Clinical Outcomes of Patients with AmpC-Beta-Lactamase-Producing Enterobacterales Bacteremia Treated with Carbapenems versus Non-Carbapenem Regimens: A Single-Center Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaubey, V.P.; Pitout, J.D.D.; Dalton, B.; Gregson, D.B.; Ross, T.; Laupland, K.B. Clinical and microbiological characteristics of bloodstream infections due to AmpC β-lactamase producing Enterobacteriaceae: An active surveillance cohort in a large centralized Canadian region. BMC Infect. Dis. 2014, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Nelson, B.C.; Mehta, M.; Seval, N.; Park, S.; Giddins, M.J.; Shi, Q.; Whittier, S.; Gomez-Simmonds, A.; Uhlemann, A.C. Piperacillin-Tazobactam versus other antibacterial agents for treatment of bloodstream infections due to AmpC β-Lactamase-producing enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Mol, P.R.; Bindayna, K.M.; Shanthi, G. Evaluation of Two Phenotypic Methods for the Detection of Plasmid-Mediated AmpC β-Lactamases among Enterobacteriaceae Isolates. J. Lab. Physicians 2021, 13, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Doi, Y.; Bonomo, R.A.; Johnson, J.K.; Simner, P.J. A Primer on AmpC β-Lactamases: Necessary Knowledge for an Increasingly Multidrug-resistant World. Clin. Infect. Dis. 2019, 69, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.A.; Sanders, C.C. Diverse potential of β-lactamase inhibitors to induce class I enzymes. Antimicrob. Agents Chemother. 1990, 34, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, R.; Bähr, T.; Gatermann, S.G. Species-specific mutation rates for ampC derepression in Enterobacterales with chromosomally encoded inducible AmpC β-lactamase. J. Antimicrob. Chemother. 2018, 73, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. AmpC Β-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Tamma, P.D.; Girdwood, S.C.T.; Gopaul, R.; Tekle, T.; Roberts, A.A.; Harris, A.D.; Cosgrove, S.E.; Carroll, K.C. The use of cefepime for treating AmpC β-lactamase-producing enterobacteriaceae. Clin. Infect. Dis. 2013, 57, 781–788. [Google Scholar] [CrossRef]
- Dangelo, R.G.; Johnson, J.K.; Bork, J.T.; Heil, E.L. Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert. Opin. Pharmacother. 2016, 17, 953–967. [Google Scholar] [CrossRef]
- Herrmann, L.; Kimmig, A.; Rödel, J.; Hagel, S.; Rose, N.; Pletz, M.W.; Bahrs, C. Early treatment outcomes for bloodstream infections caused by potential ampc beta-lactamase-producing enterobacterales with focus on piperacillin/tazobactam: A retrospective cohort study. Antibiotics 2021, 10, 665. [Google Scholar] [CrossRef]
- Meini, S.; Tascini, C.; Cei, M.; Sozio, E.; Rossolini, G.M. AmpC β-lactamase-producing Enterobacterales: What a clinician should know. Infection 2019, 47, 363–375. [Google Scholar] [CrossRef]
- Stewart, A.G.; Paterson, D.L.; Young, B.; Lye, D.C.; Davis, J.S.; Schneider, K.; Yilmaz, M.; Dinleyici, R.; Runnegar, N.; Henderson, A.; et al. Meropenem Versus Piperacillin-Tazobactam for Definitive Treatment of Bloodstream Infections Caused by AmpC β-Lactamase-Producing Enterobacter spp., Citrobacter freundii, Morganella morganii, Providencia spp., or Serratia marcescens: A Pilot Multicenter Randomized Controlled Trial (MERINO-2). Open Forum Infect. Dis. 2021, 8, ofab387. [Google Scholar]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis. 2023, ciad428. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L.; Spelman, D. Mortality impact of empirical antimicrobial therapy in ESBL- and AmpC-producing Enterobacteriaceae bacteremia in an Australian tertiary hospital. Infect. Dis. Health 2019, 24, 124–133. [Google Scholar] [CrossRef]
- Chow, J.W.; Fine, M.J.; Shlaes, D.M.; Quinn, J.P.; Hooper, D.C.; Johnson, M.P.; Ramphal, R.; Wagener, M.M.; Miyashiro, D.K.; Yu, V.L. Enterobacter bacteremia: Clinical features and emergence of antibiotic resistance during therapy. Ann. Intern. Med. 1991, 115, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.I.; Pai, H.; Kim, S.H.; Kim, H.B.; Kim, E.C.; Oh, M.D.; Choe, K.W. Cefepime and the inoculum effect in tests with Klebsiella pneumoniae producing plasmid-mediated AmpC-type β-lactamase. J. Antimicrob. Chemother. 2004, 54, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.; Dortet, L.; Delory, T.; Lafaurie, M.; Bleibtreu, A.; group for the T of A producing E study. Mutation rate of AmpC-β-lactamase-producing Enterobacterales and treatment in clinical practice: A word of caution. Clin. Infect. Dis. 2024, 79, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Núñez, M.; Lima, O.; Sousa, A.; Represa, M.; Rubiñán, P.; Celestino, P.; Garrido-Ventín, M.; García-Formoso, L.; Vasallo-Vidal, F.; Martinez-Lamas, L.; et al. Carbapenem alternatives for treatment of bloodstream infections due to AmpC producing enterobacterales. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 75. [Google Scholar] [CrossRef]
- Nisly, S.A.; McClain, D.L.; Fillius, A.G.; Davis, K.A. Oral antibiotics for the treatment of Gram-negative bloodstream infections: A retrospective comparison of three antibiotic classes. J. Glob. Antimicrob. Resist. 2020, 20, 74–77. [Google Scholar] [CrossRef]
- Tamma, P.D.; Conley, A.T.; Cosgrove, S.E.; Harris, A.D.; Lautenbach, E.; Amoah, J.; Avdic, E.; Tolomeo, P.; Wise, J.; Subudhi, S.; et al. Association of 30-Day Mortality with Oral Step-Down vs. Continued Intravenous Therapy in Patients Hospitalized with Enterobacteriaceae bacteremia. JAMA Intern. Med. 2019, 179, 316–323. [Google Scholar] [CrossRef]
- Gunter, S.G.; Barber, K.E.; Wagner, J.L.; Stover, K.R. Fluoroquinolone versus nonfluoroquinolone treatment of bloodstream infections caused by chromosomally mediated ampc-producing enterobacteriaceae. Antibiotics 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Ng, T.M.; Chew, K.L.; Yong, J.; Wu, J.E.; Yap, M.Y.; Heng, S.T.; Ng, W.H.W.; Wan, S.; Cheok, S.J.H.; et al. Outcomes of treating AmpC-producing Enterobacterales bacteraemia with carbapenems vs. non-carbapenems. Int. J. Antimicrob. Agents 2020, 55, 105860. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikov, J.; Huang, A.; Zeeck, K.; Gibble, A. Comparison of outcomes in urinary tract infections caused by AmpC-harboring organisms treated with AmpC stable versus AmpC susceptible agents. Diagn. Microbiol. Infect. Dis. 2021, 101, 115472. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Guglielmo, B.J. Diagnosis and treatment of extended-spectrum and AmpC beta-lactamase-producing organisms. Ann. Pharmacother. 2007, 41, 1427–1435. [Google Scholar] [CrossRef]
- Henderson, A.; Paterson, D.L.; Chatfield, M.D.; Tambyah, P.A.; Lye, D.C.; De, P.P.; Lin, R.T.P.; Chew, K.L.; Yin, M.; Lee, T.H.; et al. Association Between Minimum Inhibitory Concentration, Beta-lactamase Genes and Mortality for Patients Treated with Piperacillin/Tazobactam or Meropenem from the MERINO Study. Clin. Infect. Dis. 2021, 73, e3842-50. [Google Scholar] [CrossRef]
- Martinez, L.; Simonsen, G.S. The European Committee on Antimicrobial Susceptibility Testing. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. 2017, pp. 1–43. Available online: http://www.eucast.org/resistance_mechanisms/ (accessed on 1 June 2024).
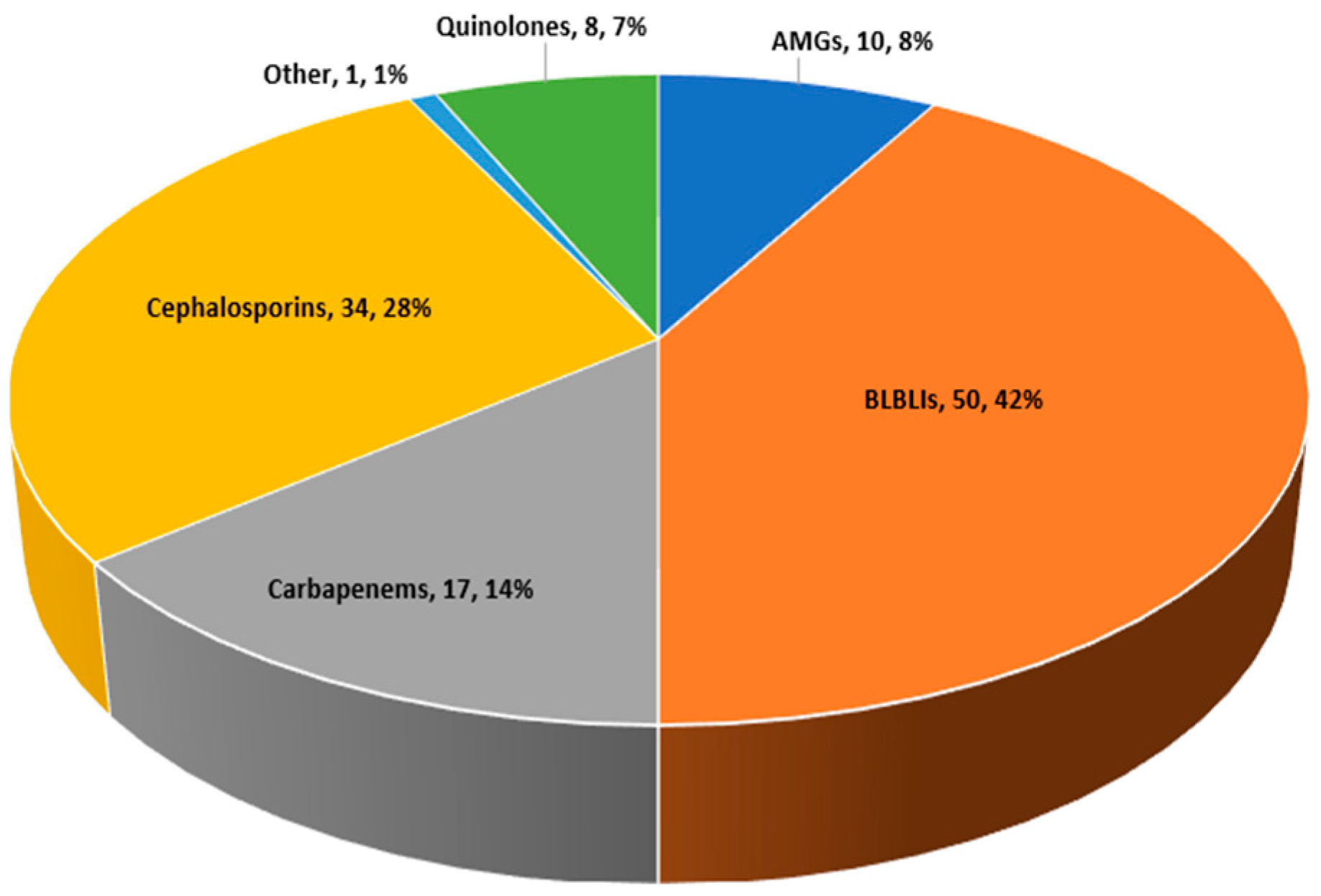
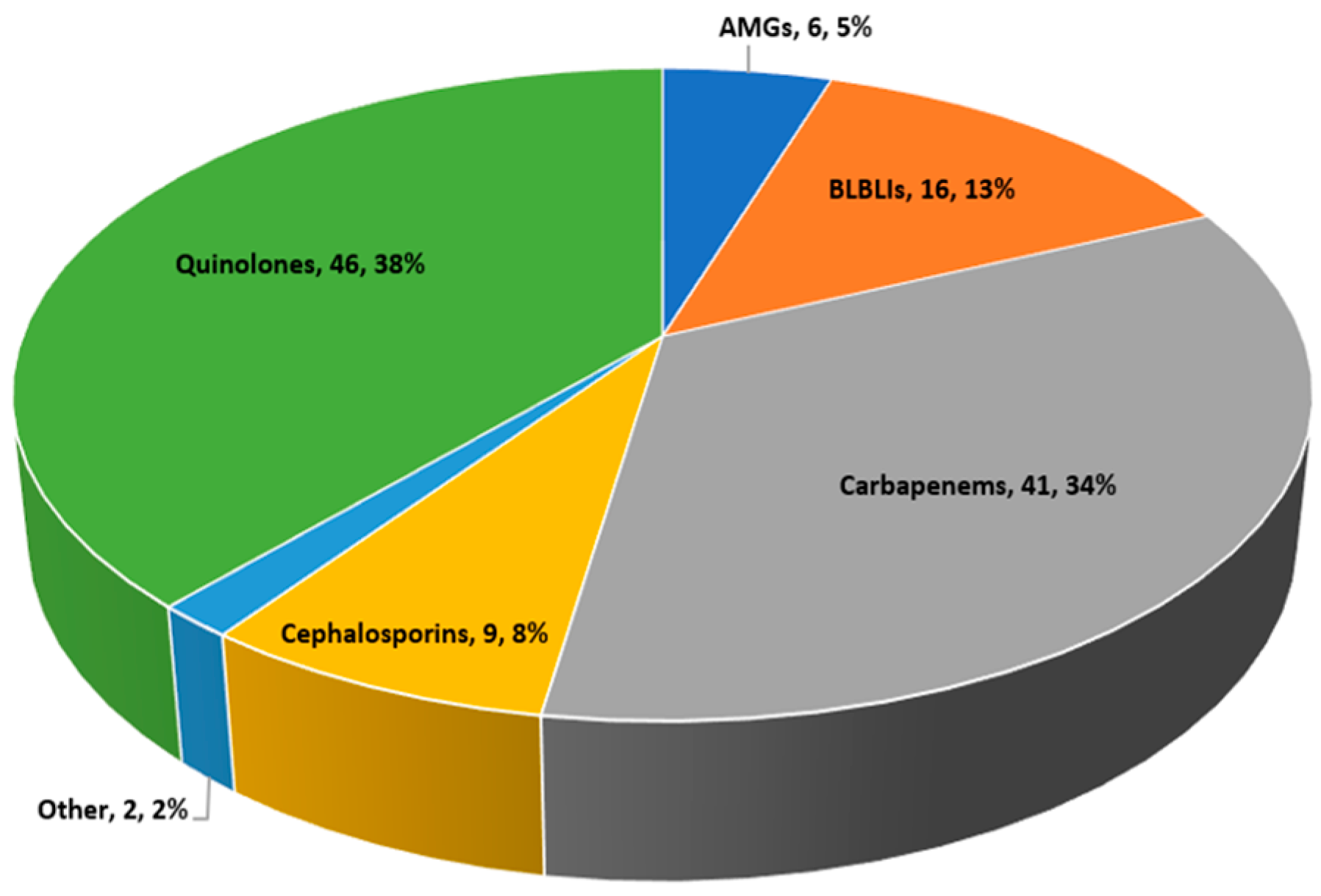
| Parameter | All Patients (n = 120) | Empiric Carbapenems, n = 17 | Empiric Non-Carbapenem Regimens, n = 103 | p Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 72.3 ± 14.9 | 67.5 ± 15.1 | 73.1 ± 14.8 | 0.15 |
| Female, n (%) | 50 (41.7) | 9 (53) | 41 (40) | 0.4 |
| Basic metabolic index (BMI) a, mean | 26.6 ± 5.2 | 25.2 ± 4 | 26.9 ± 5.3 | 0.2 |
| Residence, n (%) Home/assisted living care home Nursing home | 103 (85.8) 17 (14.1) | 14 (82.4) 3 (17.7) | 89 (86.4) 14 (13.6) | 0.7 |
| Department Medical Surgical Critical care | 95 (79.2) 19 (15.8) 6 (5) | 14 (82.3) 2 (11.8) 1 (5.9) | 81 (78.6) 17 (16.5) 5 (4.9) | 0.8 |
| Charlson score, mean ± SD | 4.2 ± 2.7 | 3.4 ± 2.9 | 4.3 ± 2.6 | 0.7 |
| Norton score, mean ± SD | 14.4 ± 4.8 | 15.8 ± 4.8 | 14.2 ± 4.8 | 0.2 |
| Previous exposure to antimicrobial therapy (90 days) | 61 (50.8) | 10 (58.8) | 51 (49.5) | 0.6 |
| Variable, n (%) | All Patients (n = 120) | Empiric Carbapenems, n = 17 | Empiric Non-Carbapenem Regimens, n = 103 | p Value |
|---|---|---|---|---|
| Pathogen Enterobacter cloacae complex Morganella morganii | 84 (70) 36 (30) | 13 (76.5) 4 (23.5) | 71 (68.9) 32 (31.1) | 0.6 |
| Likely source of bacteremia Urosepsis Abdominal Endovascular/CRBSI Bone and soft tissue Pneumonia Febrile neutropenia Other/undetermined a | 32 (26.7) 27 (22.5) 26 (21.7) 14 (11.7) 9 (7.5) 7 (5.8) 5 (4.2) | 4 (23.5) 7 (41.2) 4 (23.5) 0 0 2 (11.8) 0 | 28 (27.2) 20 (19.4) 22 (21.4) 14 (13.6) 9 (8.7) 5 (4.9) 5 (4.9) | 1 0.06 1 0.2 0.4 0.3 1 |
| Source control | 57/83 (67.7) | 11/15 (73.3) | 46/68 (67.7) | 0.8 |
| Systolic blood pressure < 90 mmHg | 44 (36.7) | 5 (29.4) | 32 (37.9) | 0.6 |
| Inotropic support | 25/120 (20.8) | 4/17 (23.5) | 21/103 (20.4) | 0.8 |
| Pitt bacteremia score, mean ± SD | 1.9 ± 2.4 | 1.9 ± 2.5 | 2 ± 2.4 | 0.9 |
| White blood cell count < 4000/uL or >12,000/uL | 66 (55) | 10 (58.8) | 56 (54.4) | 0.8 |
| C-reactive protein (mg/dL), mean ± SD b | 17.9 ± 9.7 | 17 ± 11.5 | 18 ± 9.5 | 0.7 |
| Creatinine level c | 2.3 ± 2.3 | 1.8 ± 1.4 | 2.4 ± 2.4 | 0.6 |
| Creatinine above 1.5 d | 52/115 (45.2) | 8/17 (47) | 44/98 (44.9) | 1 |
| Lactate, mean ± SD e | 3.1 ± 2 | 2.7 ± 1.5 | 3.2 ± 2 | 0.5 |
| Inappropriate empiric therapy f | 19 (15.8) | 2 (11.7) | 17 (16.5) | 1 |
| Variable, n (%) | Empiric Carbapenems (n = 17) | Empiric Non-Carbapenem Regimens (n = 103) | p Value |
|---|---|---|---|
| Modification of empiric treatment * | 1 (5.9) | 72 (70) | <0.001 |
| Length of stay ** | 15.5 ± 7.2 | 14.5 ± 7.6 | 0.6 |
| Admission to ICU within 14 days | 2 (11.8) | 16 (15.5) | 1 |
| Persistent bacteremia | 0 | 2 (2) | NA |
| Recurrent bacteremia | 0 | 4 (3.9) | NA |
| 30-day mortality | 1 (5.9) | 14 (13.6) | 0.6 |
| Variable, n (%) | Definitive Carbapenems, n = 41 | Definitive Non-Carbapenem Regimens, n = 79 | p Value |
|---|---|---|---|
| Pathogen Enterobacter cloacae complex Morganella morganii | 30 (0.73) 11 (26.8) | 54 (68.3) 25 (31.7) | 0.7 |
| Likely source of bacteremia Urosepsis Abdominal Endovascular/CRBSI Bone and soft tissue Pneumonia Febrile neutropenia Undetermined a | 12 (29.3) 9 (22) 11 (26.8) 5 (12.2) 2 (4.9) 1 (2.4) 1 (2.4) | 20 (25.3) 18 (22.8) 15 (19) 9 (11.4) 7 (8.9) 6 (7.6) 4 (5) | 0.7 0.9 0.3 0.9 0.6 0.3 0.7 |
| Source control | 23/30 (76.7) | 34/53 (64.2) | 0.3 |
| Systolic blood pressure < 90 mmHg | 17 (41.5) | 27 (34.2) | 0.4 |
| Inotropic support | 12 (29.3) | 13 (16.5) | 0.15 |
| Pitt bacteremia score, mean ± SD | 2.7 ± 2.9 | 1.6 ± 2.1 | 0.02 |
| White blood cell count < 4000/uL or >12,000/uL | 22 (53.7) | 44 (55.7) | 0.8 |
| C-reactive protein (mg/dL), mean ± SD b | 20.6 ± 11.2 (n = 35) | 16.6 ± 8.7 (n = 70) | 0.04 |
| Creatinine level c | 2.7 ± 2.3 | 2.1 ± 2.2 | 0.2 |
| Creatinine above 1.5 d | 20/37 (38.5) | 32/78 (61.5) | 0.2 |
| Lactate, mean ± SD e | 3.3 ± 2.5 (n = 25) | 3 ± 1.6 (n = 43) | 0.5 |
| Ceftriaxone resistance | 14/41 (34%) | 11/68 (14%) | 0.016 |
| Variable, n (%) | Definitive Carbapenems (n = 41) | Definitive Non-Carbapenem Regimens (n = 79) | p Value |
|---|---|---|---|
| Length of stay * | 15.7 ± 6.7 | 14.1 ± 7.9 | 0.3 |
| Admission to ICU within 14 days | 9 (22) | 9 (11.4) | 0.18 |
| Persistent bacteremia | 1 (2.4) | 1 (1.3) | 1 |
| Recurrent bacteremia | 1 (2.4) | 3 (3.8) | 1 |
| 30-day mortality | 7 (19.5) | 8 (12.7) | 0.38 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Term | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Age | 1.02 | 0.98–1.06 | 0.36 | |||
| Female gender (Yes/No) | 1.3 | 0.4–3.7 | 0.68 | |||
| Previous 90 d admission (Yes/No) | 1.9 | 0.57–6.4 | 0.3 | |||
| Previous antimicrobial therapy | 1.1 | 0.38–3.3 | 0.8 | |||
| Charlson score | 1.2 | 0.97–1.4 | 0.1 | |||
| Norton score | 0.97 | 0.86–1.09 | 0.6 | |||
| Cognitive impairment (Yes/No) | 0.3 | 0.04–2.4 | 0.3 | |||
| Pitt bacteremia score | 1.4 | 1.2–1.7 | 0.0005 | 1.4 | 1.2–1.8 | 0.0006 |
| Inotropic support (Yes/No) | 5.9 | 1.9–18.4 | 0.002 | |||
| WBC > 12,000 or <5000 | 2.5 | 0.75–8.4 | 0.1 | |||
| CRP | 1.05 | 0.99–1.1 | 0.12 | |||
| Kidney injury * | 0.8 | 0.26–2.4 | 0.7 | |||
| Lactate above 4 (n = 68) | 1.5 | 0.3–6.6 | 0.6 | |||
| Ceftriaxone resistance | 0.94 | 0.25–3.6 | 0.9 | |||
| Empiric carbapenems vs. non-carbapenem regimens | 0.4 | 0.05–3.2 | 0.4 | |||
| Definitive carbapenems vs. non-carbapenem regimens | 1.8 | 0.6–5.5 | 0.28 | |||
| Definitive treatment with carbapenems vs. ciprofloxacin ** | 0.6 | 0.17–2 | 0.53 | |||
| Inappropriate empiric antimicrobial therapy (based on in vitro AST) | 1.4 | 0.4–6.5 | 0.4 | |||
| Mean time to definite therapy | 1.3 | 0.9–1.8 | 0.13 | |||
| Enterobacter sp. (versus Morganella morganii) | 0.4 | 0.15–1.3 | 0.14 | |||
| Ceftriaxone resistance | 0.94 | 0.25–3.6 | 0.93 | |||
| Non-urinary source infection | 5.9 | 0.74–46.6 | 0.09 | 7.3 | 0.8–64.7 | 0.08 |
| Source control | 0.9 | 0.2–3.9 | 0.9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalabi, O.; Kashat, L.; Murik, O.; Zevin, S.; Assous, M.V.; Ben-Chetrit, E. Clinical Outcomes of Patients with AmpC-Beta-Lactamase-Producing Enterobacterales Bacteremia Treated with Carbapenems versus Non-Carbapenem Regimens: A Single-Center Study. Antibiotics 2024, 13, 709. https://doi.org/10.3390/antibiotics13080709
Shalabi O, Kashat L, Murik O, Zevin S, Assous MV, Ben-Chetrit E. Clinical Outcomes of Patients with AmpC-Beta-Lactamase-Producing Enterobacterales Bacteremia Treated with Carbapenems versus Non-Carbapenem Regimens: A Single-Center Study. Antibiotics. 2024; 13(8):709. https://doi.org/10.3390/antibiotics13080709
Chicago/Turabian StyleShalabi, Orjowan, Livnat Kashat, Omer Murik, Shoshana Zevin, Marc V. Assous, and Eli Ben-Chetrit. 2024. "Clinical Outcomes of Patients with AmpC-Beta-Lactamase-Producing Enterobacterales Bacteremia Treated with Carbapenems versus Non-Carbapenem Regimens: A Single-Center Study" Antibiotics 13, no. 8: 709. https://doi.org/10.3390/antibiotics13080709
APA StyleShalabi, O., Kashat, L., Murik, O., Zevin, S., Assous, M. V., & Ben-Chetrit, E. (2024). Clinical Outcomes of Patients with AmpC-Beta-Lactamase-Producing Enterobacterales Bacteremia Treated with Carbapenems versus Non-Carbapenem Regimens: A Single-Center Study. Antibiotics, 13(8), 709. https://doi.org/10.3390/antibiotics13080709







