Synergistic Combination of Aztreonam and Ceftazidime–Avibactam—A Promising Defense Strategy against OXA-48 + NDM Klebsiella pneumoniae in Romania
Abstract
1. Introduction
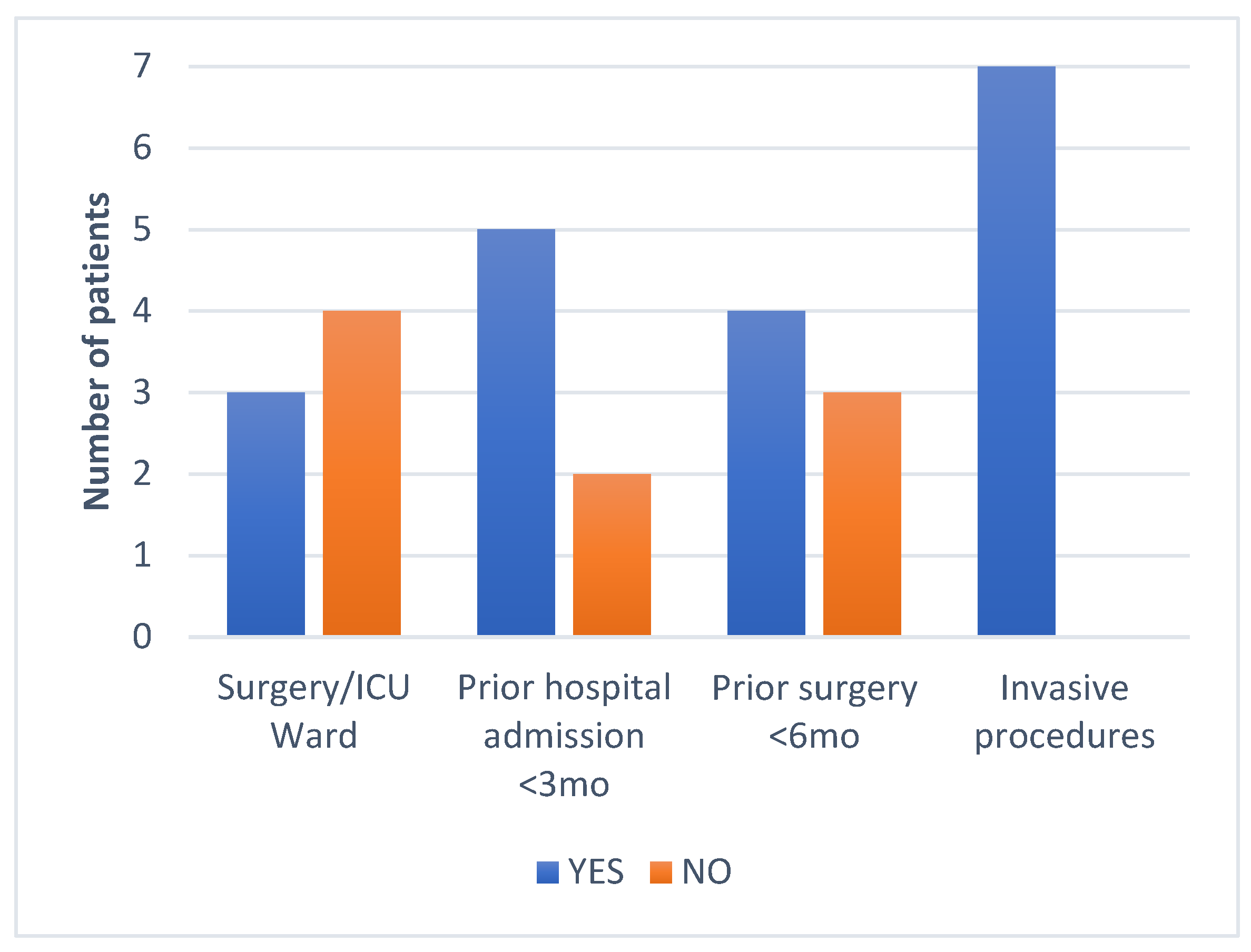
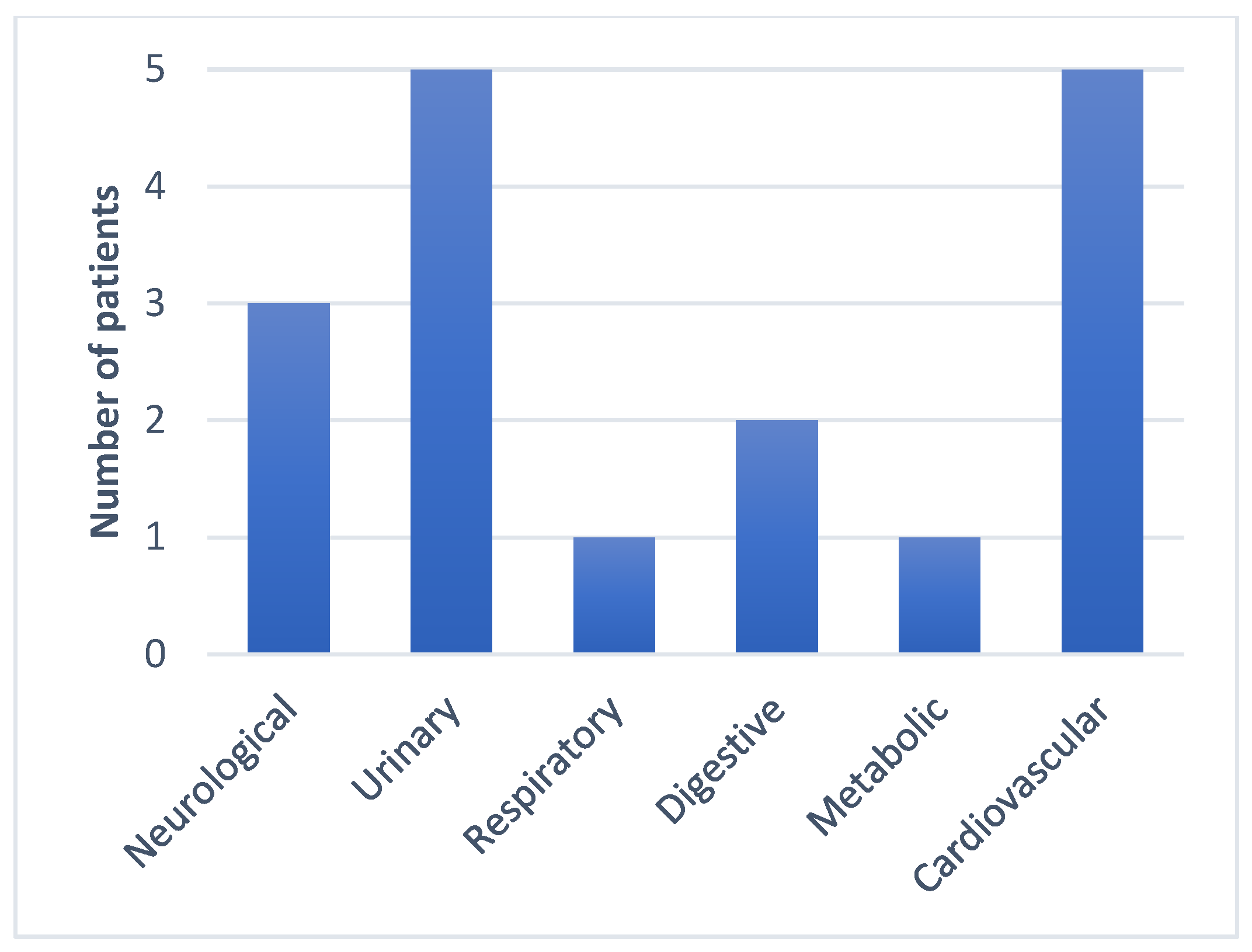
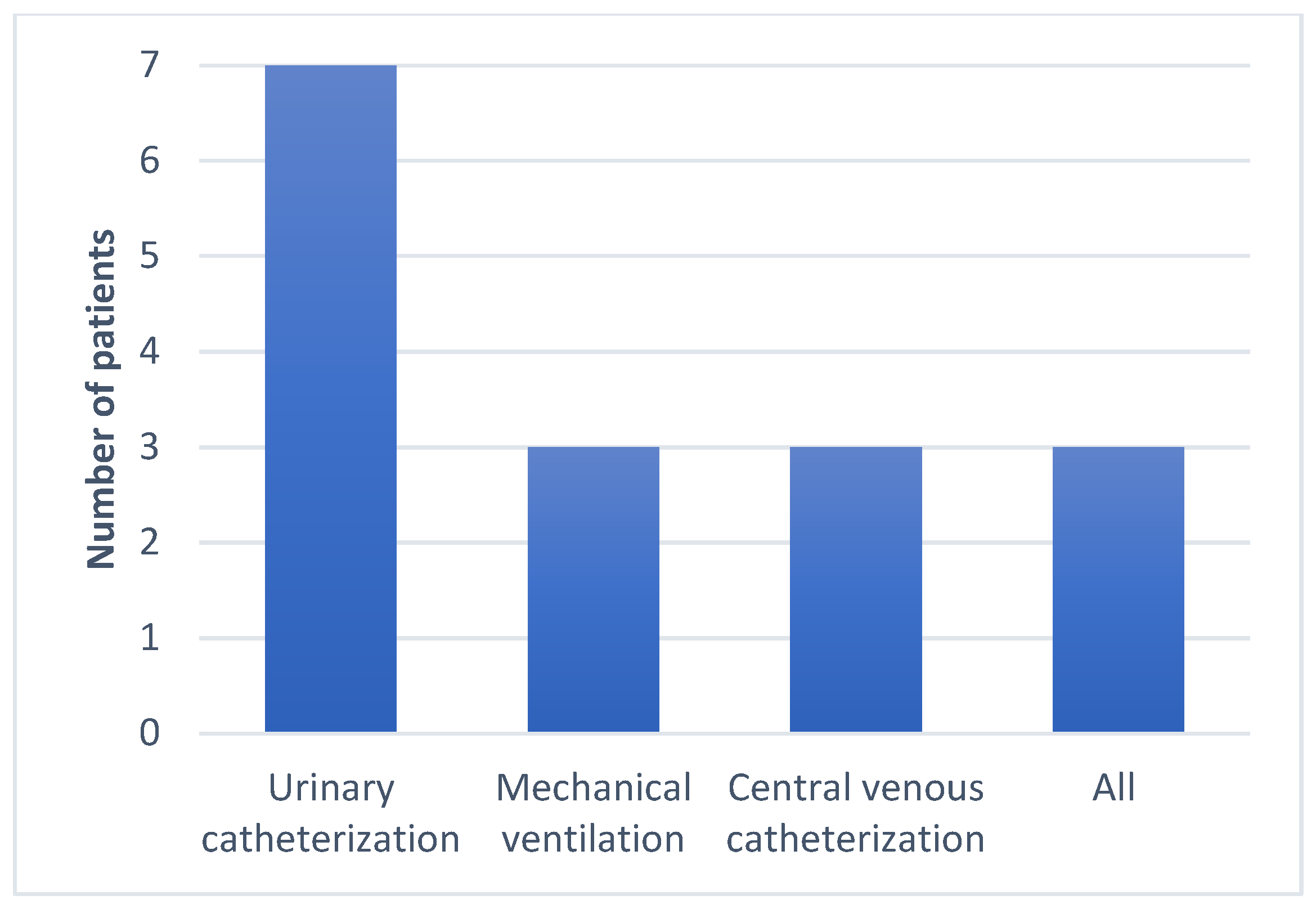
2. Results
3. Discussion
4. Materials and Methods
4.1. Setting and Patients
4.2. Microbiology
4.3. Analyzed Variables
4.4. Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Tesfa, T.; Mitiku, H.; Edae, M.; Assefa, N. Prevalence and incidence of carbapenem-resistant K. pneumoniae colonization: Systematic review and meta-analysis. Syst. Rev. 2022, 11, 240. [Google Scholar] [CrossRef]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2020 (accessed on 20 March 2024).
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2021 (accessed on 20 March 2024).
- Smith, R.M.; Lautenbach, E.; Omulo, S.; Araos, R.; Call, D.R.; Kumar, G.C.P.; Chowdhury, F.; McDonald, C.L.; Park, B.J. Human Colonization with Multidrug-Resistant Organisms: Getting to the Bottom of Antibiotic Resistance. Open Forum Infect. Dis. 2021, 8, ofab531. [Google Scholar] [CrossRef] [PubMed]
- Troeman, D.P.R.; Hazard, D.; Timbermont, L.; Malhotra-Kumar, S.; van Werkhoven, C.H.; Wolkewitz, M.; Ruzin, A.; Goossens, H.; Bonten, M.J.M.; Harbarth, S.; et al. Postoperative Staphylococcus aureus Infections in Patients with and Without Preoperative Colonization. JAMA Netw. Open 2023, 6, e2339793. [Google Scholar] [CrossRef] [PubMed]
- Radu, F.I.; Mates, I.M.; Gheorghita, V. Bucharest Mobile Military Hospital-Response to the COVID-19 Pandemic. 2020. Available online: https://www.revistamedicinamilitara.ro/wp-content/uploads/2020/04/Bucharest-mobile-military-hospital-%E2%80%93-response-to-the-COVID-19-pandemic.pdf (accessed on 22 May 2024).
- Gheorghiță, V.; Conea, I.F.; Radu, A.M.; Ștefan, I.; Mărdărescu, M.; Petrea, S.; Streinu-Cercel, A. Epidemiological trends and therapeutic challenges of malignancies in adult HIV-1-infected patients receiving combination antiretroviral therapy in a tertiary hospital from Romania: An observational retrospective study. J. Infect. Public Health 2019, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]
- CARMIAAM-ROM 2021. Available online: https://insp.gov.ro/download/carmiaam-2021/e (accessed on 22 May 2024).
- Antibiotic Resistance: A Global Threat|CDC. Available online: https://www.cdc.gov/drugresistance/solutions-initiative/stories/ar-global-threat.html (accessed on 20 March 2024).
- Operational Public Health Considerations for the Prevention and Control of Infectious Diseases in the Context of Russia’s Aggression towards Ukraine. Available online: https://www.ecdc.europa.eu/en/publications-data/operational-public-health-considerations-prevention-and-control-infectious (accessed on 20 March 2024).
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Reuter, S.; Harris, S.R.; Glasner, C.; Feltwell, T.; Argimon, S.; Abudahab, K.; Goater, R.; Giani, T.; Errico, G.; et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 2019, 4, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Docquier, J.-D.; Mangani, S. An update on β-lactamase inhibitor discovery and development. Drug Resist. Updat. 2018, 36, 13–29. [Google Scholar] [CrossRef]
- Temkin, E.; Torre-Cisneros, J.; Beovic, B.; Benito, N.; Giannella, M.; Gilarranz, R.; Jeremiah, C.; Loeches, B.; Machuca, I.; Jiménez-Martín, M.J.; et al. Ceftazidime-Avibactam as Salvage Therapy for Infections Caused by Carbapenem-Resistant Organisms. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Shlaes, D.M. New β-lactam–β-lactamase inhibitor combinations in clinical development. Ann. N. Y. Acad. Sci. 2013, 1277, 105–114. [Google Scholar] [CrossRef]
- Sandfort, M.; Hans, J.B.; Fischer, M.A.; Reichert, F.; Cremanns, M.; Eisfeld, J.; Pfeifer, Y.; Heck, A.; Eckmanns, T.; Werner, G.; et al. Increase in NDM-1 and NDM-1/OXA-48-producing Klebsiella pneumoniae in Germany associated with the war in Ukraine, 2022. Eurosurveillance 2022, 27, 2200926. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.E.; Holmes, A.; Peck, R.; Livermore, D.M.; Hope, W. OXA-48-Like β-Lactamases: Global Epidemiology, Treatment Options, and Development Pipeline. Antimicrob. Agents Chemother. 2022, 66, e0021622. [Google Scholar] [CrossRef] [PubMed]
- Glasner, C.; Albiger, B.; Buist, G.; Andrašević, A.T.; Cantón, R.; Carmeli, Y.; Friedrich, A.W.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; et al. Carbapenemase-producing Enterobacteriaceae in Europe: A survey among national experts from 39 countries, February 2013. Eurosurveillance 2013, 18, 20525. [Google Scholar] [CrossRef]
- Liu, Y.; Long, D.; Xiang, T.-X.; Du, F.-L.; Wei, D.D.; Wan, L.-G.; Deng, Q.; Cao, X.-W.; Zhang, W. Whole genome assembly and functional portrait of hypervirulent extensively drug-resistant NDM-1 and KPC-2 co-producing Klebsiella pneumoniae of capsular serotype K2 and ST86. J. Antimicrob. Chemother. 2019, 74, 1233–1240. [Google Scholar] [CrossRef]
- Wei, D.-D.; Wan, L.-G.; Liu, Y. Draft Genome Sequence of an NDM-1- and KPC-2-Coproducing Hypervirulent Carbapenem-Resistant Klebsiella pneumoniae Strain Isolated from Burn Wound Infections. Genome Announc. 2018, 6, e00192-18. [Google Scholar] [CrossRef]
- Bes, T.; Nagano, D.; Martins, R.; Marchi, A.P.; Perdigão-Neto, L.; Higashino, H.; Prado, G.; Guimaraes, T.; Levin, A.S.; Costa, S. Bloodstream Infections caused by Klebsiella pneumoniae and Serratia marcescens isolates co-harboring NDM-1 and KPC-2. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, K.; Kalyanasundaram, A. Emergence of Klebsiella pneumoniae isolate co-producing NDM-1 with KPC-2 from India. J. Antimicrob. Chemother. 2012, 67, 243–244. [Google Scholar] [CrossRef]
- Bianco, G.; Boattini, M.; Comini, S.; Casale, R.; Iannaccone, M.; Cavallo, R.; Costa, C. Occurrence of multi-carbapenemases producers among carbapenemase-producing Enterobacterales and in vitro activity of combinations including cefiderocol, ceftazidime-avibactam, meropenem-vaborbactam, and aztreonam in the COVID-19 era. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 573–580. [Google Scholar] [CrossRef]
- Guo, H.; Wu, Y.; Li, L.; Wang, J.; Xu, J.; He, F. Global emergence of carbapenem-resistant Klebsiella pneumoniae co-carrying multiple carbapenemases. Comput. Struct. Biotechnol. J. 2023, 21, 3557–3563. [Google Scholar] [CrossRef]
- Székely, E.; Damjanova, I.; Jánvári, L.; Vas, K.E.; Molnár, S.; Bilca, D.V.; Lőrinczi, L.K.; Tóth, A. First description of bla(NDM-1), bla(OXA-48), bla(OXA-181) producing Enterobacteriaceae strains in Romania. Int. J. Med. Microbiol. 2013, 303, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Flonta, M.; Boudehen, Y.-M.; Creton, E.; Bernabeu, S.; Vogel, A.; Naas, T. Dissemination of Carbapenemase-Producing Enterobacteriaceae and Pseudomonas aeruginosa in Romania. Antimicrob. Agents Chemother. 2015, 59, 7100–7103. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, I.; Czobor, I.; Chifiriuc, M.C.; Borcan, E.; Ghiţă, C.; Banu, O.; Lazăr, V.; Mihăescu, G.; Mihăilescu, D.F.; Zhiyong, Z. Molecular screening of carbapenemase-producing Gram-negative strains in Romanian intensive care units during a one year survey. J. Med. Microbiol. 2014, 63 Pt 10, 1303–1310. [Google Scholar] [CrossRef]
- Molnar, S.; Vas, K.E.; Szekely, E. Carbapenemase Producing Enterobacterales in Romania: Investigating the Origins. Rom. Rev. Lab. Med. 2020, 28, 341–348. [Google Scholar] [CrossRef]
- Lixandru, B.E.; Cotar, A.I.; Straut, M.; Usein, C.R.; Cristea, D.; Ciontea, S.; Tatu-Chitoiu, D.; Codita, I.; Rafila, A.; Nica, M.; et al. Carbapenemase-Producing Klebsiella pneumoniae in Romania: A Six-Month Survey. PLoS ONE 2015, 10, e0143214. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase–Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Rombauts, A.; Tubau, F.; Padullés, A.; Càmara, J.; Lozano, T.; Cobo-Sacristán, S.; Sabe, N.; Grau, I.; Rigo-Bonnin, R.; et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J. Antimicrob. Chemother. 2018, 73, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Hujer, A.M.; Rojas, L.J.; Papp-Wallace, K.M.; Humphries, R.M.; Spellberg, B.; Hujer, K.M.; Marshall, E.K.; Rudin, S.D.; Perez, F.; et al. Can Ceftazidime-Avibactam and Aztreonam Overcome β-Lactam Resistance Conferred by Metallo-β-Lactamases in Enterobacteriaceae? Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Davido, B.; Fellous, L.; Lawrence, C.; Maxime, V.; Rottman, M.; Dinh, A. Ceftazidime-Avibactam and Aztreonam, an Interesting Strategy to Overcome β-Lactam Resistance Conferred by Metallo-β-Lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Kohira, N.; Hackel, M.A.; Ishioka, Y.; Kuroiwa, M.; Sahm, D.F.; Sato, T.; Maki, H.; Yamano, Y. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J. Glob. Antimicrob. Resist. 2020, 22, 738–741. [Google Scholar] [CrossRef]
- Poirel, L.; Sadek, M.; Nordmann, P. Contribution of PER-Type and NDM-Type β-Lactamases to Cefiderocol Resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, AAC0087721. [Google Scholar] [CrossRef]
- Lan, P.; Lu, Y.; Chen, Z.; Wu, X.; Hua, X.; Jiang, Y.; Zhou, J.; Yu, Y. Emergence of High-Level Cefiderocol Resistance in Carbapenem-Resistant Klebsiella pneumoniae from Bloodstream Infections in Patients with Hematologic Malignancies in China. Microbiol. Spectr. 2022, 10, e0008422. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Kazmierczak, K.M.; de Jonge, B.L.M.; Hackel, M.A.; Sahm, D.F.; Bradford, P.A. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Sadouki, Z.; Vickers, A.; Livermore, D.M.; Woodford, N. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Longshaw, C.; Manissero, D.; Tsuji, M.; Echols, R.; Yamano, Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC-Antimicrob. Resist. 2020, 2, dlaa060. [Google Scholar] [CrossRef] [PubMed]
- Nurjadi, D.; Kocer, K.; Chanthalangsy, Q.; Klein, S.; Heeg, K.; Boutin, S. New Delhi Metallo-Beta-Lactamase Facilitates the Emergence of Cefiderocol Resistance in Enterobacter cloacae. Antimicrob. Agents Chemother. 2022, 66, e0201121. [Google Scholar] [CrossRef]
- Poirel, L.; Sadek, M.; Kusaksizoglu, A.; Nordmann, P. Co-resistance to ceftazidime-avibactam and cefiderocol in clinical isolates producing KPC variants. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 677–680. [Google Scholar] [CrossRef]
- Tompkins, K.; van Duin, D. Treatment for carbapenem-resistant Enterobacterales infections: Recent advances and future directions. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2053–2068. [Google Scholar] [CrossRef]
- Carmeli, Y.; Cisneros, J.-M.; Paul, M.; Daikos, G.L.; Wang, M.; Cisneros, J.T.; Singer, G.; Titov, I.; Gumenchuk, I.; Zhao, Y.; et al. 2893 A. Efficacy and Safety of Aztreonam-Avibactam for the Treatment of Serious Infections Due to Gram-Negative Bacteria, Including Metallo-β-Lactamase-Producing Pathogens: Phase 3 REVISIT Study. Open Forum Infect. Dis. 2023, 10 (Suppl. S2), ofad500.2476. [Google Scholar] [CrossRef]
- Mauri, C.; Maraolo, A.E.; Di Bella, S.; Luzzaro, F.; Principe, L. The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases. Antibiotics 2021, 10, 1012. [Google Scholar] [CrossRef]


| Patient | Previous Antibiotic/Carbapenem Exposure | Site | Ambler Class | β-Lactamases | C/A (MIC) | ATM (MIC) | SYN | Cefiderocol | Colistin (MIC) | Meropenem (MIC) | Tigecycline | Fosfomycin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No/No | Urinary | A, B + D | NDM + Oxa-48 + CTX-M | R (>16 mg/L) | R (>64 mg/L) | Yes | S | R (>16 mg/L) | R (>16 mg/L) | S | R |
| 2 | Yes/No | Urinary | A, B + D | NDM + Oxa-48 + CTX-M | R (>16 mg/L) | R (>64 mg/L) | Yes | S | S (0.5 mg/L) | R (>16 mg/L) | R | R |
| 3 | Yes/Yes | Bacteriemia | A, B + D | NDM + Oxa-48 + CTX-M | R (>16 mg/L) | R (>64 mg/L) | Yes | S | R (>16 mg/L) | R (>16 mg/L) | I | - |
| 4 | Yes/Yes | Biliary | A, B + D | NDM + Oxa-48 + CTX-M | R (>16 mg/L) | R (>64 mg/L) | Yes | S | R (>16 mg/L) | R (>16 mg/L) | R | - |
| 5 | Yes/No | Urinary | A, B + D | NDM + Oxa-48 + CTX-M | R (>16 mg/L) | R (>64 mg/L) | Yes | R | R (>16 mg/L) | R (>16 mg/L) | R | R |
| 6 | Yes/No | Urinary | A, B + D | NDM + Oxa-48 + CTX-M | R (>16 mg/L) | R (>64 mg/L) | Yes | S | R (>16 mg/L) | R (>16 mg/L) | I | R |
| 7 | Yes/Yes | Peritoneal | A, B + D | NDM + Oxa-48 + CTX-M | R (>16 mg/L) | R (>64 mg/L) | Yes | S | R (>16 mg/L) | R (>16 mg/L) | R | R |
| Patient | Duration of Treatment | Doses (C/A + ATM) | Length of Hospitalization (Days) | Clinical Recovery | Microbiological Recovery | Recurrence at 30 Days | Re-Admission (Other) at 30 Days | Death at 30 Days |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 2.5 g q8h + 2 g q8h | 12 | Yes | Yes | No | Yes | No |
| 2 | 7 | 2.5 g q8h + 2 g q8h | 10 | Yes | Yes | No | Yes | No |
| 3 | 10 | 2.5 g q8h + 2 g q8h | >30 | Yes | Yes | No | Yes | No |
| 4 | 7 | 2.5 g q8h + 2 g q8h | 28 | Yes | N/A | No | No | No |
| 5 | 14 | 2.5 g q8h + 2 g q8h | 14 | Yes | Yes | No | Yes | No |
| 6 | 12 | 1.25 q12h + 2 g q12h | 20 | Yes | Yes | No | No | No |
| 7 | 7 | 2.5 g q8h + 2 g q8h | >30 | Yes | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cismaru, I.M.; Văcăroiu, M.C.; Soium, E.; Holban, T.; Radu, A.M.; Melinte, V.; Gheorghiță, V. Synergistic Combination of Aztreonam and Ceftazidime–Avibactam—A Promising Defense Strategy against OXA-48 + NDM Klebsiella pneumoniae in Romania. Antibiotics 2024, 13, 550. https://doi.org/10.3390/antibiotics13060550
Cismaru IM, Văcăroiu MC, Soium E, Holban T, Radu AM, Melinte V, Gheorghiță V. Synergistic Combination of Aztreonam and Ceftazidime–Avibactam—A Promising Defense Strategy against OXA-48 + NDM Klebsiella pneumoniae in Romania. Antibiotics. 2024; 13(6):550. https://doi.org/10.3390/antibiotics13060550
Chicago/Turabian StyleCismaru, Ioana Miriana, Maria Cristina Văcăroiu, Elif Soium, Tiberiu Holban, Adelina Maria Radu, Violeta Melinte, and Valeriu Gheorghiță. 2024. "Synergistic Combination of Aztreonam and Ceftazidime–Avibactam—A Promising Defense Strategy against OXA-48 + NDM Klebsiella pneumoniae in Romania" Antibiotics 13, no. 6: 550. https://doi.org/10.3390/antibiotics13060550
APA StyleCismaru, I. M., Văcăroiu, M. C., Soium, E., Holban, T., Radu, A. M., Melinte, V., & Gheorghiță, V. (2024). Synergistic Combination of Aztreonam and Ceftazidime–Avibactam—A Promising Defense Strategy against OXA-48 + NDM Klebsiella pneumoniae in Romania. Antibiotics, 13(6), 550. https://doi.org/10.3390/antibiotics13060550









