding Disorders; Springer: Cham, Switzerland, 2023; pp. 3–27. [Google Scholar]
- Youn, S.; Ki, M.R.; Abdelhamid, M.A.A.; Pack, S.P. Biomimetic Materials for Skin Tissue Regeneration and Electronic Skin. Biomimetics 2024, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Rozan, H.E.; Wu, G.; Zhou, Z.; Li, Q.; Sharaf, M.; Chen, X. The complex hydrogel based on diatom biosilica and hydroxybutyl chitosan for wound healing. Colloids Surf. B Biointerfaces 2022, 216, 112523. [Google Scholar] [CrossRef]
- Lecio, G.; Ribeiro, F.V.; Pimentel, S.P.; Reis, A.A.; da Silva, R.V.C.; Nociti-Jr, F.; Moura, L.; Duek, E.; Casati, M.; Casarin, R.C.V. Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: Randomized clinical, immune and microbiological trial. Clin. Oral Investig. 2019, 24, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.O.; Ceballos, G.; Villarreal, F.J. Tetracycline compounds with non-antimicrobial organ protective properties: Possible mechanisms of action. Pharmacol. Res. 2011, 63, 102–107. [Google Scholar] [CrossRef]
- Walter, M.S.; Frank, M.J.; Satue, M.; Monjo, M.; Ronold, H.J.; Lyngstadaas, S.P.; Haugen, H.J. Bioactive implant surface with electrochemically bound doxycycline promotes bone formation markers in vitro and in vivo. Dent. Mater. 2014, 30, 200–214. [Google Scholar] [CrossRef]
- Emingil, G.; Gurkan, A.; Tervahartiala, T.; Hernandez, M.; Ozgul, S.; Sorsa, T.; Alassiri, S. Adjunctive Effects of a Sub-Antimicrobial Dose of Doxycycline on Clinical Parameters and Potential Biomarkers of Periodontal Tissue Catabolism. Dent. J. 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Fernandes, M.H. Effect of therapeutic levels of doxycycline and minocycline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch. Oral Biol. 2007, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, K.; Arampatzis, A.S.; Liasi, G.; Tsalikis, L.; Barmpalexis, P.; Christofilos, D.; Assimopoulou, A.N. 3D-Printed Antibacterial Scaffolds for the Regeneration of Alveolar Bone in Severe Periodontitis. Int. J. Mol. Sci. 2023, 24, 16754. [Google Scholar] [CrossRef] [PubMed]
- Okic Dordevic, I.; Kukolj, T.; Zivanovic, M.; Momcilovic, S.; Obradovic, H.; Petrovic, A.; Mojsilovic, S.; Trivanovic, D.; Jaukovic, A. The Role of Doxycycline and IL-17 in Regenerative Potential of Periodontal Ligament Stem Cells: Implications in Periodontitis. Biomolecules 2023, 13, 1437. [Google Scholar] [CrossRef]
- Martin, V.; Bettencourt, A.F.; Santos, C.; Fernandes, M.H.; Gomes, P.S. Unveiling the Osteogenic Potential of Tetracyclines: A Comparative Study in Human Mesenchymal Stem Cells. Cells 2023, 12, 2244. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Keshavarz Moraveji, M. Microfluidic assisted synthesis of PLGA drug delivery systems. RSC Adv. 2019, 9, 2055–2072. [Google Scholar] [CrossRef]
- Ali, M.; Walboomers, X.F.; Jansen, J.A.; Yang, F. Influence of formulation parameters on encapsulation of doxycycline in PLGA microspheres prepared by double emulsion technique for the treatment of periodontitis. J. Drug Deliv. Sci. Technol. 2019, 52, 263–271. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Akindoyo, J.O.; Jaafar, M.; Hamid, Z.A.A. Effect of Formulation Variables on the Performance of Doxycycline-Loaded PLA Microsphere. Arab. J. Sci. Eng. 2020, 45, 7419–7428. [Google Scholar] [CrossRef]
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29083794 (accessed on 2 June 2024).
- Chaves, B.J.; Tadi, P. Gentamicin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557550/ (accessed on 2 June 2024).
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Patel, R.S.; Parmar, M. Doxycycline Hyclate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555888/ (accessed on 2 June 2024).
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef]
- Gonzalez-Lizarraga, F.; Socias, S.B.; Avila, C.L.; Torres-Bugeau, C.M.; Barbosa, L.R.; Binolfi, A.; Sepulveda-Diaz, J.E.; Del-Bel, E.; Fernandez, C.O.; Papy-Garcia, D.; et al. Repurposing doxycycline for synucleinopathies: Remodelling of alpha-synuclein oligomers towards non-toxic parallel beta-sheet structured species. Sci. Rep. 2017, 7, 41755. [Google Scholar] [CrossRef]
- Jamzad, S.; Fassihi, R. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide—A technical note. AAPS PharmSciTech 2006, 7, E33. [Google Scholar] [CrossRef]
- DRUGBANK Gentamycin Sulfate. Available online: https://go.drugbank.com/salts/DBSALT000690 (accessed on 27 June 2024).
- DRUGBANK Doxycycline HCl. Available online: https://go.drugbank.com/salts/DBSALT000896 (accessed on 27 June 2024).
- Shariati, S.; Yamini, Y.; Esrafili, A. Carrier mediated hollow fiber liquid phase microextraction combined with HPLC-UV for preconcentration and determination of some tetracycline antibiotics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 393–400. [Google Scholar] [CrossRef]
- Andrade, C.A.; Zambrano-Intriago, L.A.; Oliveira, N.S.; Vieira, J.S.; Quiroz-Fernández, L.S.; Rodríguez-Díaz, J.M. Adsorption Behavior and Mechanism of Oxytetracycline on Rice Husk Ash: Kinetics, Equilibrium, and Thermodynamics of the Process. Water Air Soil. Pollut. 2020, 231, 103. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Son, R.G.; Park, K.S.; Pack, S.P. Oriented multivalent silaffin-affinity immobilization of recombinant lipase on diatom surface: Reliable loading and high performance of biocatalyst. Colloids Surf. B Biointerfaces 2022, 219, 112830. [Google Scholar] [CrossRef]
- Frew, A. General principles of investigating and managing drug allergy. Br. J. Clin. Pharmacol. 2011, 71, 642–646. [Google Scholar] [CrossRef]
- Ki, M.-R.; Kim, S.H.; Rho, S.; Kim, J.K.; Min, K.H.; Yeo, K.B.; Lee, J.; Lee, G.; Jun, S.-H.; Pack, S.P. Use of biosilica to improve loading and delivery of bone morphogenic protein 2. Int. J. Biol. Macromol. 2024, 254, 127876. [Google Scholar] [CrossRef]
- Spasovski, S.; Belazelkoska, Z.; Popovska, M.; Atanasovska-Stojanovska, A.; Radojkova-Nikolovska, V.; Muratovska, I.; Toseska-Spasova, N.; Dzipunova, B.; Nikolovski, B. Clinical Therapeutic Effects of the Application of Doxycycline in the Treatment of Periodontal Disease. Open Access Maced. J. Med. Sci. 2016, 4, 152–157. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-alpha Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; Papadia, F.; Tummolo, A.; Fischetto, R.; Nicastro, F.; Piacente, L.; Ventura, A.; Mori, G.; Oranger, A.; Gigante, I.; et al. Impaired bone remodeling in children with osteogenesis imperfecta treated and untreated with bisphosphonates: The role of DKK1, RANKL, and TNF-alpha. Osteoporos. Int. 2016, 27, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Tarasiuk, O.; Miceli, M.; Di Domizio, A.; Nicolini, G. AMPK and Diseases: State of the Art Regulation by AMPK-Targeting Molecules. Biology 2022, 11, 1041. [Google Scholar] [CrossRef]
- Kewalramani, G.; Puthanveetil, P.; Wang, F.; Kim, M.S.; Deppe, S.; Abrahani, A.; Luciani, D.S.; Johnson, J.D.; Rodrigues, B. AMP-activated protein kinase confers protection against TNF-alpha-induced cardiac cell death. Cardiovasc. Res. 2009, 84, 42–53. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, M.Y.; Song, E.K.; Kim, E.K.; Moon, W.S.; Han, M.K.; Park, J.W.; Kwon, K.B.; Park, B.H. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes 2009, 58, 344–351. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Mukherjee, S.; Chatterjee, S.K.; Chattopadhyay, D.; Das, S.; Majumdar, S.S.; Mukhopadhyay, S.; Mukherjee, S.; Bhattarcharya, S. Impairment of energy sensors, SIRT1 and AMPK, in lipid induced inflamed adipocyte is regulated by Fetuin A. Cell Signal 2018, 42, 67–76. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Uthaiwat, P.; Priprem, A.; Puthongking, P.; Daduang, J.; Nukulkit, C.; Chio-Srichan, S.; Boonsiri, P.; Thapphasaraphong, S. Characteristic Evaluation of Gel Formulation Containing Niosomes of Melatonin or Its Derivative and Mucoadhesive Properties Using ATR-FTIR Spectroscopy. Polymers 2021, 13, 1142. [Google Scholar] [CrossRef]
- Nematidil, N.; Nezami, S.; Mirzaie, F.; Ebrahimi, E.; Sadeghi, M.; Farmani, N.; Sadeghi, H. Fabrication and characterization of a novel nanoporous nanoaerogel based on gelatin as a biosorbent for removing heavy metal ions. J. Solgel Sci. Technol. 2021, 97, 721–733. [Google Scholar] [CrossRef]
- Sæbø, I.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef]
- Cheng, H.; Pan, X.; Shi, Z.; Huang, X.; Zhong, Q.; Liu, H.; Chen, Y.; Lian, Q.; Wang, J.; Shi, Z. Chitin/corn stalk pith sponge stimulated hemostasis with erythrocyte absorption, platelet activation, and Ca(2+)-binding capabilities. Carbohydr. Polym. 2022, 284, 118953. [Google Scholar] [CrossRef]
- Su, C.; Jiang, C.; Sun, X.; Cao, Z.; Mu, Y.; Cong, X.; Qiu, K.; Lin, J.; Chen, X.; Feng, C. Diatomite hemostatic particles with hierarchical porous structure for rapid and effective hemostasis. Colloids Surf. B Biointerfaces 2022, 219, 112809. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Ghavimi, M.A.; Maleki Dizaj, S.; Sharifi, S.; Sajjadi, S.S.; Jamei Khosroshahi, A.R. Efficacy of a New Hemostatic Dental Sponge in Controlling Bleeding, Pain, and Dry Socket Following Mandibular Posterior Teeth Extraction-A Split-Mouth Randomized Double-Blind Clinical Trial. J. Clin. Med. 2023, 12, 4578. [Google Scholar] [CrossRef]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.A.; Makila, E.M.; da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef]
- Delasoie, J.; Zobi, F. Natural diatom biosilica as microshuttles in drug delivery systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef]
- Mhase, P.P. Cytotoxicity assessment of nanoparticulate doxycycline (DH LNP) and rifampicin against human macrophage (U 937). Int. J. Pure Appl. Biosci. 2018, 6, 921–929. [Google Scholar] [CrossRef]
- Gruber, S.; Nickel, A. Toxic or not toxic? The specifications of the standard ISO 10993-5 are not explicit enough to yield comparable results in the cytotoxicity assessment of an identical medical device. Front. Med. Technol. 2023, 5, 1195529. [Google Scholar] [CrossRef]
- Molecular Probes. Manual: Live/Dead Viability/Cytotoxicity Kit for Mammalian Cells. Catalog Number L3224. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp03224.pdf (accessed on 18 July 2024).
- Min, K.H.; Shin, J.W.; Ki, M.-R.; Pack, S.P. Green synthesis of silver nanoparticles on biosilica diatomite: Well-dispersed particle formation and reusability. Process Biochem. 2023, 125, 232–238. [Google Scholar] [CrossRef]
- Ki, M.R.; Kim, S.H.; Park, T.I.; Pack, S.P. Self-Entrapment of Antimicrobial Peptides in Silica Particles for Stable and Effective Antimicrobial Peptide Delivery System. Int. J. Mol. Sci. 2023, 24, 16423. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- Zuluaga, A.F.; Agudelo, M.; Rodriguez, C.A.; Vesga, O. Application of microbiological assay to determine pharmaceutical equivalence of generic intravenous antibiotics. BMC Clin. Pharmacol. 2009, 9, 1. [Google Scholar] [CrossRef]
- Ki, M.R.; Kim, S.H.; Nguyen, T.K.M.; Son, R.G.; Jun, S.H.; Pack, S.P. BMP2-Mediated Silica Deposition: An Effective Strategy for Bone Mineralization. ACS Biomater. Sci. Eng. 2023, 9, 1823–1833. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lee, S.; Lee, T.A.; Song, S.J.; Park, T.; Park, B. Hyperproduction of IL-6 caused by aberrant TDP-43 overexpression in high-fat diet-induced obese mice. FEBS Lett. 2015, 589, 1825–1831. [Google Scholar] [CrossRef]
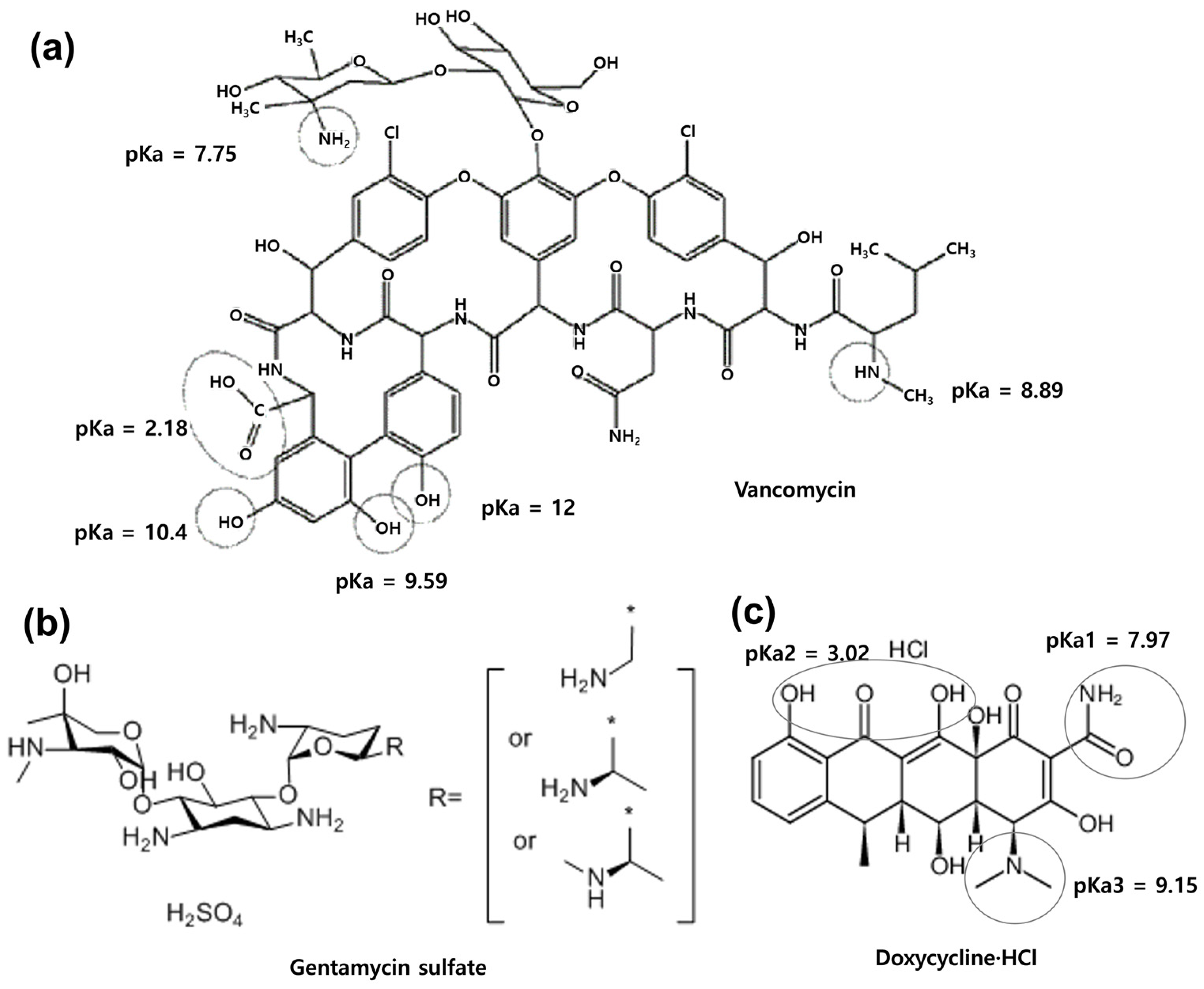



| Initial Antibiotics (μg) | Adsorbed Antibiotics (μg) | DB Matrix (mg) | LE a (%) | AE b (%) | |
|---|---|---|---|---|---|
| VM@SP | 300 | 53.27 ± 8.70 | 1 | 5.05 ± 0.45 | 17.75 ± 2.88 |
| VM@DB | 300 | 180.08 ± 2.24 | 1 | 15.26 ± 0.09 | 60.03 ± 0.75 |
| GEN@DB | 300 | 271.88 ± 9.38 | 1 | 21.38 ± 0.33 | 90.63 ± 3.13 |
| DC@DB | 300 | 277.29 ± 18.83 | 1 | 21.70 ± 0.66 | 92.43 ± 6.28 |
| Antibiotics | E. coli | a p Value | S. aureus | a p Value |
|---|---|---|---|---|
| VM | 115.22 ± 51.53 | 3.75 ± 1.26 | ||
| VM@DB | 56.25 ± 15.73 | 0.085 | 4.89 ± 2.05 | 0.328 |
| GEN | 0.60 ± 0.04 | 0.48 ± 0.11 | ||
| GEN@DB | 4.36 ± 0.20 | >0.001 | 2.18 ± 0.10 | >0.001 |
| DC | 1.48 ± 0.31 | 0.09 ± 0.02 | ||
| DC@DB | 7.94 ± 0.54 | >0.001 | 0.34 ± 0.12 | 0.100 |
| Sponge Sample | 2H | 4H | 8H | 16H | 32H | 64H |
|---|---|---|---|---|---|---|
| GA | 9.83 ± 0.02 | 8.74 ± 0.06 | 8.41 ± 1.14 | 8.03 ± 0.03 | 5.54 ± 0.32 | 4.06 ± 0.56 |
| GDA | 8.32 ± 0.56 | 7.53 ± 0.36 | 8.03 ± 0.24 | 7.69 ± 0.13 | 7.70 ± 0.50 | 7.14 ± 0.53 |
| Sponge Sample | 2H | 4H | 8H | 16H | 32H | 64H |
|---|---|---|---|---|---|---|
| GA | 11.66 ± 0.68 | 12.89 ± 0.35 | 13.40 ± 0.57 | 12.41 ± 0.37 | 10.82 ± 0.48 | 9.55 ± 0.07 |
| GDA | 9.74 ± 0.32 | 11.51 ± 0.29 | 11.56 ± 0.32 | 11.21 ± 0.32 | 11.87 ± 0.41 | 11.30 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, S.; Ki, M.-R.; Min, K.H.; Abdelhamid, M.A.A.; Pack, S.P. Antimicrobial and Hemostatic Diatom Biosilica Composite Sponge. Antibiotics 2024, 13, 714. https://doi.org/10.3390/antibiotics13080714
Youn S, Ki M-R, Min KH, Abdelhamid MAA, Pack SP. Antimicrobial and Hemostatic Diatom Biosilica Composite Sponge. Antibiotics. 2024; 13(8):714. https://doi.org/10.3390/antibiotics13080714
Chicago/Turabian StyleYoun, Sol, Mi-Ran Ki, Ki Ha Min, Mohamed A. A. Abdelhamid, and Seung Pil Pack. 2024. "Antimicrobial and Hemostatic Diatom Biosilica Composite Sponge" Antibiotics 13, no. 8: 714. https://doi.org/10.3390/antibiotics13080714
APA StyleYoun, S., Ki, M.-R., Min, K. H., Abdelhamid, M. A. A., & Pack, S. P. (2024). Antimicrobial and Hemostatic Diatom Biosilica Composite Sponge. Antibiotics, 13(8), 714. https://doi.org/10.3390/antibiotics13080714







