Efficacy of Short Novel Antimicrobial Peptides in a Mouse Model of Staphylococcus pseudintermedius Skin Infection
Abstract
1. Introduction
2. Results
2.1. Skin Infection Model
2.1.1. Establishment of a Skin S. pseudintermediu Infection Model
2.1.2. Trauma Observation
2.1.3. Wound Area Measurement
2.2. Bacterial Enumeration
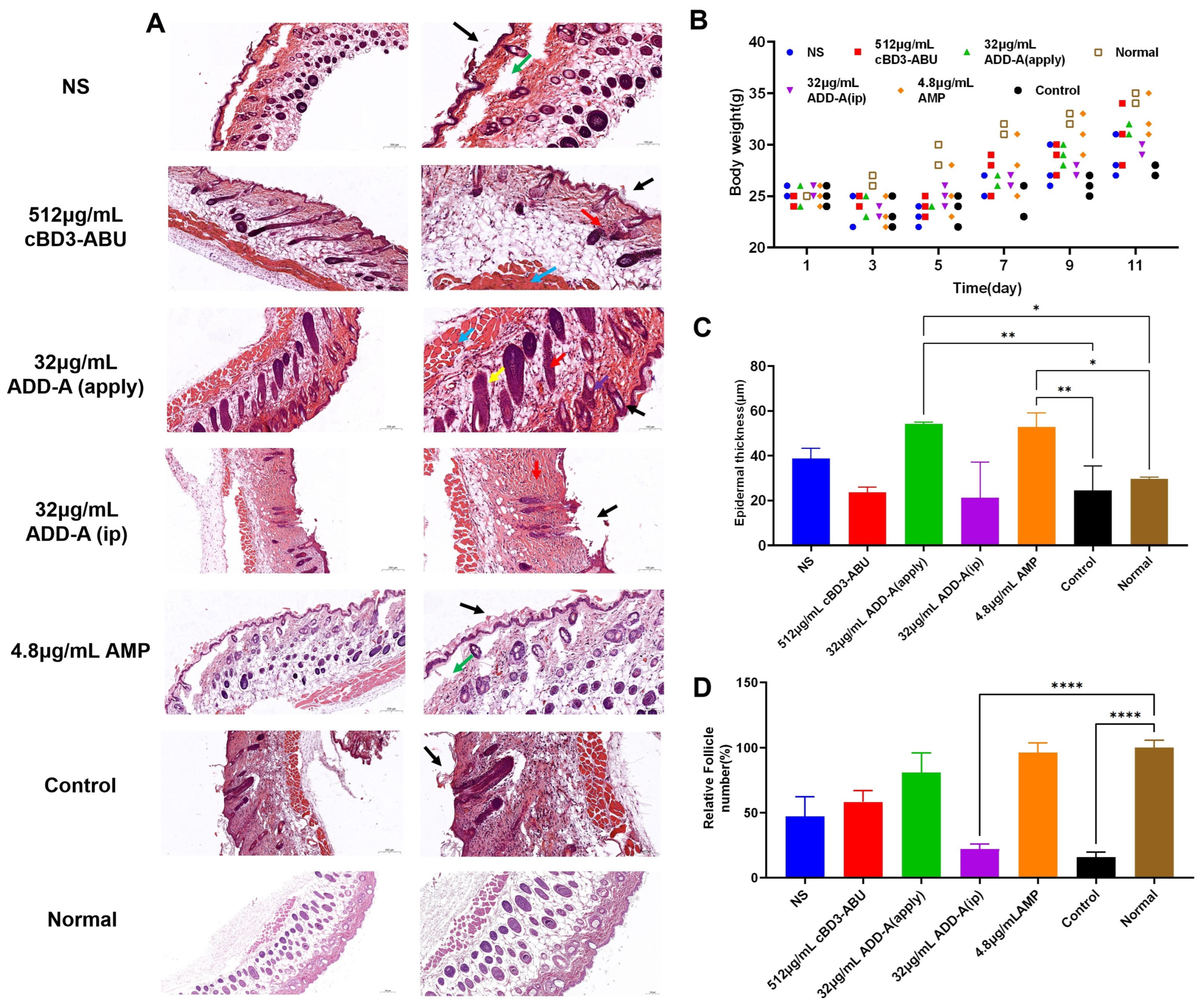
2.3. Hematoxylin-Eosin (HE) Staining Results
2.4. Weight of Mice
2.5. Epidermal Thickness
2.6. Relative Follicle Number
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolate
4.2. Antimicrobial Peptides
4.3. Animals
4.4. Main Reagents
4.5. Preparation of Bacterial Suspensions
4.6. Observations on the Bacteriostatic Effect of ADD-A on Mice with S. pseudintermediu Infection Model
4.6.1. Mouse Skin Infection Model
4.6.2. Animal Grouping and Handling
4.6.3. Bacterial Enumeration
4.6.4. Observation of Pathological Tissue Sections
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wegener, A.; Broens, E.M.; Zomer, A.; Spaninks, M.; Wagenaar, J.A.; Duim, B. Comparative genomics of phenotypic antimicrobial resistances in methicillin-resistant Staphylococcus pseudintermedius of canine origin. Vet. Microbiol. 2018, 225, 125–131. [Google Scholar] [CrossRef]
- Guimaraes, L.; Teixeira, I.M.; da Silva, I.T.; Antunes, M.; Pesset, C.; Fonseca, C.; Santos, A.L.; Cortes, M.F.; Penna, B. Epidemiologic case investigation on the zoonotic transmission of Methicillin-resistant Staphylococcus pseudintermedius among dogs and their owners. J. Infect. Public Health 2023, 16 (Suppl. 1), 183–189. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.L.; Morris, D.O.; Griffeth, G.C.; Shofer, F.S.; Rankin, S.C. Surveillance of healthy cats and cats with inflammatory skin disease for colonization of the skin by methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi ssp. schleiferi. Vet. Dermatol. 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Ganiere, J.P.; Medaille, C.; Mangion, C. Antimicrobial drug susceptibility of Staphylococcus intermedius clinical isolates from canine pyoderma. J. Vet. Med. Ser. B-Infect. Dis. Vet. Public Health 2005, 52, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.M.; Waisglass, S.E.; Dick, H.L.N.; Weese, J.S. Prevalence of meticillin-resistant Staphylococcus pseudintermedius (MRSP) from skin and carriage sites of dogs after treatment of their meticillin-resistant or meticillin-sensitive staphylococcal pyoderma. Vet. Dermatol. 2012, 23, 369-e67. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, J.; Williams, N.; Carter, S.; McEwan, N.; Nuttall, T. Heterogeneity of Staphylococcus pseudintermedius isolates from atopic and healthy dogs. Vet. Dermatol. 2010, 21, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Papic, B.; Golob, M.; Zdovc, I.; Kusar, D.; Avbersek, J. Genomic insights into the emergence and spread of methicillin-resistant Staphylococcus pseudintermedius in veterinary clinics. Vet. Microbiol. 2021, 258, 109119. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H.; Pages, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Soares Filipe, J.F.; Drago, L.; Martino, P.A. Investigation on Antibiotic-Resistance, Biofilm Formation and Virulence Factors in Multi Drug Resistant and Non Multi Drug Resistant Staphylococcus pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef]
- Lozano, C.; Rezusta, A.; Ferrer, I.; Perez-Laguna, V.; Zarazaga, M.; Ruiz-Ripa, L.; Revillo, M.J.; Torres, C. Staphylococcus pseudintermedius Human Infection Cases in Spain: Dog-to-Human Transmission. Vector Borne Zoonotic Dis. 2017, 17, 268–270. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert. Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Geitani, R.; Ayoub Moubareck, C.; Touqui, L.; Karam Sarkis, D. Cationic antimicrobial peptides: Alternatives and/or adjuvants to antibiotics active against methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa. BMC Microbiol. 2019, 19, 54. [Google Scholar] [CrossRef]
- Yamauchi, R.; Kawano, K.; Yamaoka, Y.; Taniguchi, A.; Yano, Y.; Takasu, K.; Matsuzaki, K. Development of Antimicrobial Peptide–Antibiotic Conjugates to Improve the Outer Membrane Permeability of Antibiotics Against Gram-Negative Bacteria. ACS Infect. Dis. 2022, 8, 2339–2347. [Google Scholar] [CrossRef]
- Brahma, B.; Patra, M.C.; Karri, S.; Chopra, M.; Mishra, P.; De, B.C.; Kumar, S.; Mahanty, S.; Thakur, K.; Poluri, K.M.; et al. Diversity, Antimicrobial Action and Structure-Activity Relationship of Buffalo Cathelicidins. PLoS ONE 2015, 10, e0144741. [Google Scholar] [CrossRef]
- Lau, Q.Y.; Ng, F.M.; Cheong, J.W.; Yap, Y.Y.; Tan, Y.Y.; Jureen, R.; Hill, J.; Chia, C.S. Discovery of an ultra-short linear antibacterial tetrapeptide with anti-MRSA activity from a structure-activity relationship study. Eur. J. Med. Chem. 2015, 105, 138–144. [Google Scholar] [CrossRef]
- Alvarez-Bravo, J.; Kurata, S.; Natori, S. Novel synthetic antimicrobial peptides effective against methicillin-resistant Staphylococcus aureus. Biochem. J. 1994, 302, 535–538. [Google Scholar] [CrossRef]
- Sang, Y.; Ortega, M.T.; Blecha, F.; Prakash, O.; Melgarejo, T. Molecular cloning and characterization of three beta-defensins from canine testes. Infect. Immun. 2005, 73, 2611–2620. [Google Scholar] [CrossRef]
- Santoro, D.; Bunick, D.; Graves, T.K.; Campbell, K.L. Expression and distribution of antimicrobial peptides in the skin of healthy beagles. Vet. Dermatol. 2011, 22, 61–67. [Google Scholar] [CrossRef]
- Kluver, E.; Schulz-Maronde, S.; Scheid, S.; Meyer, B.; Forssmann, W.G.; Adermann, K. Structure-activity relation of human beta-defensin 3: Influence of disulfide bonds and cysteine substitution on antimicrobial activity and cytotoxicity. Biochemistry 2005, 44, 9804–9816. [Google Scholar] [CrossRef]
- Ishibashi, J.; Saido-Sakanaka, H.; Yang, J.; Sagisaka, A.; Yamakawa, M. Purification, cDNA cloning and modification of a defensin from the coconut rhinoceros beetle, Oryctes rhinoceros. Eur. J. Biochem. 1999, 266, 616–623. [Google Scholar] [CrossRef]
- Saido-Sakanaka, H.; Ishibashi, J.; Momotani, E.; Amano, F.; Yamakawa, M. In vitro and in vivo activity of antimicrobial peptides synthesized based on the insect defensin. Peptides 2004, 25, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Seo, M.; Lee, H.J.; Baek, M.; Kim, I.W.; Kim, S.Y.; Kim, M.A.; Kim, S.H.; Hwang, J.S. Anti-Inflammatory Activity of Antimicrobial Peptide Allomyrinasin Derived from the Dynastid Beetle, Allomyrina dichotoma. J. Microbiol. Biotechnol. 2019, 29, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Motobu, M.; Hikosaka, K.; Yamada, M.; Nakamura, K.; Saido-Sakanaka, H.; Asaoka, A.; Yamakawa, M.; Sekikawa, K.; Kitani, H.; et al. Protective effects of antimicrobial peptides derived from the beetle Allomyrina dichotoma defensin on endotoxic shock in mice. Int. Immunopharmacol. 2006, 6, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-R.; Ouyang, M.-Y.; Zhao, Y.-X.; Wang, Y.-Q.; Hu, C.-M. Analogues designed from cBD3 against multidrug resistant bacteria from canine skin. Anim. Breed. Feed. 2022; accepted. [Google Scholar]
- Zhao, T.; Shao, J.; Wang, Z.; Yan, R.; Lei, Y.; Liu, X.; Hu, C. Isolation and identification of bacterial pathogens of canine and feline dermatosis and analysis of the inhibitory effect of natural products. China Anim. Husb. Vet. Med. 2022, 49, 1556–1567. [Google Scholar] [CrossRef]
- Zamora-Carreras, H.; Strandberg, E.; Muhlhauser, P.; Burck, J.; Wadhwani, P.; Jimenez, M.A.; Bruix, M.; Ulrich, A.S. Alanine scan and 2H NMR analysis of the membrane-active peptide BP100 point to a distinct carpet mechanism of action. Biochim. Biophys. Acta 2016, 1858, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Maroti, G.; Kereszt, A.; Kondorosi, E.; Mergaert, P. Natural roles of antimicrobial peptides in microbes, plants and animals. Res. Microbiol. 2011, 162, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Lin, S.; Beuerman, R.W.; Liu, S. Recent advances in synthetic lipopeptides as anti-microbial agents: Designs and synthetic approaches. Amino Acids 2017, 49, 1653–1677. [Google Scholar] [CrossRef]
- Greber, K.E.; Zielińska, J.; Nierzwicki, Ł.; Ciura, K.; Kawczak, P.; Nowakowska, J.; Bączek, T.; Sawicki, W. Are the short cationic lipopeptides bacterial membrane disruptors? Structure-Activity Relationship and molecular dynamic evaluation. Biochim. Biophys. Acta Biomembr. 2019, 1861, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Latendorf, T.; Gerstel, U.; Wu, Z.; Bartels, J.; Becker, A.; Tholey, A.; Schroder, J.M. Cationic Intrinsically Disordered Antimicrobial Peptides (CIDAMPs) Represent a New Paradigm of Innate Defense with a Potential for Novel Anti-Infectives. Sci. Rep. 2019, 9, 3331. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.C.; Strandberg, E.; Grau-Campistany, A.; Wadhwani, P.; Reichert, J.; Bürck, J.; Rabanal, F.; Auger, M.; Paquin, J.F.; Ulrich, A.S. Influence of the Length and Charge on the Activity of α-Helical Amphipathic Antimicrobial Peptides. Biochemistry 2017, 56, 1680–1695. [Google Scholar] [CrossRef] [PubMed]
- Grau-Campistany, A.; Strandberg, E.; Wadhwani, P.; Reichert, J.; Burck, J.; Rabanal, F.; Ulrich, A.S. Hydrophobic mismatch demonstrated for membranolytic peptides, and their use as molecular rulers to measure bilayer thickness in native cells. Sci. Rep. 2015, 5, 9388. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Perez-Paya, E.; Houghten, R.A.; Blondelle, S.E. The role of amphipathicity in the folding, self-association and biological activity of multiple subunit small proteins. J. Biol. Chem. 1995, 270, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cebrian, R.; Montalban-Lopez, M.; Ren, H.; Wu, W.; Kuipers, O.P. Outer-membrane-acting peptides and lipid II-targeting antibiotics cooperatively kill Gram-negative pathogens. Commun. Biol. 2021, 4, 31. [Google Scholar] [CrossRef]


| Groups | Quantities | Traumatic Injury | Bacterial Infection | Dosages | Mode of Infection | Antimicrobial Treatment | Route of Administration |
|---|---|---|---|---|---|---|---|
| Group 1 | 5 | Haemorrhage under the skin | S. pseudintermediu bacterial solution | 1 mL | Cotton swab application | saline | Cotton swab application |
| Group 2 | 5 | Haemorrhage under the skin | S. pseudintermediu bacterial solution | 1 mL | Cotton swab application | 512 μg/mL cBD3-ABU | Cotton swab application |
| Group 3 | 5 | Haemorrhage under the skin | S. pseudintermediu bacterial solution | 1 mL | Cotton swab application | 32 μg/mL ADD-A | Cotton swab application |
| Group 4 | 5 | Haemorrhage under the skin | S. pseudintermediu bacterial solution | 1 mL | Cotton swab application | 32 μg/mL ADD-A | intraperitoneal injection |
| Group 5 | 5 | Haemorrhage under the skin | S. pseudintermediu bacterial solution | 1 mL | Cotton swab application | 4.8 μg/mL ampicillin sodium | Cotton swab application |
| Group 6 | 5 | Haemorrhage under the skin | Not handled | ||||
| Group 7 | 5 | Not handled |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, M.; Wu, F.; Hu, C. Efficacy of Short Novel Antimicrobial Peptides in a Mouse Model of Staphylococcus pseudintermedius Skin Infection. Antibiotics 2024, 13, 508. https://doi.org/10.3390/antibiotics13060508
Ouyang M, Wu F, Hu C. Efficacy of Short Novel Antimicrobial Peptides in a Mouse Model of Staphylococcus pseudintermedius Skin Infection. Antibiotics. 2024; 13(6):508. https://doi.org/10.3390/antibiotics13060508
Chicago/Turabian StyleOuyang, Mingyu, Fangrong Wu, and Changmin Hu. 2024. "Efficacy of Short Novel Antimicrobial Peptides in a Mouse Model of Staphylococcus pseudintermedius Skin Infection" Antibiotics 13, no. 6: 508. https://doi.org/10.3390/antibiotics13060508
APA StyleOuyang, M., Wu, F., & Hu, C. (2024). Efficacy of Short Novel Antimicrobial Peptides in a Mouse Model of Staphylococcus pseudintermedius Skin Infection. Antibiotics, 13(6), 508. https://doi.org/10.3390/antibiotics13060508





