Exploring the Molecular Mechanisms of Macrolide Resistance in Laboratory Mutant Helicobacter pylori
Abstract
1. Introduction
2. Results
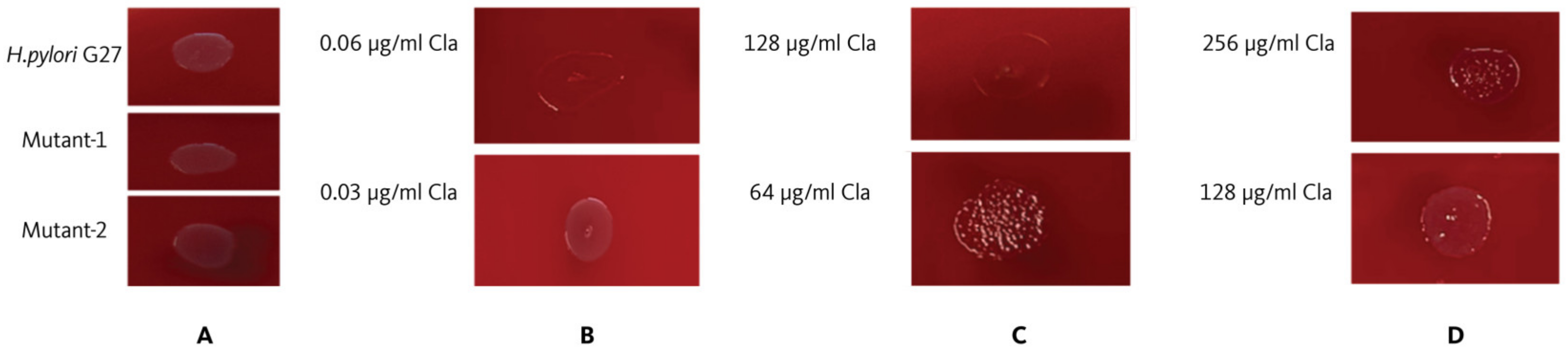
2.1. Clarithromycin Minimum Inhibitory Concentration (MIC) Value for Helicobacter pylori G27
2.2. Establishment of Resistant Strains
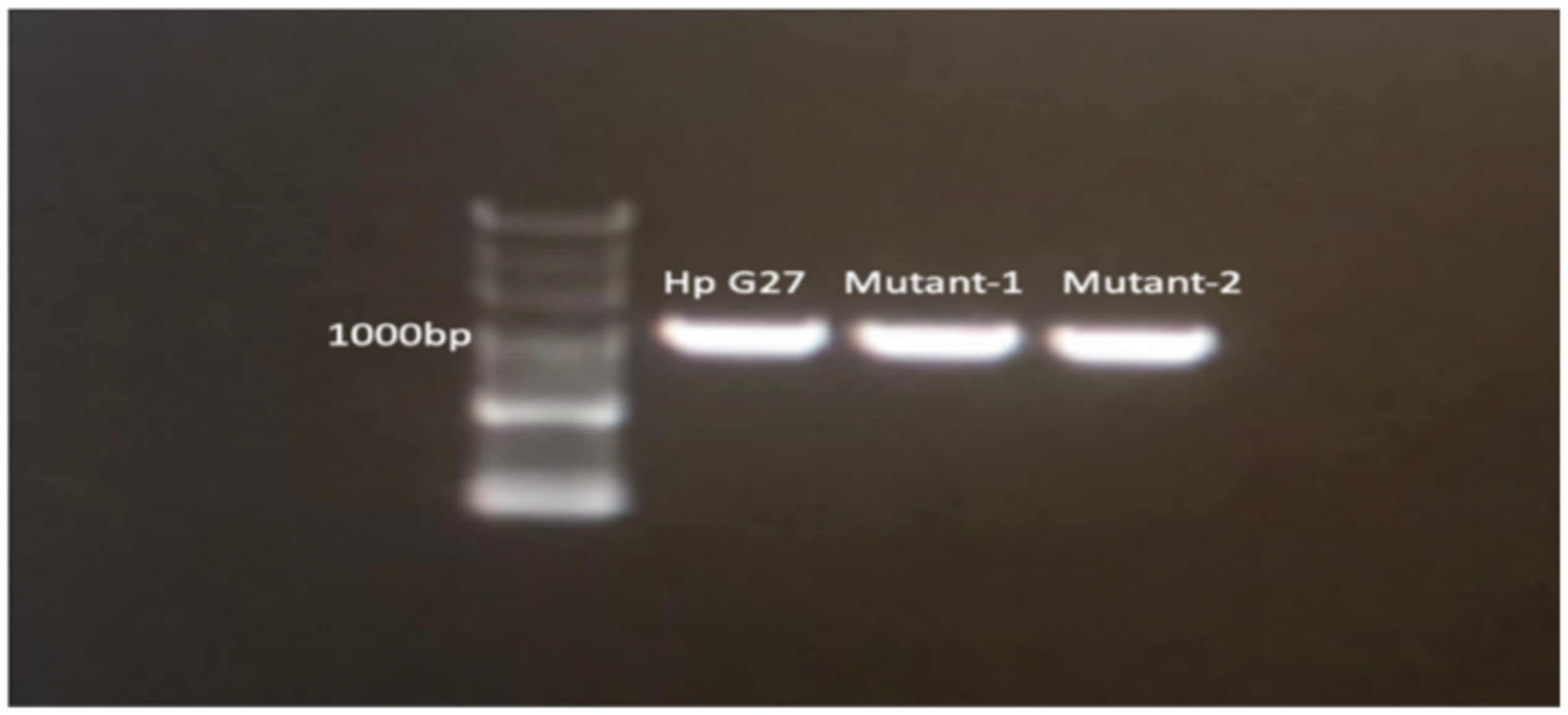
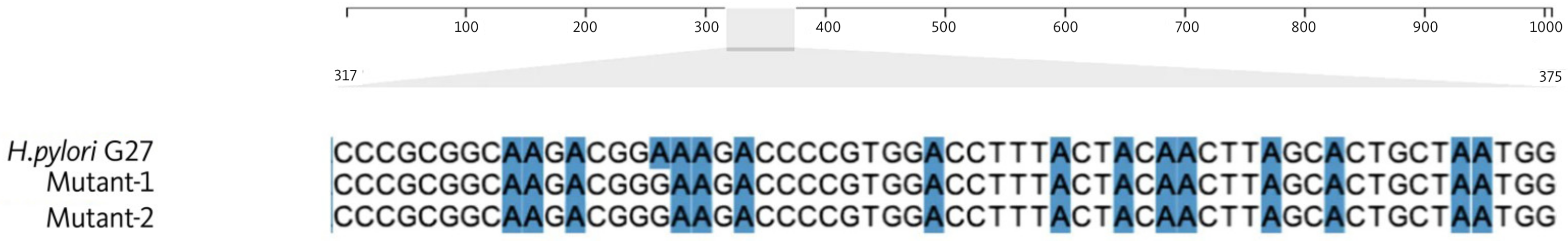
2.3. Sanger Sequencing
2.4. Next-Generation Sequencing (NGS) for Detecting Mutations in the Resistant Strains
3. Discussion
4. Materials and Methods
4.1. Bacteria
4.2. Subculture of H. pylori
4.3. Agar Dilution Susceptibility Tests
4.4. Inoculation of Bacteria and Interpretations of Results
4.5. In Vitro Selection of Clarithromycin-Resistant Strains
4.6. DNA Isolation from H. pylori G27 and Mutant Strains
4.7. Sanger Sequencing
4.8. WGS and Bioinformatics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241.
- Abrams, J.A.; Wang, T.C. Adenocarcinoma and other tumors of the stomach. In Sleisenger and Fordtran’s Gastrointestinal and Liver Disease Pathophysiology/Diagnosis/Management, 9th ed.; Feldman, M., Friedman, L.S., Brandt, L.J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2010. [Google Scholar]
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014, 348, g3174. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, G.I.; Rothenbacher, D.; Brenner, H. Epidemiology of Helicobacter pylori infection. Helicobacter 2004, 9 (Suppl. S1), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pounder, R.E.; Ng, D. The prevalence of Helicobacter pylori infection in different countries. Aliment. Pharmacol. Ther. 1995, 9 (Suppl. S2), 33–39. [Google Scholar] [PubMed]
- Kivi, M.; Johansson, A.L.; Reilly, M.; Tindberg, Y. Helicobacter pylori status in family members as risk factors for infection in children. Epidemiol. Infect. 2005, 133, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Nouraie, M.; Latifi-Navid, S.; Rezvan, H.; Radmard, A.R.; Maghsudlu, M.; Zaer-Rezaii, H.; Amini, S.; Siavoshi, F.; Malekzadeh, R. Childhood hygienic practice and family education status determine the prevalence of Helicobacter pylori infection in Iran. Helicobacter 2009, 14, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.M.; Sharaf, R.R.; Aziz, R.K. Helicobacter pylori: A poor man’s gut pathogen? Gut Pathog. 2010, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Malfertheiner, P.; Lee, Y.C.; Sheu, B.S.; Sugano, K.; Cheng, H.C.; Yeoh, K.-G.; Hsu, P.-I.; Goh, K.-L.; Mahachai, V.; et al. Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM). Screening and eradication of Helicobacter pylori for gastric cancer prevention: The Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Adeloye, D.; Luk, T.T.; Huang, L.; He, Y.; Xu, Y.; Ye, X.; Yi, Q.; Song, P.; Rudan, I. The global prevalence of and factors associated with Helicobacter pylori infection in children: A systematic review and meta-analysis. Lancet Child Adolesc. Heal. 2022, 6, 185–194. [Google Scholar] [CrossRef]
- Fiedorek, S.C.; Malaty, H.M.; Evans, D.L.; Pumphrey, C.L.; Casteel, H.B.; Evans, D.J., Jr.; Graham, D.Y. Factors influencing the epidemiology of Helicobacter pylori infection in children. Pediatrics 1991, 88, 578–582. [Google Scholar] [CrossRef]
- Laarej, K.; Alami, R.; Bentaleb, L.; Jbilou, M.; El Kabbaj, S.; Elmaouardi, M.; Bouklouze, A. Epidemiological study of Helicobacter pylori infection in a population in the Rabat-sale-Zamour-Zaer region. Am. J. Epidemiol. 2020, 4, 6–9. [Google Scholar]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Cave, D.R. Transmission and epidemiology of Helicobacter pylori. Am. J. Med. 1996, 100, 12S–17S; discussion 17S. [Google Scholar] [CrossRef]
- Mégraud, F. Transmission of Helicobacter pylori: Faecal-oral versus oral-oral route. Aliment. Pharmacol. Ther. 1995, 9 (Suppl. S2), 85–91. [Google Scholar] [PubMed]
- Perry, S.; de la Luz Sanchez, M.; Yang, S.; Haggerty, T.D.; Hurst, P.; Perez-Perez, G.; Parsonnet, J. Gastroenteritis and transmission of Helicobacter pylori infection in households. Emerg. Infect. Dis. 2006, 12, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; Angelis, G.L.D. Helicobacter pylori, transmission routes and recurrence of infection: State of the art. Acta Biomed. 2018, 89, 72–76. [Google Scholar] [PubMed]
- Graham, D.Y.; Malaty, H.M.; Evans, D.G.; Evans, D.J., Jr.; Klein, P.D.; Adam, E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology 1991, 100, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; El-Omar, E.M. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fisch-Bach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–441. [Google Scholar]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Tanoglu, A.; Duzenli, T.; Tozun, A.N. Helicobacter pylori treatment in Turkey: Current status and rational treatment options. North. Clin. Istanb. 2020, 7, 87–94. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 30 March 2024).
- Liu, W.Z.; Xiao, S.D.; Hu, P.J.; Lu, H.; Cui, Y.; Tytgat, G.N. A new quadruple therapy for Helicobacter pylori using tripotassium dicitrato bismuthate, furazolidone, josamycin and famotidine. Aliment. Pharmacol. Ther. 2000, 14, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F. H. pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut 2004, 53, 1374–1384. [Google Scholar] [CrossRef]
- Francesco, V.D.; Zullo, A.; Hassan, C.; Giorgio, F.; Rosania, R.; Ierardi, E. Mechanisms of Helicobacter pylori antibiotic resistance: An updated appraisal. World J. Gastrointest. Pathophysiol. 2011, 2, 35–41. [Google Scholar] [CrossRef]
- Mégraud, F.; Lehours, P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 2007, 20, 280–322. [Google Scholar] [CrossRef]
- Gerrits, M.M.; van Vliet, A.H.; Kuipers, E.J.; Kusters, J.G. Helicobacter pylori and antimicrobial resistance: Molecular mechanisms and clinical implications. Lancet Infect. Dis. 2006, 6, 699–709. [Google Scholar] [CrossRef]
- Versalovic, J.; Osato, M.S.; Spakovsky, K.; Dore, M.P.; Reddy, R.; Stone, G.G.; Shortridge, D.; Flamm, R.K.; Tanaka, S.K.; Graham, D.Y. Point mutations in the 23S rRNA gene of Helicobacter pylori associated with different levels of clarithromycin resistance. J. Antimicrob. Chemother. 1997, 40, 283–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsugawa, H.; Suzuki, H.; Muraoka, H.; Ikeda, F.; Hirata, K.; Matsuzaki, J.; Saito, Y.; Hibi, T. Enhanced bacterial efflux system is the first step to the development of metronidazole resistance in Helicobacter pylori. Biochem. Biophys. Res. Commun. 2011, 404, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, J.F.; Sirous, M.; Daryani, N.E.; Eshraghi, S.; Akbari, B.; Shirazi, M.H. Assessing the role of the RND efflux pump in metronidazole resistance of Helicobacter pylori by RT-PCR assay. J. Infect. Dev. Ctries. 2011, 5, 88–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bina, J.E.; Alm, R.A.; Uria-Nickelsen, M.; Thomas, S.R.; Trust, T.J.; Hancock, R.E. Helicobacter pylori uptake and efflux: Basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob. Agents Chemother. 2000, 44, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Binh, T.T.; Shiota, S.; Suzuki, R.; Matsuda, M.; Trang, T.T.; Kwon, D.H.; Iwatani, S.; Yamaoka, Y. Discovery of novel mutations for clarithromycin resistance in Helicobacter pylori by using next-generation sequencing. J. Antimicrob. Chemother. 2014, 69, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kusano, C.; Horii, T.; Ichijima, R.; Ikehara, H. The ideal Helicobacter pylori treatment for the present and the future. Digestion 2022, 103, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Vester, B.; Douthwaite, S. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob. Agents Chemother. 2001, 45, 1–12. [Google Scholar] [CrossRef]
- Tomb, J.F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. Erratum: The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 1997, 389, 412. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Abadi, A.T.B. Resistance to clarithromycin and gastroenterologist’s persistence roles in nomination for Helicobacter pylori as high priority pathogen by World Health Organization. World J. Gastroenterol. 2017, 23, 6379–6384. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Tshibangu-Kabamba, E.; Ngoma-Kisoko, P.J.; Tuan, V.P.; Matsumoto, T.; Akada, J.; Kido, Y.; Tshimpi-Wola, A.; Tshiamala-Kashala, P.; Ahuka-Mundeke, S.; Ngoyi, D.M.; et al. Next-generation sequencing of the whole bacterial genome for tracking molecular insight into the broad-spectrum antimicrobial resistance of Helicobacter pylori clinical isolates from the Democratic Republic of Congo. Microorganisms 2020, 8, 887. [Google Scholar] [CrossRef]
- Weinitschke, S.; Denger, K.; Cook, A.M.; Smits, T.H.M. The DUF81 protein TauE in Cupriavidus necator H16, a sulfite exporter in the metabolism of C2 sulfonates. Microbiology 2007, 153, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, A.L.; Ermakova, A.Y.; Beletsky, A.V.; Petrova, M.; Mardanov, A.V.; Ravin, N.V. Genome analysis of Acinetobacter lwoffii strains isolated from permafrost soils aged from 15 thousand to 1.8 million years revealed their close relationships with present-day environmental and clinical isolates. Biology 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Seithel, A.; Eberl, S.; Singer, K.; Auge, D.; Heinkele, G.; Wolf, N.B.; Dörje, F.; Fromm, M.F.; König, J. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab. Dispos. 2007, 35, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Maier, R.J. A novel DNA-binding protein plays an important role in Helicobacter pylori stress tolerance and survival in the host. J. Bacteriol. 2015, 197, 973–982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed; CLSI guideline M45; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2024, Version 14.0. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 8 April 2024).
- Alavifard, H.; Mirzaei, N.; Yadegar, A.; Baghaei, K.; Smith, S.M.; Sadeghi, A.; Zali, M.R. Investigation of clarithromycin resistance-associated mutations and virulence genotypes of Helicobacter pylori isolated from Iranian population: A cross-sectional study. Curr. Microbiol. 2021, 78, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program, for Windows 95/98/NT; Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; pp. c1979–c2000. [Google Scholar]
- BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?BLAST_SPEC=blast2seq&LINK_LOC=align2seq&PAGE_TYPE=BlastSearch (accessed on 1 January 2023).
- Bushnell, B. Bbtools Software Package. Available online: http://sourceforge.net/projects/bbmap (accessed on 20 December 2023).
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes de novo assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, Y.; Fujisawa, T.; Nakamura, Y. DFAST: A flexible prokaryotic genome annotation pipeline for faster genome publication. Bioinformatics 2018, 34, 1037–1039. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef] [PubMed]
| Gene | Position of Mutations | Mutation Type | H. pylori G27 | Mutation | Mutant-1 | Mutant-2 | AA Change |
|---|---|---|---|---|---|---|---|
| 23SrRNA | 2142 | SNP | A | G | + | + | - |
| 23SrRNA | 1490 | SNP | T | A | + | − | No |
| 23SrRNA | 1494 | SNP | T | A | + | − | No |
| 23SrRNA | 1495 | SNP | T | A | + | − | No |
| 23SrRNA | 1476 | SNP | T | A | + | − | No |
| 23SrRNA | 1472 | SNP | G | T | + | − | No |
| Sulfite exporter TauE/SafE family protein | 35 | SNP | A | G | + | − | L > P |
| DUF874 Family proteinGene-1 | 589 | SNP | T | C | − | + | H > R |
| DUF874 Family proteinGene-1 | 587 | SNP | G | A | − | + | No |
| DUF874 Family proteinGene-1 | 572 | SNP | G | T | − | + | No |
| DUF874 Family proteinGene-1 | 553 | SNP | G | A | − | + | T* > I |
| DUF874 Family proteinGene-1 | 549 | SNP | T | G | − | + | T* > A |
| DUF874 Family proteinGene-2 | 941 | SNP | T | C | − | + | No |
| DUF874 Family proteinGene-2 | 890 | SNP | C | T | − | + | No |
| DUF874 Family proteinGene-2 | 703 | SNP | G | A | − | + | A** > V |
| DUF874 Family proteinGene-2 | 701 | SNP | G | A | − | + | No |
| Primers | PCR Conditions |
|---|---|
| 23S-F 5′-AGCACCGTAAGTTCGCGATAAG-3′ |
|
| 23S-R 5′-CTTTCAGCAGTTATCACATCC-3′ |
| Tools | Intended Purpose | References |
|---|---|---|
| BBtools v39.01—BBDuk | Quality trimming and filtering | [54] |
| Spades v3.15.4 | Genome assembly | [55] |
| Dfast v1.6.0 | Annotation of the assembled contigs | [56] |
| BBtools v39.01—BBmap | Mapping of H. pylori reads to the reference G27 genome | [54] |
| BBtools v39.01—Call Variants | Variant calling from sam files with callvariants.sh. | [57] |
| EMBOSS Transeq v6.6.0.0 | Conversion of SNP-bearing contigs and regions for phenotypic change | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayaş, M.; Oktem-Okullu, S.; Özcan, O.; Kocagöz, T.; Gürol, Y. Exploring the Molecular Mechanisms of Macrolide Resistance in Laboratory Mutant Helicobacter pylori. Antibiotics 2024, 13, 396. https://doi.org/10.3390/antibiotics13050396
Ayaş M, Oktem-Okullu S, Özcan O, Kocagöz T, Gürol Y. Exploring the Molecular Mechanisms of Macrolide Resistance in Laboratory Mutant Helicobacter pylori. Antibiotics. 2024; 13(5):396. https://doi.org/10.3390/antibiotics13050396
Chicago/Turabian StyleAyaş, Meltem, Sinem Oktem-Okullu, Orhan Özcan, Tanıl Kocagöz, and Yeşim Gürol. 2024. "Exploring the Molecular Mechanisms of Macrolide Resistance in Laboratory Mutant Helicobacter pylori" Antibiotics 13, no. 5: 396. https://doi.org/10.3390/antibiotics13050396
APA StyleAyaş, M., Oktem-Okullu, S., Özcan, O., Kocagöz, T., & Gürol, Y. (2024). Exploring the Molecular Mechanisms of Macrolide Resistance in Laboratory Mutant Helicobacter pylori. Antibiotics, 13(5), 396. https://doi.org/10.3390/antibiotics13050396







