Essential Oils against Candida auris—A Promising Approach for Antifungal Activity
Abstract
1. Introduction
2. EOs and Their Chemical Constituents with Antifungal Activity—Mechanisms of Action
2.1. Disruption of Cell Membrane
2.2. Induction of Oxidative Stress
2.3. Mitochondrial Dysfunction
2.4. Inhibition of Cell Wall Synthesis
2.5. Interaction with Membrane Proteins
2.6. Biofilm Inhibition
3. EOs and Their Compounds against C. auris
3.1. Diverse Sources of EOs and Their Compounds
3.2. Methods of Antifungal Analysis
3.3. Proposed Mechanisms of EO Action against C. auris
3.4. Findings
4. Limitations, Challenges, and Future Directions
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
- CDC’s Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 8 May 2024).
- Rhodes, J.; Fisher, M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019, 52, 84–89. [Google Scholar] [CrossRef]
- Spivak, E.S.; Hanson, K.E. Candida auris: An emerging fungal pathogen. J. Clin. Microbiol. 2018, 56, e01588-17. [Google Scholar] [CrossRef]
- Day, A.M.; McNiff, M.M.; da Silva Dantas, A.; Gow, N.A.R.; Quinna, J. Hog1 Regulates stress tolerance and virulence in the emerging fungal pathogen Candida auris. MBP 2018, 3, e00506-18. [Google Scholar]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef]
- Biswal, M.; Rudramurthy, S.M.; Jain, N.; Shamanth, A.S.; Sharma, D.; Jain, K.; Yaddanapudi, L.N.; Chakrabarti, A. Controlling a possible outbreak of Candida auris infection: Lessons learnt from multiple interventions. J. Hosp. Infect. 2017, 97, 363–370. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Johnson, J.C.; Davis, M.; Huttenlocher, A.; Kernien, A.F.; Nett, J.E. Emerging fungal pathogen Candida auris evades neutrophil attack. mBIO 2018, 9, e01403-18. [Google Scholar]
- Navarro-Arias, M.J.; Hernández-Chávez, M.J.; García-Carnero, L.C.; Amezcua-Hernández, D.G.; Lozoya-Pérez, N.E.; Estrada-Mata, E.; Martínez-Duncker, I.; Franco, B.; Mora-Montes, H.M. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect. Drug Resist. 2019, 12, 783–794. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Dominguez, E.G.; Zarnowski, R.; Choy, H.L.; Zhao, M.; Sanchez, H.; Nett, J.E.; Andes, D.R. Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. mSphere 2019, 4, 2–8. [Google Scholar] [CrossRef]
- Romera, D.; Aguilera-Correa, J.J.; Gadea, I.; Viñuela-Sandoval, L.; García-Rodríguez, J.; Esteban, J. Candida auris: A comparison between planktonic and biofilm susceptibility to antifungal drugs. J. Med. Microbiol. 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018, 9, 5346. [Google Scholar] [CrossRef]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef]
- Kean, R.; McKloud, E.; Townsend, E.M.; Sherry, L.; Delaney, C.; Jones, B.L.; Williams, C.; Ramage, G. The comparative efficacy of antiseptics against Candida auris biofilms. Int. J. Antimicrob. Agents 2018, 52, 673–677. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Córdoba, S.; Vivot, W.; Szusz, W.; Albo, G. Antifungal activity of essential oils against Candida species isolated from clinical samples. Mycopathologia 2019, 184, 615–623. [Google Scholar] [CrossRef]
- Rashed, A.A.; Rathi, D.N.G.; Nasir, N.A.H.A.; Rahman, A.Z.A. Antifungal properties of essential oils and their compounds for application in skin fungal infections: Conventional and nonconventional approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef]
- Hertiani, T.; Burhan, F.; Bafadal, M.; Pratiwi, U.S.T. Membrane cell disruption of Candida albicans by Masoyi bark essential oil. IJRPS 2020, 11, 2598–2602. [Google Scholar] [CrossRef]
- Shahina, Z.; Ndlovu, E.; Persaud, O.; Sultana, T. Candida albicans reactive oxygen species (ROS)-dependent. Microbiol. Spectr. 2022, 10, e03183-22. [Google Scholar] [CrossRef]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A Review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Q.; Wu, H.; Huang, J.; Ju, J. Synergistic antifungal mechanism of effective components from essential oil against Penicillium roqueforti. Eng. Microbiol. 2023, 3, 100057. [Google Scholar] [CrossRef]
- Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal activity of natural compounds vs. Candida spp.: A mixture of cinnamaldehyde and eugenol shows promising in vitro results. Antibiotics 2022, 11, 73. [Google Scholar]
- Andrade-Ochoa, S.; Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Rivera-Chavira, B.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Differential antimicrobial effect of essential oils and their main components: Insights based on the cell membrane and external structure. Membranes 2021, 11, 405. [Google Scholar] [CrossRef]
- Mandras, N.; Roana, J.; Scalas, D.; Del Re, S.; Cavallo, L.; Ghisetti, V.; Tullio, V. The inhibition of non-albicans Candida species and uncommon yeast pathogens by selected essential oils and their major compounds. Molecules 2021, 26, 4937. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.S.; El-Ganiny, A.M. Back to nature: Combating Candida albicans biofilm, phospholipase and hemolysin using plant essential oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H. Anti-Candida and antibiofilm activity of selected lamiaceae essential oils. Front. Biosci.-Landmark 2023, 28, 28. [Google Scholar] [CrossRef]
- McGregor, R.C.; Parker, K.A.; Hornby, J.M.; Latta, L.C. Microbial population dynamics under microdoses of the essential oil arborvitae. BMC Complement. Altern. Med. 2019, 19, 247. [Google Scholar] [CrossRef]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In vitro antifungal activity of cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef]
- Hamdy, R.; Fayed, B.; Hamoda, A.M.; Rawas-Qalaji, M.; Haider, M.; Soliman, S.S.M. Essential oil-based design and development of novel anti- Candida azoles formulation. Molecules 2020, 25, 1463. [Google Scholar] [CrossRef]
- De Alteriis, E.; Maione, A.; Falanga, A.; Bellavita, R.; Galdiero, S.; Albarano, L.; Salvatore, M.M.; Galdiero, E.; Guida, M. Activity of free and liposome-encapsulated essential oil from Lavandula angustifolia against persister-derived biofilm of Candida auris. Antibiotics 2022, 11, 26. [Google Scholar] [CrossRef]
- Parker, R.A.; Gabriel, K.T.; Graham, K.D.; Butts, B.K.; Cornelison, C.T. Antifungal activity of select essential oils against Candida auris and their interactions with antifungal drugs. Pathogens 2022, 11, 821. [Google Scholar] [CrossRef]
- Fernandes, L.; Ribeiro, R.; Costa, R.; Henriques, M.; Rodrigues, M.E. Essential oils as a good weapon against drug-resistant Candida auris. Antibiotics 2022, 11, 977. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Donadu, M.G.; Usai, M.; Marchetti, M.; Ferrari, M.; Mazzarello, V.; Zanetti, S.; Nagy, F.; Kovács, R. Evaluation of the antimicrobial and antivirulent potential of essential oils isolated from Juniperus oxycedrus L. ssp. macrocarpa aerial parts. Microorganisms 2022, 10, 758. [Google Scholar] [CrossRef]
- Ribeiro, R.; Fernandes, L.; Costa, R.; Cavaleiro, C.; Salgueiro, L.; Henriques, M.; Rodrigues, M.E. Comparing the effect of Thymus spp. essential oils on Candida auris. Ind. Crops Prod. 2022, 178, 114667. [Google Scholar] [CrossRef]
- Baldim, I.; Paziani, M.H.; Grizante Barião, P.H.; von Zeska Kress, M.R.; Oliveira, W.P. Nanostructured lipid carriers loaded with lippia sidoides essential oil as a strategy to combat the multidrug-resistant Candida auris. Pharmaceutics 2022, 14, 180. [Google Scholar] [CrossRef]
- Di Vito, M.; Garzoli, S.; Rosato, R.; Mariotti, M.; Gervasoni, J.; Santucci, L.; Ovidi, E.; Cacaci, M.; Lombarini, G.; Torelli, R.; et al. A new potential resource in the fight against Candida auris: The Cinnamomum zeylanicum essential oil in synergy with antifungal drug. Microbiol. Spectr. 2023, 11, e0438522. [Google Scholar] [CrossRef]
- Rosato, R.; Napoli, E.; Granata, G.; Di Vito, M.; Garzoli, S.; Geraci, C.; Rizzo, S.; Torelli, R.; Sanguinetti, M.; Bugli, F. Study of the chemical profile and anti-fungal activity against Candida auris of Cinnamomum aassia essential oil and of its nano-formulations based on polycaprolactone. Plants 2023, 12, 358. [Google Scholar] [CrossRef]
- Ruiz-Duran, J.; Torres, R.; Stashenko, E.E.; Ortiz, C. Antifungal and antibiofilm activity of colombian essential oils against different Candida strains. Antibiotics 2023, 12, 668. [Google Scholar] [CrossRef]
- Zapata-Zapata, C.; Loaiza-Oliva, M.; Martínez-Pabón, M.C.; Stashenko, E.E.; Mesa-Arango, A.C. In vitro activity of essential oils distilled from colombian plants against Candida auris and other Candida species with different antifungal susceptibility profiles. Molecules 2022, 27, 6837. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Horn, C.; Vediyappan, G. Anticapsular and antifungal activity of α-cyperone. Antibiotics 2021, 10, 51. [Google Scholar] [CrossRef]
- Maione, A.; La Pietra, A.; de Alteriis, E.; Mileo, A.; De Falco, M.; Guida, M.; Galdiero, E. Effect of myrtenol and its synergistic interactions with antimicrobial drugs in the inhibition of single and mixed biofilms of Candida auris and Klebsiella pneumoniae. Microorganisms 2022, 10, 1773. [Google Scholar] [CrossRef]
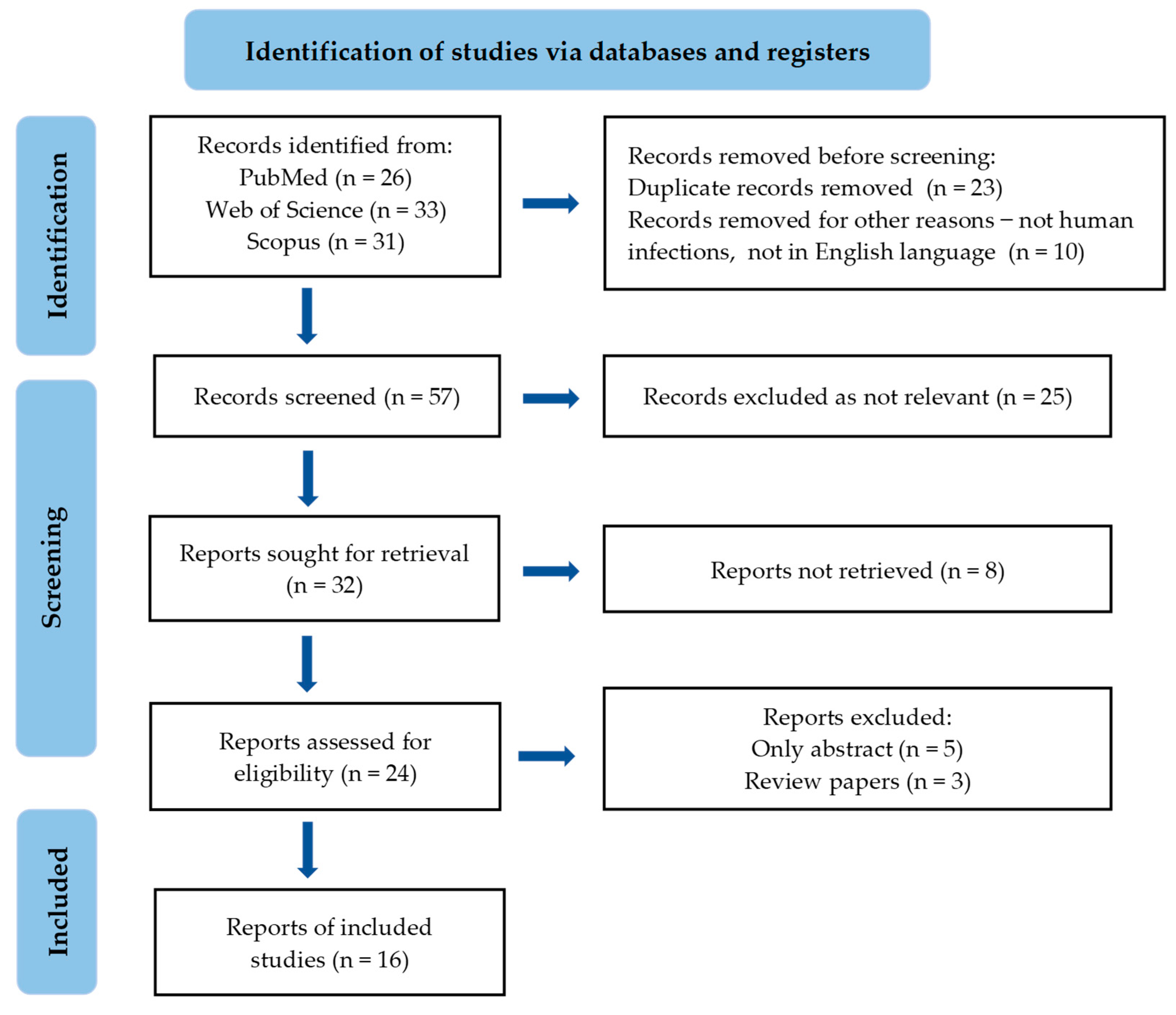


| References | Source of Essential Oil/Compound | Methods of Analysis | Mechanisms of Action against C. auris | Results |
|---|---|---|---|---|
| [30] | Thuja plicata |
| N/A |
|
| [31] | Cinnamomum zeylanicum bark and leaf |
|
|
|
| [32] | Structural modifications of natural cuminaldehyde isolated from Calligonum comosum |
| N/A |
|
| [33] | Lavandula angustifolia (linalyl acetate, linalool) free and liposome-enveloped EOs |
|
|
|
| [34] | Melaleuca alternifoli, Pelargonium graveolens, Citrus aurantifolia peel, Eucalyptus spp., Mentha piperita, Leptospermum scoparium, Syzygium aromaticum bud, Commiphora abyssinica, Mentha spicata, Cinnamomum zeylanicum, Citrus bergamium, Citrus limon, Boswellia spp., Coriandrum sativum, Citrus × aurantium subsp. amara, Citrus × aurantium var. paradisi, Lavandula spp., Zingiber officinale, Ocimum basilicum, and Cymbopogon schoenanthus |
| N/A |
|
| [35] | Melaleuca leucadendra, Melaleuca viridiflora, Melaleuca alternifolia, and Thymus zygis |
|
|
|
| [36] | Juniperus oxycedrus ssp. macrocarpa (α-pinene) |
|
|
|
| [37] | Thymus vulgaris (thymol 63.1%), Thymus zygis (thymol 26.5%), Thymus satureioides (borneol 29.3%), and Thymus mastichina (linalool 31.9%) |
| N/A |
|
| [38] | Lippia sidoides (thymol 68.22%), encapsulated EO in nanostructured lipid carrier |
|
|
|
| [39] | Cymbopogon martini, Cymbopogon citratus, Elettaria cardamomum, Coriandrum sativum, Anethum graveolens, Helichrysum italicum, Cuminum cyminum, Mentha piperita, Melaleuca alternifolia, Rosmarinus officinalis, Thymus vulgaris with thymol chemotype Cinnamomum zeylanicum (bark), Pelargonium graveolens, Cinnamomum camphora, and Lavandula angustifolia |
|
|
|
| [40] | Cinnamomum cassia and its nano-formulations based on polycaprolactone |
|
|
|
| [41] | Lippia alba (carvone-limonene chemotype) and L. origanoides (thymol chemotype), L. micromera (p-cymene chemotype) |
| N/A |
|
| [42] | Lippia origanoides different chemotypes |
| N/A |
|
| [43] | carvacrol, thymol, eugenol, methyl eugenol |
|
|
|
| [44] | α-cyperone |
| N/A |
|
| [45] | myrtenol |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A. Essential Oils against Candida auris—A Promising Approach for Antifungal Activity. Antibiotics 2024, 13, 568. https://doi.org/10.3390/antibiotics13060568
Kowalczyk A. Essential Oils against Candida auris—A Promising Approach for Antifungal Activity. Antibiotics. 2024; 13(6):568. https://doi.org/10.3390/antibiotics13060568
Chicago/Turabian StyleKowalczyk, Adam. 2024. "Essential Oils against Candida auris—A Promising Approach for Antifungal Activity" Antibiotics 13, no. 6: 568. https://doi.org/10.3390/antibiotics13060568
APA StyleKowalczyk, A. (2024). Essential Oils against Candida auris—A Promising Approach for Antifungal Activity. Antibiotics, 13(6), 568. https://doi.org/10.3390/antibiotics13060568






