Detection of KPC-216, a Novel KPC-3 Variant, in a Clinical Isolate of Klebsiella pneumoniae ST101 Co-Resistant to Ceftazidime-Avibactam and Cefiderocol
Abstract
1. Introduction
2. Results
2.1. Strain Collection
2.2. Antimicrobial Susceptibility Testing
2.3. Whole-Genome Sequencing and In Silico Analysis
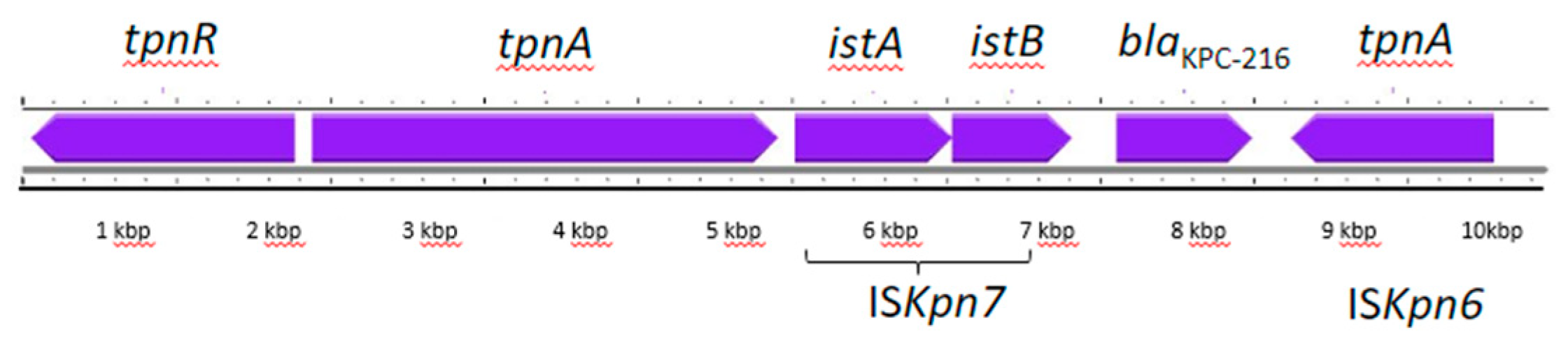
2.4. Plasmid Analysis
2.5. Virulence Factors Analysis
3. Discussion
4. Materials and Methods
4.1. Phenotypic Analysis
4.2. Whole-Genome Sequencing and In Silico Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2017, 3, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Fahrbach, K.; Zhao, Q.; Lodise, T. Association between Carbapenem Resistance and Mortality Among Adult, Hospitalized Patients with Serious Infections Due to Enterobacteriaceae: Results of a Systematic Literature Review and Meta-analysis. Open Forum Infect. Dis. 2018, 5, ofy150. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Earley, M.; Chen, L.; Hanson, B.M.; Yu, Y.; Liu, Z.; Salcedo, S.; Cober, E.; Li, L.; Kanj, S.S.; et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): A prospective, multicentre, cohort study. Lancet Infect. Dis. 2022, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- El-Lababidi, R.M.; Rizk, J.G. Cefiderocol: A Siderophore Cephalosporin. Ann. Pharmacother. 2020, 54, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Giske, C.G.; Gramatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-lactam- β-lactamase inhibitor combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Q.; Zhou, P.; Deng, S. Ceftazidime-avibactam versus polymyxins in treating patients with carbapenem-resistant Enterobacteriaceae infections: A systematic review and meta-analysis. Infection 2024, 52, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.A.; Pierrat, G.; Tenaillon, O.; Bonacorsi, S.; Bercot, B.; Jaouen, E.; Jacquier, H.; Birgy, A. Klebsiella pneumoniae Carbapenemase Variants Resistant to Ceftazidime-Avibactam: An Evolutionary Overview. Antimicrob. Agents Chemother. 2022, 66, e0044722. [Google Scholar] [CrossRef]
- Shen, Z.; Ding, B.; Ye, M.; Wang, P.; Bi, Y.; Wu, S.; Xu, X.; Guo, Q.; Wang, M. High ceftazidime hydrolysis activity and porin OmpK35 deficiency contribute to the decreased susceptibility to ceftazidime/avibactam in KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2017, 72, 1930–1936. [Google Scholar] [CrossRef]
- Di Pilato, V.; Principe, L.; Andriani, L.; Aiezza, N.; Coppi, M.; Ricci, S.; Giani, T.; Luzzaro, F.; Rossolini, G.M. Deciphering variable resistance to novel carbapenem-based β-lactamase inhibitor combinations in a multi-clonal outbreak caused by Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae resistant to ceftazidime/avibactam. Clin. Microbiol. Infect. 2022, 29, 537.e1–537.e8. [Google Scholar] [CrossRef]
- Hernández-García, M.; Castillo-Polo, J.A.; Cordero, D.G.; Pérez-Viso, B.; García-Castillo, M.; de la Fuente, J.S.; Morosini, M.I.; Cantón, R.; Ruiz-Garbajosa, P. Impact of Ceftazidime-Avibactam Treatment in the Emergence of Novel KPC Variants in the ST307-Klebsiella pneumoniae High-Risk Clone and Consequences for Their Routine Detection. J. Clin. Microbiol. 2022, 60, e0224521. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.A.; Cointe, A.; Jacquier, H.; Choudhury, A.; Magnan, M.; Courroux, C.; Tenaillon, O.; Bonacorsi, S.; Birgy, A. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC β-lactamase mutants and the inoculum effect. Clin. Microbiol. Infect. 2021, 27, 1172.e7–1172.e10. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Lu, Y.; Jiang, Y.; Wu, X.; Yu, Y.; Zhou, J. Catecholate siderophore receptor CirA impacts cefiderocol susceptibility in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2022, 60, 106646. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Arcari, G.; Bibbolino, G.; Sacco, F.; Tomolillo, D.; Di Lella, F.M.; Trancassini, M.; Faino, L.; Venditti, M.; Antonelli, G.; et al. Evolutionary Trajectories toward Ceftazidime-Avibactam Resistance in Klebsiella pneumoniae Clinical Isolates. Antimicrob. Agents Chemother. 2021, 65, e0057421. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. Interplay between β-lactamases and new β-lactamase inhibitors. Nat. Rev. Microbiol. 2019, 17, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-Avibactam Use for Klebsiella pneumoniae Carbapenemase–Producing K. pneumoniae Infections: A Retrospective Observational Multicenter Study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Chen, L.; Kreiswirth, B.N.; Clancy, C.J. Emergence of Ceftazidime-Avibactam Resistance and Restoration of Carbapenem Susceptibility in Klebsiella pneumoniae Carbapenemase-Producing K pneumoniae: A Case Report and Review of Literature. Open Forum Infect. Dis. 2017, 4, ofx101. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shen, S.; Chen, J.; Tian, Z.; Shi, Q.; Han, R.; Guo, Y.; Hu, F. Klebsiella pneumoniae carbapenemase variants: The new threat to global public health. Clin. Microbiol. Rev. 2023, 36, e0000823. [Google Scholar] [CrossRef]
- David, S.; Wong, J.L.C.; Sanchez-Garrido, J.; Kwong, H.-S.; Low, W.W.; Morecchiato, F.; Giani, T.; Rossolini, G.M.; Brett, S.J.; Clements, A.; et al. Widespread emergence of OmpK36 loop 3 insertions among multidrug-resistant clones of Klebsiella pneumoniae. PLoS Pathog. 2022, 18, e1010334. [Google Scholar] [CrossRef]
- Haidar, G.; Clancy, C.J.; Shields, R.K.; Hao, B.; Cheng, S.; Nguyen, M.H. Mutations in bla KPC-3 That Confer Ceftazidime-Avibactam Resistance Encode Novel KPC-3 Variants That Function as Extended-Spectrum β-Lactamases. Antimicrob. Agents Chemother. 2017, 61, e02534-16. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Sadek, M.; Kusaksizoglu, A.; Nordmann, P. Co-resistance to ceftazidime-avibactam and cefiderocol in clinical isolates producing KPC variants. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.; Poirel, L.; Juhas, M.; Nordmann, P. KPC-Mediated Resistance to Ceftazidime-Avibactam and Collateral Effects in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2021, 65, AAC0089021. [Google Scholar] [CrossRef]
- Mathers, A.J.; Peirano, G.; Pitout, J.D.D. The Role of Epidemic Resistance Plasmids and International High-Risk Clones in the Spread of Multidrug-Resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Cuzon, G.; Villegas, M.-V.; Lartigue, M.-F.; Quinn, J.P.; Nordmann, P. Genetic Structures at the Origin of Acquisition of the β-Lactamase bla KPC Gene. Antimicrob. Agents Chemother. 2008, 52, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Codda, G.; Niccolai, C.; Willison, E.; Wong, J.L.; Coppo, E.; Frankel, G.; Marchese, A.; Rossolini, G.M. Functional features of KPC-109, a novel 270-loop KPC-3 mutant mediating resistance to avibactam-based β-lactamase inhibitor combinations and cefiderocol. Int. J. Antimicrob. Agents 2024, 63, 107030. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Errico, G.; Monaco, M.; Giani, T.; Del Grosso, M.; Antonelli, A.; David, S.; Lindh, E.; Camilli, R.; Aanensen, D.M.; et al. The changing epidemiology of carbapenemase-producing Klebsiella pneumoniaein Italy: Toward polyclonal evolution with emergence of high-risk lineages. J. Antimicrob. Chemother. 2020, 76, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, M.; Rossini, A.; Scudeller, L.; Zabzuni, D.; Errico, G.; Fogato, E.; D’Angelo, R.; Silverj, F.G.; Cesana, E.; Bergamaschini, L.C.; et al. Dynamics of carbapenemase-producing Enterobacterales intestinal colonisation in the elderly population after hospital discharge, Italy, 2018-2020. Int. J. Antimicrob. Agents 2022, 59, 106594. [Google Scholar] [CrossRef]
- Lim, C.J.; Cheng, A.C.; Kennon, J.; Spelman, D.; Hale, D.; Melican, G.; Sidjabat, H.E.; Paterson, D.L.; Kong, D.C.M.; Peleg, A.Y. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: A nested case-control study. J. Antimicrob. Chemother. 2014, 69, 1972–1980. [Google Scholar] [CrossRef]
- EUCAST. EUCAST Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. Available online: http://www.eucast.org (accessed on 23 April 2024).
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
| Antibiotics | MIC (mg/L)/Susceptibility Category 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| KP_RM_1 (October 2022) | KP_RM_BSI (September 2022) | KP_RM_UTI (September 2022) | EC 2_RM_BSI (September 2022) | |||||
| Amikacin | ≥32 | R | na 3 | na | na | na | na | na |
| Ampicillin | ≥16 | R | na | na | na | na | na | na |
| Amoxicillin/clavulanic acid | 16/2 | R | ≥32 | R | ≥32 | R | ≥32 | R |
| Cefepime | ≥8 | R | ≥32 | R | ≥32 | R | 16 | R |
| Ceftazidime | ≥8 | R | ≥64 | R | ≥64 | R | ≥64 | R |
| Ceftazidime/avibactam (CAZ-AVI) | 16/4 | R | 4 | S | na | na | ≤0.12 | S |
| Cefotaxime | ≥4 | R | na | na | na | na | na | na |
| Cefiderocol (FDC) | ≥8 | R | na | na | na | na | na | na |
| Ciprofloxacin | ≥1 | R | ≥4 | R | ≥4 | R | ≥4 | R |
| Colistin | ≤0.5 | S | 0.5 | S | na | na | 0.5 | S |
| Ertapenem | ≥2 | R | na | na | na | na | na | na |
| Imipenem | ≤1 | S | ≥16 | R | 8 | R | ≤0.125 | S |
| Imipenem–relebactam (IMR) | 0.25/4 | S | na | na | na | na | na | na |
| Gentamicin | ≥8 | R | ≥16 | R | ≥16 | R | ≥16 | R |
| Levofloxacin | ≥2 | R | na | na | na | na | na | na |
| Meropenem | 2 | S | ≥16 | R | ≥16 | R | ≤0.125 | S |
| Meropenem–vaborbactam (MVB) | 0.5/8 | S | 4 | S | na | na | na | na |
| Nitrofurantoin | ≥64 | R | na | na | na | na | na | na |
| Piperacillin/tazobactam | 16/4 | R | ≥128 | R | ≥128 | R | 8 | S |
| Tigecycline | ≤0.5 | S | na | na | na | na | na | na |
| Tobramycin | ≥8 | R | ≥16 | R | na | na | ≥16 | R |
| Trimethoprim/sulphamethoxazole | ≤1/19 | S | ≤20 | S | ≤20 | S | ≥320 | R |
| Resistant to | Localization | |
|---|---|---|
| Resistance gene | ||
| armA | Aminoglycosides | Plasmid |
| blaSHV-1 | β-lactams | Chromosome |
| blaKPC-216 | β-lactams | Plasmid |
| msr(E) | Macrolides, lincosamides and streptogramins (MLS) | Plasmid |
| mph(E) | Macrolides, lincosamides and streptogramins (MLS) | Plasmid |
| Protein substitution | ||
| GyrA S83Y | Quinolones | Chromosome |
| GyrA D87N | Quinolones | Chromosome |
| ParC S80I | Quinolones | Chromosome |
| OmpK35 AA63 stop | β-lactams | Chromosome |
| OmpK36 134TD duplication | β-lactams | Chromosome |
| Plasmid ID | Length (bp) | Plasmid Replicon | pMLST | Resistance Genes |
|---|---|---|---|---|
| P003 | 5356 | Col440II | - | |
| P014 | 7585 | Col156 | - | |
| P026 | 9289 | ColRNAI | - | |
| P040 | 5592 | ColRNAI | - | |
| P007 | 72,398 | IncFIA(HI1), IncFII(K), IncR | [K2:A13:B-] | blaKPC-216, armA, mph(E), msr(E) |
| VFclass | Virulence Factors | Related Genes |
|---|---|---|
| Adherence | Type 3 fimbriae | mrkA, mrkB, mrkC, mrkD, mrkF, mrkH, mrkI, mrkJ |
| Type I fimbriae | fimA, fimB, fimC, fimD, fimE, fimF, fimG, fimH, fimI, fimK | |
| Type IV pili (Yersinia) | pilW | |
| Antiphagocytosis | Capsule | wiz |
| Efflux pump | AcrAB | acrA, acrB |
| Iron uptake | Aerobactin | iutA |
| Ent siderophore | entA, entB, entC, entD, entE, entF, entS, fepA, fepB, fepC, fepD, fepG, fes | |
| Salmochelin | iroE, iroN | |
| Yersiniabactin | fyuA, irp1, irp2, ybtA, ybtE, ybtP, ybtQ, ybtS, ybtT, ybtU, ybtX | |
| Regulation | RcsAB | rcsA, rcsB |
| Secretion system | T6SS-I | clpV/tssH, dotU/tssL, hcp/tssD, icmF/tssM, impA/tssA, ompA, sciN/tssJ, tssF, tssG, vasE/tssK, vgrG/tssI, vipA/tssB, vipB/tssC |
| T6SS-II | clpV, impH, impJ, sciN, vasA/impG | |
| T6SS-III | dotU, icmF, impA, impF, impG, impH, impJ, ompA, sciN, vgrG | |
| Serum resistance | LPS rfb locus | rfb |
| Fimbrial adherence determinants | Stb (Salmonella) | stbA, stbB, stbC, stbD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giufrè, M.; Errico, G.; Del Grosso, M.; Pagnotta, M.; Palazzotti, B.; Ballardini, M.; Pantosti, A.; Meledandri, M.; Monaco, M. Detection of KPC-216, a Novel KPC-3 Variant, in a Clinical Isolate of Klebsiella pneumoniae ST101 Co-Resistant to Ceftazidime-Avibactam and Cefiderocol. Antibiotics 2024, 13, 507. https://doi.org/10.3390/antibiotics13060507
Giufrè M, Errico G, Del Grosso M, Pagnotta M, Palazzotti B, Ballardini M, Pantosti A, Meledandri M, Monaco M. Detection of KPC-216, a Novel KPC-3 Variant, in a Clinical Isolate of Klebsiella pneumoniae ST101 Co-Resistant to Ceftazidime-Avibactam and Cefiderocol. Antibiotics. 2024; 13(6):507. https://doi.org/10.3390/antibiotics13060507
Chicago/Turabian StyleGiufrè, Maria, Giulia Errico, Maria Del Grosso, Michela Pagnotta, Bernardetta Palazzotti, Milva Ballardini, Annalisa Pantosti, Marcello Meledandri, and Monica Monaco. 2024. "Detection of KPC-216, a Novel KPC-3 Variant, in a Clinical Isolate of Klebsiella pneumoniae ST101 Co-Resistant to Ceftazidime-Avibactam and Cefiderocol" Antibiotics 13, no. 6: 507. https://doi.org/10.3390/antibiotics13060507
APA StyleGiufrè, M., Errico, G., Del Grosso, M., Pagnotta, M., Palazzotti, B., Ballardini, M., Pantosti, A., Meledandri, M., & Monaco, M. (2024). Detection of KPC-216, a Novel KPC-3 Variant, in a Clinical Isolate of Klebsiella pneumoniae ST101 Co-Resistant to Ceftazidime-Avibactam and Cefiderocol. Antibiotics, 13(6), 507. https://doi.org/10.3390/antibiotics13060507






