Voriconazole Admixed with PMMA—Impact on Mechanical Properties and Efficacy
Abstract
1. Introduction
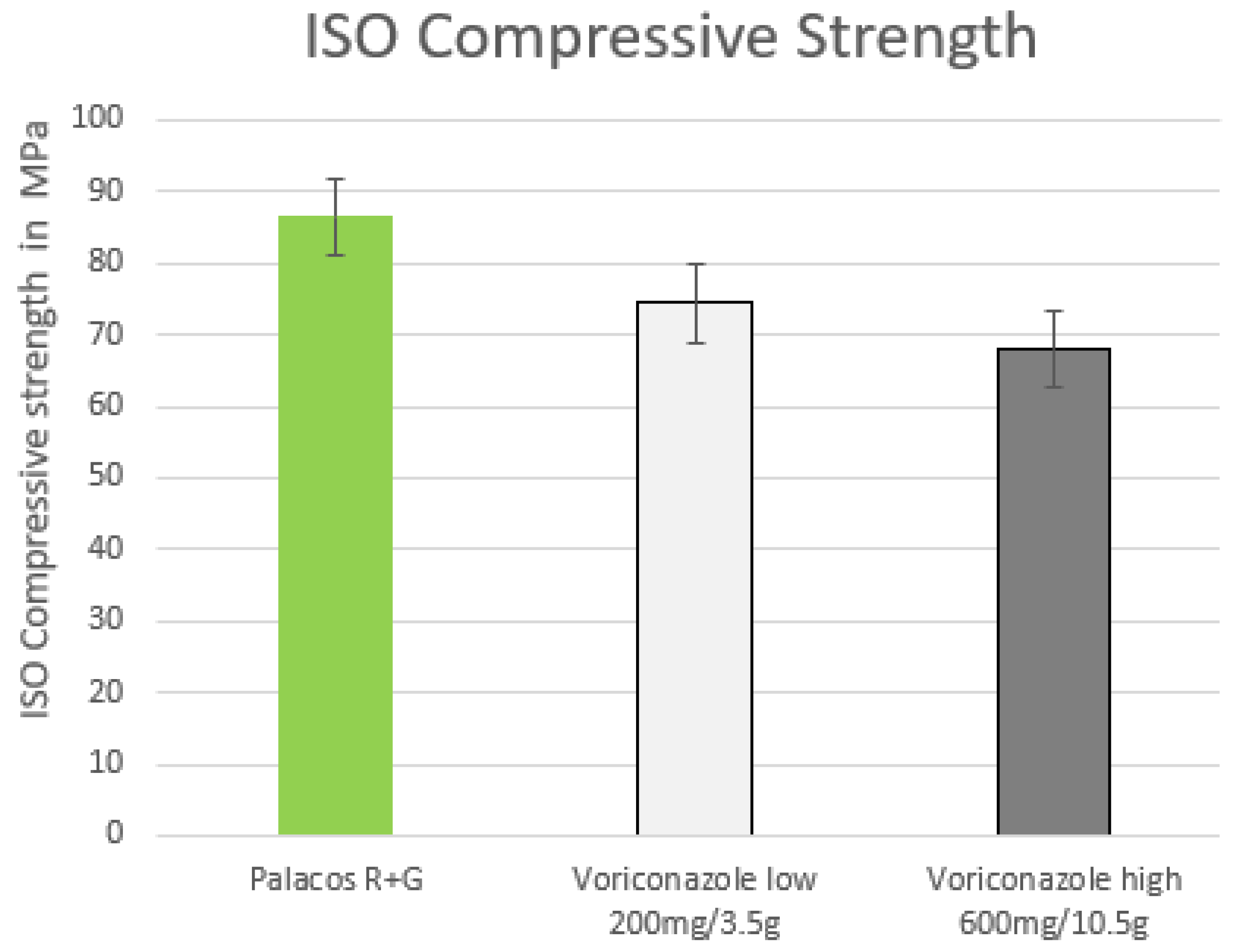
2. Results
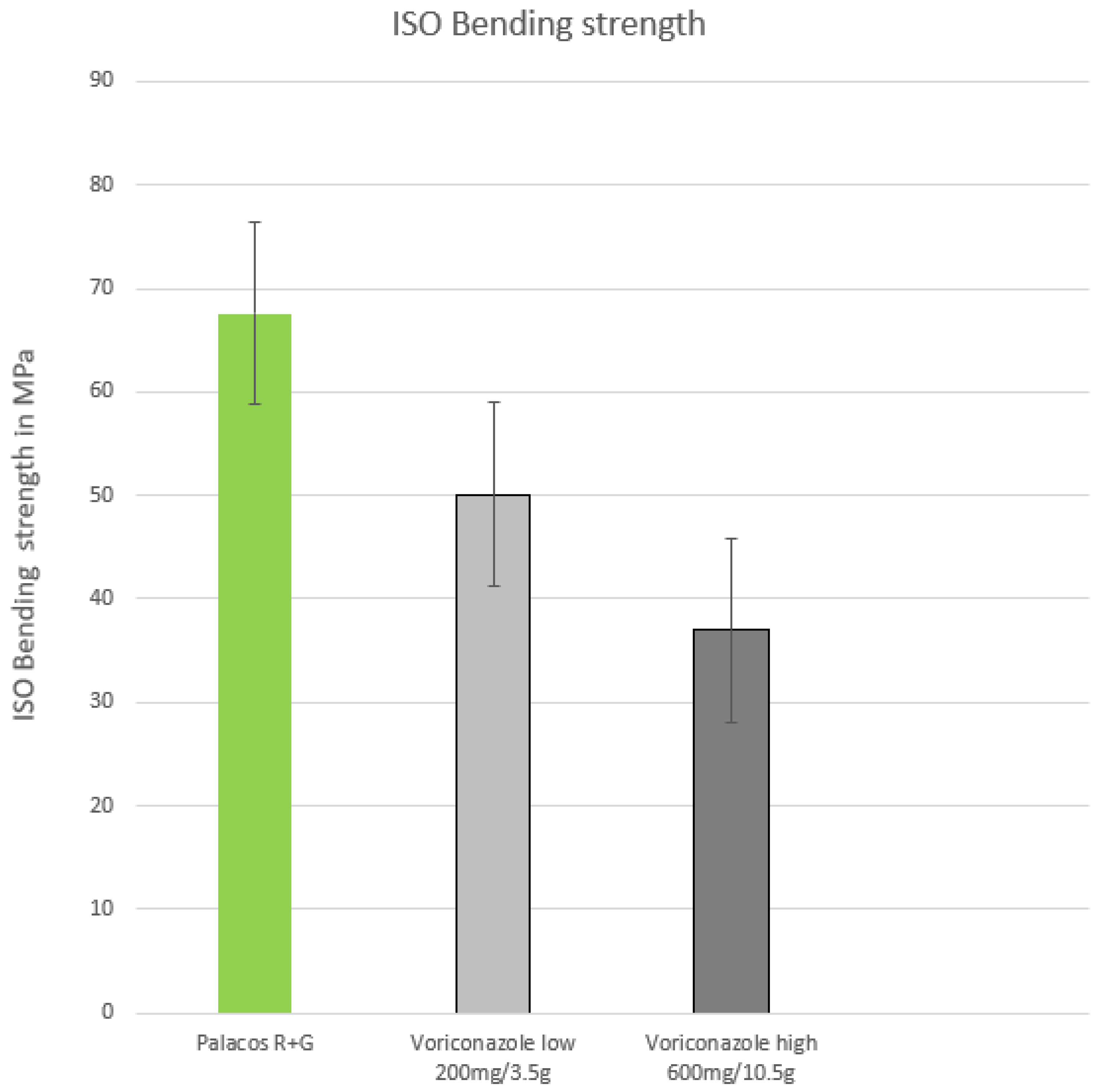
2.1. ISO Bending Strength
2.2. ISO Bending Modulus
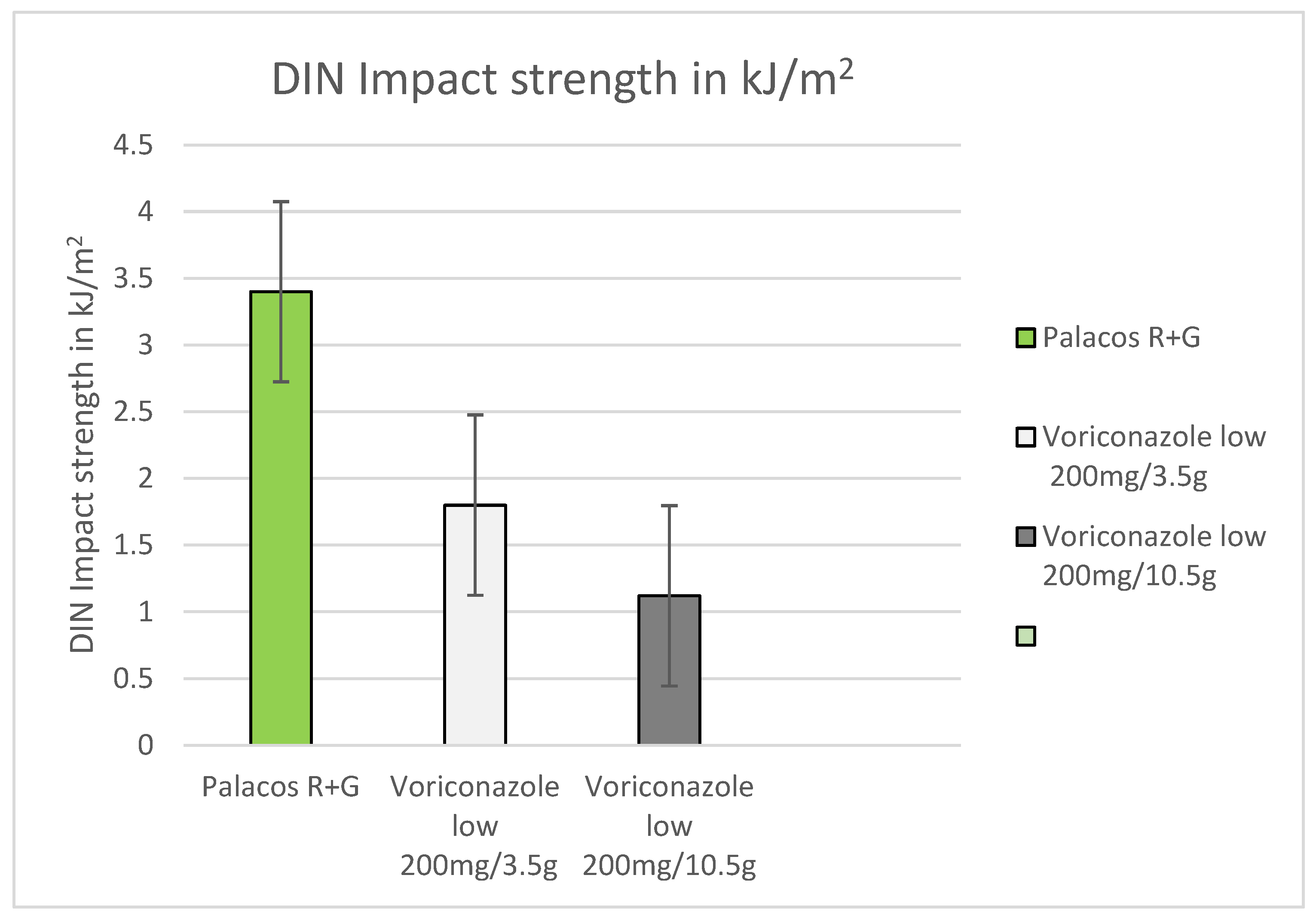
2.3. DIN Impact Strength (Dynstat)
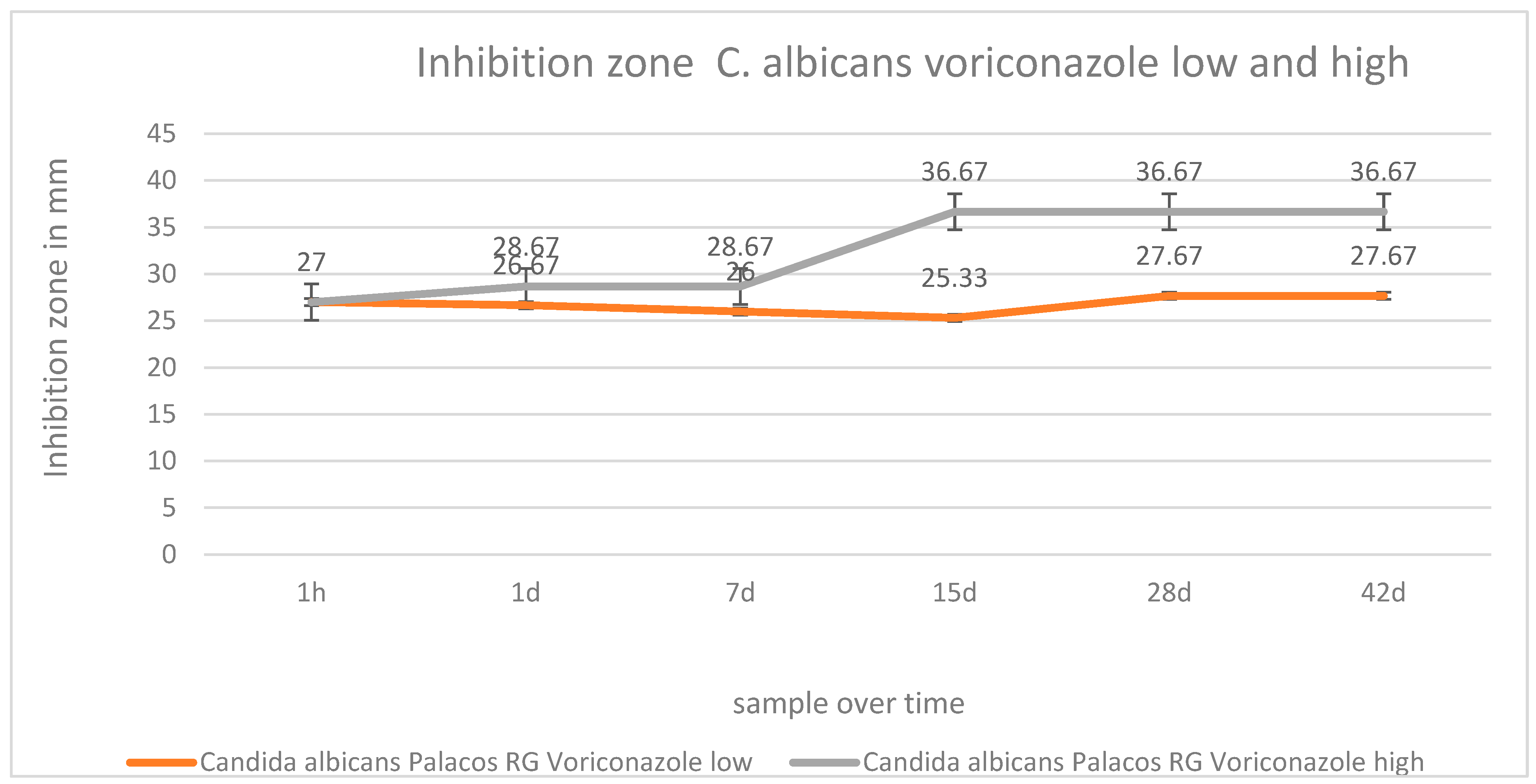
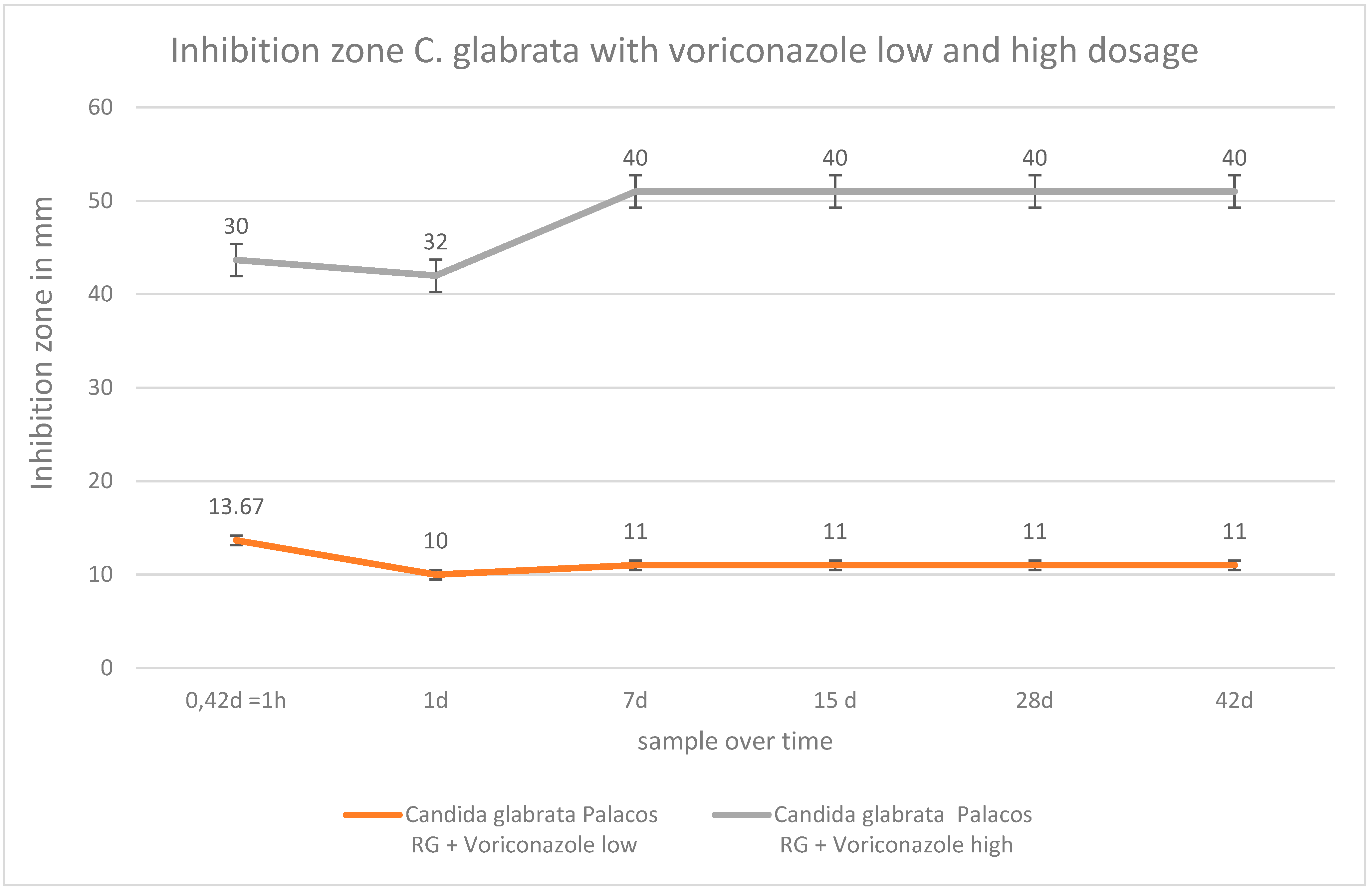
2.4. Inhibition Zone Tests
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Mechanical Testing
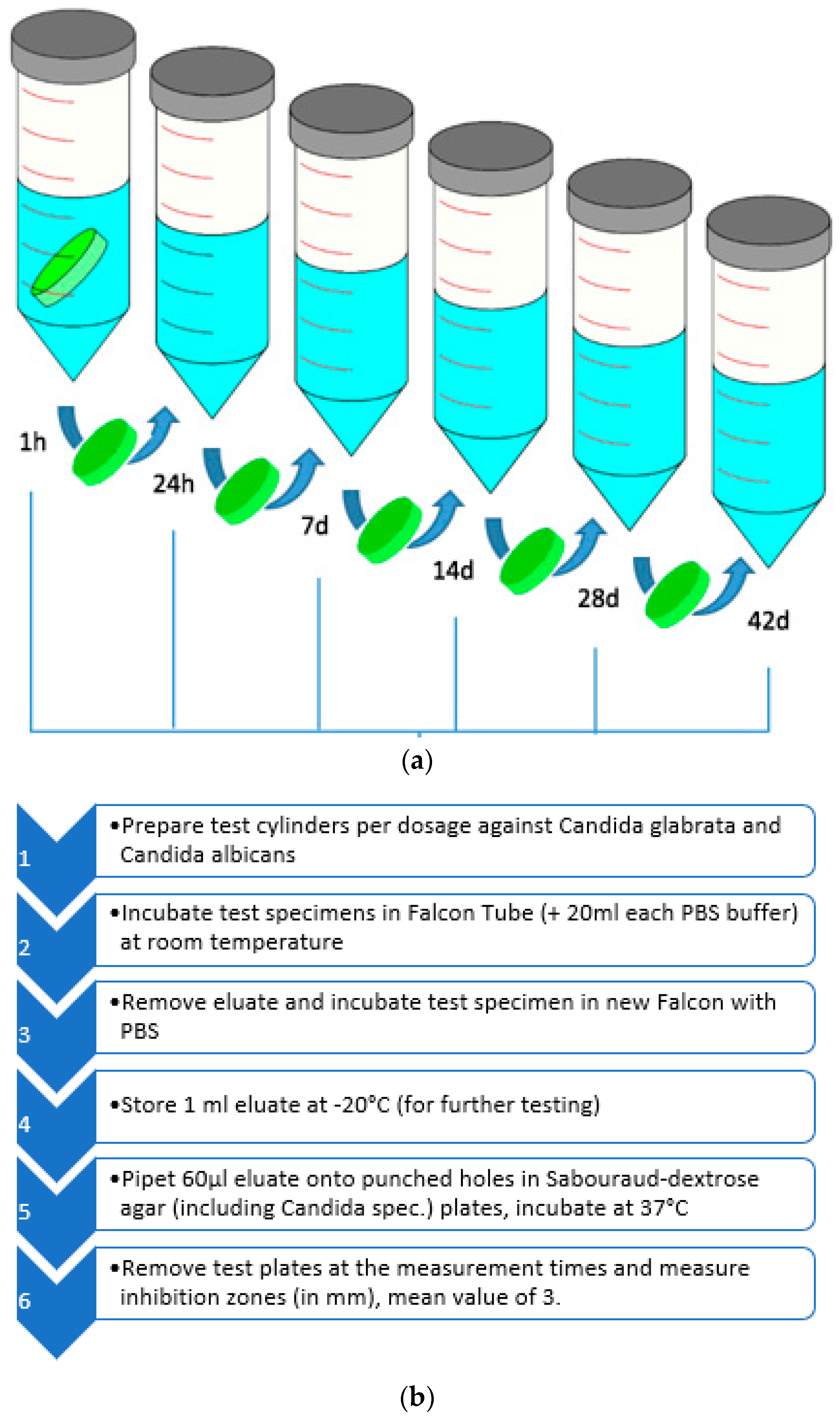
3.2.2. Inhibition Zone Testing
4. Discussion
4.1. Considerations for Local Application of Voriconazole in PMMA
4.2. Miscibility of Antifungals and Orthopedic Cement
4.3. Future Considerations about Antifungal Dosage
4.4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, R. Periprosthetic Joint Infection. N. Engl. J. Med. 2023, 388, 251–262. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Becker, S.L.; Sahan, I. Antifungal-Loaded Acrylic Bone Cement in the Treatment of Periprosthetic Hip and Knee Joint Infections: A Review. Antibiotics 2022, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Adeli, B.; Parvizi, J. Strategies for the prevention of periprosthetic joint infection. J. Bone Jt. Surg. Br. 2012, 94 (Suppl. A11), 42–46. [Google Scholar] [CrossRef]
- Azzam, K.; McHale, K.; Austin, M.; Purtill, J.J.; Parvizi, J. Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin. Orthop. Relat. Res. 2009, 467, 1706–1714. [Google Scholar] [CrossRef]
- Kuiper, J.W.P.; van den Bekerom, M.P.J.; van der Stappen, J.; Nolte, P.A.; Colen, S. 2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infections. Acta Orthop. 2013, 84, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.T.; Jakobs, T.F.; Wallnöfer, A.; Reiser, M.F.; Helmberger, T.K. Perkutane Vertebroplastie: Indikationen, Kontraindikationen und Technik. Radiologe 2003, 43, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, O.; Schoof, B.; Klatte, T.O.; Schmidl, S.; Fensky, F.; Guenther, D.; Frommelt, L.; Gehrke, T.; Gebauer, M. Fungal periprosthetic joint infection in total knee arthroplasty: A systematic review. Orthop. Rev. 2015, 7, 5623. [Google Scholar] [CrossRef]
- Nace, J.; Siddiqi, A.; Talmo, C.T.; Chen, A.F. Diagnosis and Management of Fungal Periprosthetic Joint Infections. J. Am. Acad. Orthop. Surg. 2019, 27, e804–e818. [Google Scholar] [CrossRef]
- Enz, A.; Müller, S.; Mittelmeier, W.; Klinder, A. Severe polymicrobial and fungal periprosthetic osteomyelitis persisting after hip disarticulations treated with caspofungin in risk patients: A case series. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Grzelecki, D.; Grajek, A.; Dudek, P.; Olewnik, Ł.; Zielinska, N.; Fulin, P.; Czubak-Wrzosek, M.; Tyrakowski, M.; Marczak, D.; Kowalczewski, J. Periprosthetic Joint Infections Caused by Candida Species-A Single-Center Experience and Systematic Review of the Literature. J. Fungi 2022, 8, 797. [Google Scholar] [CrossRef]
- De Meo, D.; Cera, G.; Ceccarelli, G.; Castagna, V.; Aronica, R.; Pieracci, E.M.; Persiani, P.; Villani, C. Candida fracture-related infection: A systematic review. J. Bone Jt. Infect. 2021, 6, 321–328. [Google Scholar] [CrossRef]
- Riaz, T.; Tande, A.J.; Steed, L.L.; Demos, H.A.; Salgado, C.D.; Osmon, D.R.; Marculescu, C.E. Risk Factors for Fungal Prosthetic Joint Infection. J. Bone Jt. Infect. 2020, 5, 76–81. [Google Scholar] [CrossRef]
- Miller, R.B.; McLaren, A.C.; Pauken, C.; Clarke, H.D.; McLemore, R. Voriconazole is delivered from antifungal-loaded bone cement. Clin. Orthop. Relat. Res. 2013, 471, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kweon, C.; McLaren, A.C.; Leon, C.; McLemore, R. Amphotericin B delivery from bone cement increases with porosity but strength decreases. Clin. Orthop. Relat. Res. 2011, 469, 3002–3007. [Google Scholar] [CrossRef]
- Fusini, F.; Aprato, A.; Massé, A.; Bistolfi, A.; Girardo, M.; Artiaco, S. Candida periprosthetic infection of the hip: A systematic review of surgical treatment and clinical outcomes. Int. Orthop. 2020, 44, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pocket Guide to Diagnosis & Treatment of Periprosthetic Joint Infection (PJI). Available online: http://pro-implant.org/tool/Pocket-guide/1 (accessed on 30 August 2022).
- Dennes, E.; Fiorenza, F.; Saint-Marcoux, F.; Megherbi, M.; Dupon, M.; Weinbreck, P. Voriconazole stability in cement spacers. Med. Mal. Infect. 2012, 42, 567–568. [Google Scholar] [CrossRef]
- Koutserimpas, C.; Naoum, S.; Alpantaki, K.; Raptis, K.; Dretakis, K.; Vrioni, G.; Samonis, G. Fungal Prosthetic Joint Infection in Revised Knee Arthroplasty: An Orthopaedic Surgeon’s Nightmare. Diagnostics 2022, 12, 1606. [Google Scholar] [CrossRef] [PubMed]
- ISO 5833:2002; Implants for Surgery—Acrylic Resin Cement. ISO: Geneva, Switzerland, 2002.
- DIN 53435:1983; Testing of Plastics Bending Test and Impact Test on Dynstat Tests Specimens. GlobalSpec: Albany, NY, USA, 1983.
- Renz, N.; Cabric, S.; Janz, V.; Trampuz, A. Sonication in the diagnosis of periprosthetic infections: Significance and practical implementation. Orthopaede 2015, 44, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M.; McLaren, A.; Vernon, B.; McLemore, R. Strength of antimicrobial bone cement decreases with increased poragen fraction. Clin. Orthop. Relat. Res. 2010, 468, 2101–2106. [Google Scholar] [CrossRef]
- Dunne, N.J.; Hill, J.; McAfee, P.; Kirkpatrick, R.; Patrick, S.; Tunney, M. Incorporation of large amounts of gentamicin sulphate into acrylic bone cement: Effect on handling and mechanical properties, antibiotic release, and biofilm formation. Proc. Inst. Mech. Eng. H 2008, 222, 355–365. [Google Scholar] [CrossRef]
- Von Hertzberg-Boelch, S.P.; Luedemann, M.; Rudert, M.; Steinert, A.F. PMMA Bone Cement: Antibiotic Elution and Mechanical Properties in the Context of Clinical Use. Biomedicines 2022, 10, 1830. [Google Scholar] [CrossRef]
- Hansen, E.; Kühn, K.D. (Eds.) Essentials of Cemented Knee Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Sprowson, A.P.; Jensen, C.; Chambers, S.; Parsons, N.R.; Aradhyula, N.M.; Carluke, I.; Inman, D.; Reed, M.R. The use of high-dose dual-impregnated antibiotic-laden cement with hemiarthroplasty for the treatment of a fracture of the hip: The Fractured Hip Infection trial. Bone Jt. J. 2016, 98, 1534–1541. [Google Scholar] [CrossRef]
- Kühn, K.D.; Renz, N.; Trampuz, A. Local antibiotic therapy. Unfallchirurg 2017, 120, 561–572. (In German) [Google Scholar] [CrossRef]
- Czuban, M.; Wulsten, D.; Wang, L.; Di Luca, M.; Trampuz, A. Release of different amphotericin B formulations from PMMA bone cements and their activity against Candida biofilm. Colloids Surf. B Biointerfaces 2019, 183, 110406. [Google Scholar] [CrossRef]
- Hetzmannseder, S.; Chang, Y.; Kittinger, C.; Kühn, K. Properties of Orthopaedic Cements Biomechanically Little Affected by Exceptional Use of Liquid Antibiotics. Orthop. Surg. 2021, 13, 2153–2162. [Google Scholar] [CrossRef]
- Chang, Y.H.; Tai, C.L.; Hsu, H.Y.; Hsieh, P.H.; Lee, M.S.; Ueng, S.W. Liquid antibiotics in bone cement: An effective way to improve the efficiency of antibiotic release in antibiotic loaded bone cement. Bone Jt. Res. 2014, 3, 246–251. [Google Scholar] [CrossRef]
- Deelstra, J.J.; Neut, D.; Jutte, P.C. Successful treatment of Candida albicans-infected total hip prosthesis with staged procedure using an antifungal-loaded cement spacer. J. Arthroplast. 2013, 28, 374.e5–374.e8. [Google Scholar] [CrossRef]
- Beredaki, M.I.; Georgiou, P.C.; Siopi, M.; Kanioura, L.; Arendrup, M.C.; Mouton, J.W.; Meletiadis, J. Voriconazole efficacy against Candida glabrata and Candida krusei: Preclinical data using a validated in vitro pharmacokinetic/pharmacodynamic model. J. Antimicrob. Chemother. 2020, 75, 140–148. [Google Scholar] [CrossRef]
- WHO. Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. (Electronic Version); WHO: Geneva, Switzerland, 2022; ISBN 978-92-4-006024-1. [Google Scholar]
- Parvizi, J.; Gehrke, T. Proceedings of the Second International Consensus Meeting on Musculoskeletal Infection Consensus of Periprosthetic Joint Infection. Section 3 Treatment. 2013. Available online: https://upload.orthobullets.com/documents/temp/73c7b918-5f54-46d2-87cd-1ab75e3c3a9d50Proceedings%20of%20the%20Second%20International%20Consensus%20Meeting%20on%20Musculoskeletal%20Infection.pdf (accessed on 25 June 2020).
- Grimsrud, C.; Raven, R.; Fothergill, A.W.; Kim, H.T. The in vitro elution characteristics of antifungal-loaded PMMA bone cement and calcium sulfate bone substitute. Orthopedics 2011, 34, e378–e381. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef]
- Chisari, E.; Lin, F.; Fei, J.; Parvizi, J. Fungal periprosthetic joint infection: Rare but challenging problem. Chin. J. Traumatol. 2022, 25, 63–66. [Google Scholar] [CrossRef]
- Sambri, A.; Zunarelli, R.; Fiore, M.; Bortoli, M.; Paolucci, A.; Filippini, M.; Zamparini, E.; Tedeschi, S.; Viale, P.; De Paolis, M. Epidemiology of Fungal Periprosthetic Joint Infection: A Systematic Review of the Literature. Microorganisms 2023, 11, 84. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, D.Y.; Kang, D.W.; Ro, D.H.; Lee, M.C.; Han, H.S. Efficacy of antifungal-impregnated cement spacer against chronic fungal periprosthetic joint infections after total knee arthroplasty. Knee 2018, 25, 631–637. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]


| Pure Voriconazole in mg | Voriconazole (Rotexmedica) Powder for IV Solutions/in g (including Pharmaceutical Excipients) | Cement Powder Quantity after Adding Low and High Dosages of Voriconazole in g | |
|---|---|---|---|
| 40.5 g PMMA reference/sachet | - | - | 40.5 g |
| 40.5 g PMMA reference/sachet | +200 (low dose) | +3.5 | 44 |
| 40.5 g PMMA reference/sachet | +600 (high dose) | +10.5 | 51 |
| Test Series Overview | Voriconazole Low (200 mg) in PMMA Reference | Voriconazole High (600 mg) in PMMA Reference | PMMA Reference |
|---|---|---|---|
| Methods: | |||
| ISO compression 70 MPa | + | + | + |
| ISO bending 50 MPa | + | + | + |
| ISO bending modulus | |||
| 1800 MPa | + | + | + |
| DIN impact strength (Dynstat) | + | + | + |
| Inhibition zone test | + | + | + |
| C. albicans | + | + | _ |
| C. glabrata | + | + | _ |
| Voriconazole | Dosage Form | Manufacturer/ Product Name | Voriconazole in mg Active Substance | Excipient in mg | Total Weight | The Active Substance with Respect to Total Weight | Publication Mentioned |
|---|---|---|---|---|---|---|---|
| Voriconazole | Powder for infusion | Hikma (before Hospira)/ Voriconazole 200 mg Hikma | 200 mg | Each vial contains 217.6 mg of sodium. Each vial contains 3200 mg Sulphobutylether beta cyclodextrin sodium (SBECD) (powder for infusion). | 3.417 mg | 5.8% of the total weight is voriconazole. 93.5% of the total weight is SBECD. | Miller et al., 2013 [12] |
| Voriconazole | N/A | N/A | 1000 mg | N/A | N/A | N/A | Deelstra et al., 2013 [32] |
| Voriconazole | Powder for infusion | Ratiopharm/ Voriconazole 200 mg Ratiopharm | 200 mg | Hydroxypropylbetadex: 2500 mg | 2.700 mg | 7.4% of total weight is voriconazole. 92.6% of the total weight is hydroxypropylbetadex. | SmPC (Feb 2022) |
| Voriconazole | Powder for infusion | Rotexamedica | 200 mg | Hydroxypropylbetadex (HPBCD): 3300 mg | 3.500 mg | 5.7% of total weight is voriconazole. 94.2% of the total weight is SBECD. | N/A |
| Voriconazole | Powder for infusion | Pfizer VFEND 200 mg | 200 mg | Natrium: 221 mg Beta-cyclodextrin-sulfobutylether (SBECD): 3200 mg | 3.621 mg | 5.5% of the total weight is voriconazole. 94% of the total weight is SBECD. | SmPC (May 2022) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krampitz, B.; Steiner, J.; Trampuz, A.; Kühn, K.-D. Voriconazole Admixed with PMMA—Impact on Mechanical Properties and Efficacy. Antibiotics 2023, 12, 848. https://doi.org/10.3390/antibiotics12050848
Krampitz B, Steiner J, Trampuz A, Kühn K-D. Voriconazole Admixed with PMMA—Impact on Mechanical Properties and Efficacy. Antibiotics. 2023; 12(5):848. https://doi.org/10.3390/antibiotics12050848
Chicago/Turabian StyleKrampitz, Barbara, Julia Steiner, Andrej Trampuz, and Klaus-Dieter Kühn. 2023. "Voriconazole Admixed with PMMA—Impact on Mechanical Properties and Efficacy" Antibiotics 12, no. 5: 848. https://doi.org/10.3390/antibiotics12050848
APA StyleKrampitz, B., Steiner, J., Trampuz, A., & Kühn, K.-D. (2023). Voriconazole Admixed with PMMA—Impact on Mechanical Properties and Efficacy. Antibiotics, 12(5), 848. https://doi.org/10.3390/antibiotics12050848







