Abstract
The eradication of Helicobacter pylori is a valid strategy for preventing gastric cancer; however, the therapeutic failure of first-line treatments in Colombia is associated with high resistance to metronidazole and amoxicillin. This study explored alternative antibiotics and analyzed point mutations in resistance genes to furazolidone and rifampicin in order to include them in rescue therapy regimens. A total of 239 complete genomes of Helicobacter pylori Colombian strains were compared to that of the ATCC 26695 strain to identify mutations in the rpoB and porD genes for rifampicin and furazolidinone resistance, respectively. While rifampicin resistance mutations were not found, only 0.84% of the isolates showed the porD gene, suggesting that Helicobacter pylori is sensitive to these antibiotics. A phylogenomic analysis of Helicobacter pylori revealed an independent lineage in Colombia (hspColombia). The absence of point mutations in the rpoB gene, together with the scarce mutations identified in the porD gene of Helicobacter pylori, suggest that the hspColombia isolates are sensitive to rifampicin and furazolidone, which could be key to including these antibiotics in the rescue therapies against Helicobacter pylori.
1. Introduction
Helicobacter pylori (H. pylori) is a bacterium that triggers an inflammatory process, which begins with chronic gastritis and progresses to peptic ulcer, atrophic gastritis, intestinal metaplasia, gastric dysplasia, and adenocarcinoma or MALT lymphoma [1,2]. The natural history of this infection is closely connected to the pathogenesis of gastric cancer, beginning at childhood when it is acquired by vertical transmission (from parents to children) and persisting throughout the life span of the host if the infection is not properly treated [3]. Even though H. pylori is classified as a Type I carcinogen, the prevalence of the infection is not directly correlated with the incidence of gastric cancer. Indeed, the development of gastric lesions is associated with the type of strain, host genetic susceptibility, host-bacterium coevolution, diet, as well as socioeconomic and environmental conditions [4,5].
The incidence of gastric cancer varies between different geographic regions worldwide. For instance, the incidence of cancer in Latin America ranges from 20 to 30 cases per 100,000 inhabitants, being more frequent in mountainous areas and less common in coastal regions [6]. In Colombia, two regions within the Department of Nariño clearly show these marked differences in the risk of gastric cancer. In the Andean region of Tuquerres, the incidence of gastric cancer is among the highest in the country, affecting 150/100,000 inhabitants. In contrast, Tumaco, which is located on the Pacific coast, shows a relatively low incidence (6/100,000) [7]. Interestingly, both regions have a similarly high prevalence of H. pylori infection (~90%) [8].
The eradication of H. pylori is the current recognized and effective strategy to reduce the incidence of gastric cancer. In Colombia, the antimicrobial resistance rate to first-line treatments against H. pylori is rising. Bacterial resistance to metronidazole, levofloxacin and amoxicillin has increased [9]. Consequently, the search for alternative antibiotics, such as furazolidone and rifampicin, has been proposed to replace those showing high resistance rates.
Nevertheless, in Colombia there is a limited number of studies focused on the bacterial resistance to furazolidone and rifampicin due to their restricted use in H. pylori eradication treatment. Furthermore, H. pylori is a microorganism that is difficult to grow under laboratory conditions, and the antibiotic susceptibility tests require longer periods of time. Therefore, novel methods that identify mutations in SNPs associated with antibiotic resistance, such as qPCR and Next Generation Sequencing (NGS), have become more precise approaches to providing better treatment options to mitigate infections caused by H. pylori.
Furazolidone, one of the nitrofurans used in the treatment of H. pylori as a rescue therapy, acts as a bacteriostatic agent with a mechanism of action similar to nitroimidazoles. Resistance to this antibiotic is mediated by nitroreductases such as flavodoxin pyruvate oxidoreductase (encoded by the porD gene). Mutations linked to furazolidone resistance in the porD gene involve guanine-to-adenine transitions at position 353 (G353A), adenine-to-guanine at position 356 (A356G), and cytosine-to-thymine at position 357 (C357T) [10].
Rifampicin binds to the β subunit of the DNA-dependent RNA polymerase, which is encoded by the rpoB gene of H. pylori, forming a stable complex that disrupts RNA synthesis. Point mutations in the V149F, Q527R, D530, and H540 codons of the rpoB gene can confer resistance to rifamycins by reducing the subunit’s affinity [11].
The purpose of this study is to conduct genomic surveillance of circulating strains and determine the resistance rate to furazolidone and rifampicin in H. pylori in Colombia.
2. Results
The bioinformatics analysis of H. pylori sequences revealed that the 16 isolates showed, on average, 115 contigs. The genomes had sizes ranging from 1,595,752 bp to 1,700,565 bp, with a mean of 1,663,692 bp ± 7,365,295. The analysis revealed a mean G/C content of 39.1% ± 0.05, with minimum and maximum values of 38.8% and 39.5%, respectively. Furthermore, the average number of genes in H. pylori was 1640, fluctuating between 1561 and 1796 (Table 1).

Table 1.
Summary of reported Helicobacter pylori genome sequences.
Finally, a total of 239 H. pylori isolates were analyzed in this study, of which 173 came from the central and mountainous Andean region of Colombia, mainly from the city of Bogota. Likewise, 57 isolates came from the Department of Nariño, whereas 7 were from the Department of Tolima.
The analysis of point mutations in the rpoB gene for rifampicin resistance did not identify any mutation in the Colombian isolates. The similar analysis for furazolidone revealed two isolates (2/239) with the C357T mutation (0.84%).
In addition, the C357G mutation was observed in four isolates of H. pylori. Nevertheless, to date, and to the best of our knowledge, there are no published data regarding the association of this mutation with H. pylori resistance to furazolidone.
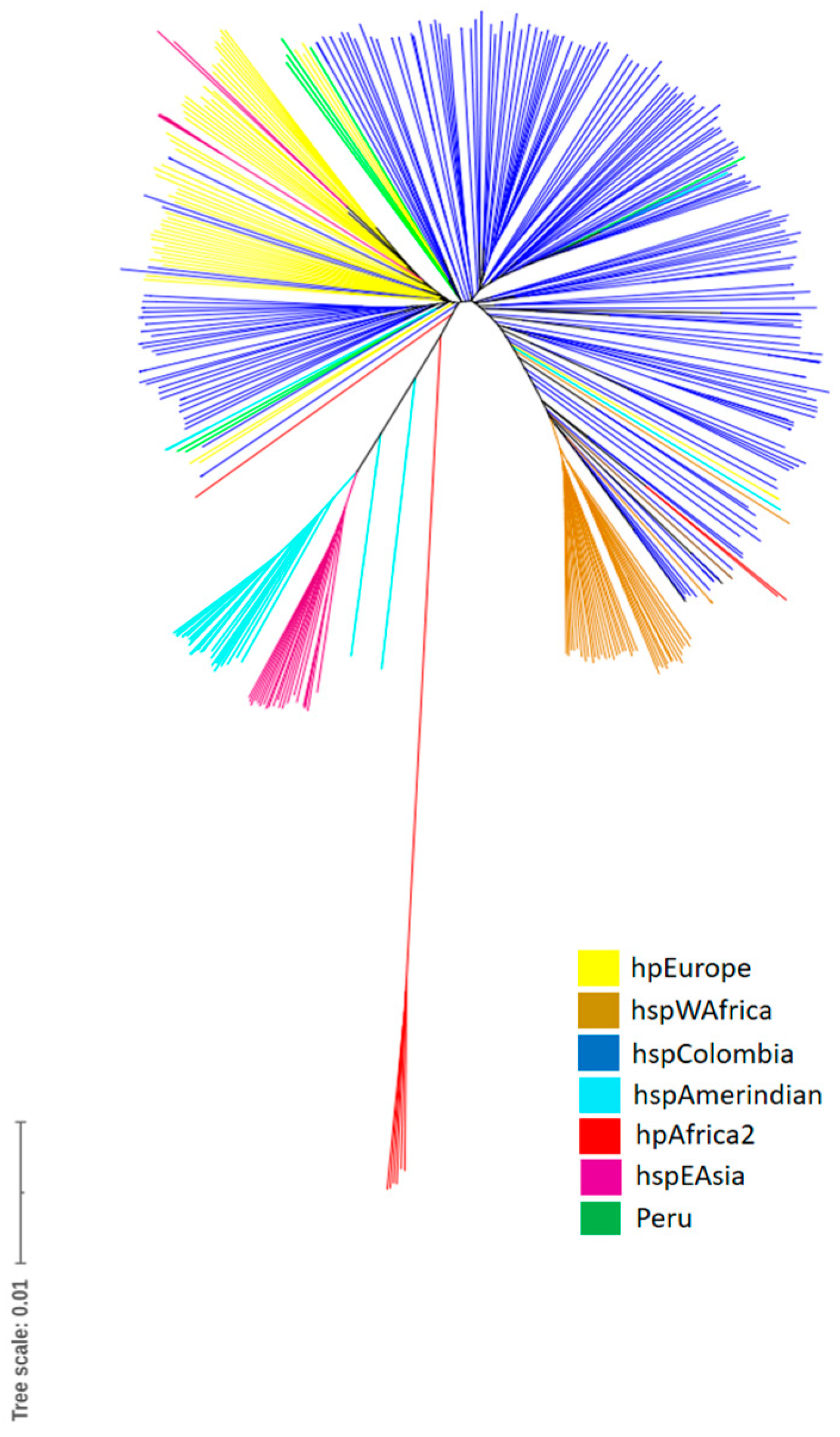
The tree that originated from the phylogenomic analysis based on the core genome also shows the formation of independent clades of hpEurope, hspAmerindian, hspEAsia, hspWAfrica, and hspColombia, with hpAfrica2 being the most ancestral group. Some strains from the Pacific region of Colombia were included within a clade grouped with African strains (hpAfrica). The Colombian strains were also observed to be grouped with strains of European origin, which indicates that the horizontal exchange of genetic material between strains from geographically isolated populations appeared after the colonization of the Americas by the Spaniards (Figure 1).
Figure 1.
Phylogenomic tree based on the core genome of Helicobacter pylori isolates. The different populations (hp) and subpopulations are described on the right side of the phylogenetic tree.
3. Discussion
In Colombia, the arrival of Europeans to the American continent triggered the most notable miscegenation in human history, merging genetic pools from Africa, Europe, and America [12]. The Colombian Pacific coast shows a strong African influence in terms of both population ancestry and the H. pylori strains that infect these communities, which have ancestral similarities to bacterial variants found in Africa [13]. It is important to highlight that H. pylori has accompanied humanity since the dawn of Homo sapiens on the African plains, and this extended co-evolution period has resulted in a relatively low incidence of gastric cancer in the African populations. This phenomenon is known as the “African enigma” [14].
This enigma has been extrapolated to the Pacific coast of Nariño, Colombia, as one of the possible theoretical explanations for the low incidence of gastric cancer in this region [13]. Moreover, H. pylori strains from the Pacific have a higher resistance rate to first- and second-line antibiotics compared to strains found in the Andean region, which can be the result of a prolonged period of co-evolution of bacterium and host, as well as the frequent treatments for different local infections [4,13,15].
In contrast, the Colombian Andean region, where the incidence of gastric cancer is high, has an ancestral composition that originated the mixing between Europeans and Native American populations [13]. This mixture has given rise to a new environment for bacteria [16]. Specifically for H. pylori, the gastric niche present in these mestizo populations has triggered the appearance of a new strain known as hspColombia, which may have arisen from horizontal gene transfer between hpEurope and hspAmerindian, intragenomic recombination, new neo-tropical dietary habits, immune response, and/or the renewed gastric ecosystem [4,16]. All the aforementioned factors created a selective pressure on these bacterial communities.
Previous studies have reported that H. pylori from the Colombian Andean region has a vacA gene with a higher frequency of s1m1 alleles (Higher risk), whereas it is more common to find alleles s2m2 (Lower risk) in bacteria from the Pacific region of the country [17]. In addition, dietary factors may play an essential role in developing gastric cancer, as the Andean region consumes mainly foods with high carbohydrate content, while the Pacific coast diet is characterized by being rich in fruits with antioxidants, fresh vegetables, and seafood [18].
In Colombia, it has been observed that the presence of the 1082 (A/G) point mutation in the IL-10 gene (79%), together with another mutation in position 511 of the IL-1β gene (72%) and the Th1 immune response, increase the risk for gastric cancer in human populations [4]. These findings highlight the complex interactions between genetic, environmental, and dietary factors that predispose the different regions of Colombia to developing gastric cancer.
This study focused on assessing H. pylori resistance to antimicrobial treatments based on furazolidone and rifampicin, with the purpose of exploring potential therapeutic alternatives. In terms of antibiotic resistance, the Colombian isolates did not exhibit mutations in the rpoB gene that were associated with rifampicin resistance, which suggests a potential effectiveness of this antibiotic in the treatment of H. pylori infections. This finding is consistent with a previous study carried out in Colombia that applied phenotypic techniques and DNA sequencing methods, reporting equally low resistance to furazolidone and rifampicin and supporting the results presented here [19].
In addition, mutations were identified in the porD gene that are associated with furazolidone resistance. Nevertheless, the incidence of resistant isolates reported in this study was minimum, representing only 0.84% of the total, which suggests that this antibiotic could be an excellent therapeutic alternative. It is important to highlight that a study about the phenotypic resistance to this antibiotic carried out in Bogota, Colombia, reported a resistance rate of 4.8% [10].
In Colombia, the treatment of H. pylori typically relies on a combination of amoxicillin, metronidazole, and clarithromycin as first-line therapy in conjunction with Proton Pump Inhibitors (PPIs). However, depending on the success or failure of empirical therapy, tetracycline or levofloxacin is used as second-line therapy, depending on the resistance rate of H. pylori to metronidazole or amoxicillin.
H. pylori resistance to first- and second-line antibiotics has increased in Colombia, which is demonstrated by the resistance rates being higher than the threshold established for an optimal treatment. Specifically, metronidazole, amoxicillin, and levofloxacin resistance rates have reached 93%, 25.9%, and 12.04%, respectively [9]. Given this scenario, and considering the high incidence and mortality of gastric cancer in the country, it is imperative to find new therapeutic strategies to eliminate H. pylori from gastric mucosa.
Given the increasing problem of H. pylori resistance, rifampicin and furazolidone have been proposed as alternatives for eradicating the bacteria. Under this scheme, a 14-day regimen has been suggested that includes the following components for furazolidone: PPIs, tetracycline, bismuth, and furazolidone. In the case of rifampicin, a similar 14-day regimen has been proposed that consists of PPIs, amoxicillin, bismuth, and rifampicin [20].
H. pylori is a genetically diverse microorganism, and treatment regimens often fail due to human genetic variability, stomach physiology (such as pH), and H. pylori’s own evolutionary lineage; therefore, it is essential to conduct further phenotypic and genotypic studies on clinical strains. These studies will help understand the most optimal treatment. Additionally, it is crucial to integrate susceptibility testing alongside empirical regimens in the bacteria eradication protocol [20].
This study provides a fundamental approach that can be applied in other countries in the region or anywhere in the world. The use of Next Generation Sequencing techniques in combination with genomic analysis makes it possible to carry out accurate diagnoses and determine the molecular epidemiological status, including the antimicrobial resistance of pathogens relevant to public health [9].
In the future, real time PCR commercial kits could be developed for rpoB and porD genes, which could be used to identify phenotypic resistance using samples obtained from gastric biopsy tissues of fecal samples [10,21]. Given the difficulties of growing H. pylori in vitro and the prolonged incubation times, this diagnosis and treatment strategy could represent a novel and efficient alternative in the fight against this microorganism in Colombia.
Nevertheless, a limitation of our study lies in the possibility that there are other genes with multiple mutation sites that could also affect antibiotic resistance levels. These mutations have not yet been identified by phenotypic or molecular testing, which suggests the need for additional research in this area to fully understand the antimicrobial resistance landscape in H. pylori.
4. Materials and Methods
4.1. Samples Provided by This Study
The 16 whole genome sequences correspond to H. pylori isolates collected between 2017 and 2022 in Nariño, Colombia. All participants signed the informed consent. The average age of patients was 52 years old, with a range from 30 to 68 years old. Regarding the gender of participants, 3 were male and 13 were female. Based on the histopathology results, 3 patients showed active chronic gastritis, 8 atrophic gastritis, and 5 intestinal metaplasia (Table 2).

Table 2.
Description of origin and clinical source of the Colombian genomes analyzed in this study.
4.2. DNA Extraction and Bioinformatics Analysis
H. pylori was grown in Petri dishes containing Columbia agar (Oxoid, Basingstoke, UK) supplemented with 10% defibrinated lamb blood, Dent selective supplement (Oxoid, UK), and 1% Isovitalex enrichment supplement (Oxoid, UK). Plates were incubated at 10% CO2 and 37 °C for 7–10 days. DNA extraction was carried out using the QIAmp DNA Mini kit (QIAGEN, Hilden, Germany). The MiSeq TM platform (Illumina, San Diego, CA, USA) was used for whole genome sequencing. The library was prepared through the Nextera XT (Illumina) kit, followed by 2 × 300 bp paired ends, which generated a 40X coverage. The FastQC v 0.12 software was used for quality control of the raw sequencing data [22]. The Trim Galore v 6.6 and Cutadapt v 4.3 programs were used to clean the data and to ensure its integrity and accuracy [23,24]. The de novo genome assembly was achieved through the SPAdes v 13.3 program [25]. Finally, the genomes were annotated at the NCBI database using the Prokaryotic Genome Annotation Pipeline (PGAP) [26]. All H. pylori complete genome sequences are available at NCBI (BioProject: PRJNA984677).
4.3. Antibiotic Resistance Mutations
An analysis of H. pylori genomes was carried out using data from Colombia that are available on the PubMLST platform (https://pubmlst.org/), which keeps relevant genetic information of several bacterial strains. We accessed 223 H. pylori specific genomes from Colombian strains registered at PubMLST (https://pubmlst.org/) together with the 16 isolates that were sequenced by our research group. Ultimately, the 16 genomes sequenced for this study, combined with the 223 genomes obtained from the database, totaled 239 genomes for Colombia in this analysis.
We used the 26695 reference strain as a starting point to investigate mutations in the H. pylori isolates. The proteins for mutation analysis were obtained from the reference strain 26695. The protein sequence (Ref: A0A2I8V4C0) was used for the rpoB gene, and the protein (Ref: O25737) was used for the porD gene, retrieved from UniProt (https://www.uniprot.org/). We studied the rpoB gene, as it has been associated with rifampicin resistance. Specifically, we assessed mutations in positions V149F, Q527R, D530, and H540. Meanwhile, we analyzed positions G353A, A356G, and C357T of the porD gene because of their association with furazolidone resistance (Table 3).

Table 3.
Gene mutations that confer rifampicin and furazolidone resistance in Helicobacter pylori.
4.4. Phylogenomic Analysis
The genome sequences of H. pylori isolates were obtained from BIGSdb (Bacteria Isolate Genome Sequence Database) [30]. Subsequently, a gene-by-gene alignment was carried out using DCSs (DNA Coding Sequences) of the J99 H. pylori reference strain (ID: NZ_JABFHN010000001.1). The resulting genome comparison matrix, containing 1694 genes of the H. pylori core genome, was used to construct a phylogenomic tree through the MEGA X software v. 10 [31] with 1000 bootstrap replicates. The phylogenomic tree was visualized and edited using iTOL v5 [32].
5. Conclusions
The absence of point mutations in the rpoB gene and the low number of mutations in the porD gene of H. pylori suggest that the Colombian isolates (hspColombia) are sensitive to rifampicin and furazolidone. This low resistance rate is a strong evidence to support the inclusion of furazolidone and rifampicin in rescue therapies against H. pylori in Colombia. Given that a H. pylori lineage (hspColombia) was found to be specific to Colombia, the resistance exhibited against antimicrobials is related to the local regimen administered in the country. This finding could provide essential information to design better treatment strategies against the hspColombia strain in the future.
Author Contributions
Conceptualization, K.A.G.; methodology, K.A.G.; software, K.A.G.; validation, K.A.G., A.H. and A.J.P.; formal analysis, K.A.G.; investigation, K.A.G.; resources, K.A.G., A.H. and A.J.P.; data curation, K.A.G. and A.J.P.; writing—original draft preparation, K.A.G.; writing—review and editing, A.H. and A.J.P.; visualization, K.A.G.; funding acquisition, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study received support from the Vicerectoria de Investigación e Interacción Social at the University of Nariño, through Agreement No. 15 dated 8 March 2022, as well as from the Center for Health Studies of the University of Nariño.
Institutional Review Board Statement
The Ethics Committee of Universidad de Nariño (No. 58, 2019) approved this study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://pubmlst.org/organisms?title=Helicobacter+pylori (accessed on 8 August 2023).
Acknowledgments
We would like to thank the reviewers for their constructive comments and guidelines.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef]
- Alipour, M. Molecular mechanism of Helicobacter pylori-induced gastric cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Duan, M.; Li, Y.; Liu, J.; Zhang, W.; Dong, Y.; Han, Z.; Wan, M.; Lin, M.; Lin, B.; Kong, Q.; et al. Transmission routes and patterns of Helicobacter pylori. Helicobacter 2023, 28, e12945. [Google Scholar] [CrossRef]
- Guzman, K.; Pazos, A. Helicobacter pylori: Pathogenic or mutualistic microorganism in Colombian populations. Univ. Salud 2023, 25, 1–6. [Google Scholar] [CrossRef]
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.I.; Kook, M.C.; Park, B.; Joo, J. Family history of gastric cancer and Helicobacter pylori treatment. New Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef]
- Piazuelo, M.B.; Correa, P. Gastric cancer: Overview. Colomb. Medica 2013, 44, 192–201. [Google Scholar]
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. N. Am. 2013, 42, 211. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B. Gastric cancer: The colombian enigma. Rev. Colomb. Gastroenterol. 2010, 25, 334–337. [Google Scholar]
- Guzman, K.; Montenegro, L.; Pazos, A. The Helicobacter pylori single nucleotide polymorphisms SNPs associated with multiple therapy resistance in Colombia. Front. Microbiol. 2023, 14, 1198325. [Google Scholar] [CrossRef]
- Atehortúa-Rendón, J.D.; Martínez, A.; Pérez-Cala, T. Helicobacter pylori susceptibility to six commonly used antibiotics in Colombia. Rev. Colomb. Gastroenterol. 2020, 35, 351–361. [Google Scholar]
- Arango-Gil, I.S.; Martínez, A.; Chica, J.E.; Pérez-Cala, T.L. Rifabutina: Terapia de rescate eficaz para la infección por Helicobacter pylori, revisión de la literatura actual. Medicas UIS 2022, 35, 31–42. [Google Scholar]
- Conley, A.B.; Rishishwar, L.; Norris, E.T.; Valderrama-Aguirre, A.; Mariño-Ramírez, L.; Medina-Riva, M.A.; Jordan, I.K. A comparative analysis of genetic ancestry and admixture in the Colombian populations of Choco and Medellin. G3 Genes Genomes Genet. 2017, 7, 3435–3447. [Google Scholar] [CrossRef]
- Kodaman, N.; Pazos, A.; Schneider, B.G.; Piazuelo, M.B.; Mera, R.; Sobota, R.S.; Sicinschi, L.A.; Shaffer, C.L.; Romero-Gallo, J.; de Sablet, T.; et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc. Natl. Acad. Sci. USA 2014, 111, 1455–1460. [Google Scholar] [CrossRef]
- Holcombe, C. Helicobacter pylori: The African enigma. Gut 1992, 33, 429. [Google Scholar] [CrossRef]
- Matta, A.J.; Zambrano, D.C.; Pazos, A.J. Punctual mutations in 23S rRNA gene of clarithromycin-resistant Helicobacter pylori in Colombian populations. World J. Gastroenterol. 2018, 24, 1531. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, A.J.; Trujillo, E.; Acevedo, O.; Bravo, M.M. Phylogenomics of Colombian Helicobacter pylori isolates. Gut Pathog. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Bravo, L.E.; van Doorn, L.J.; Realpe, J.L.; Correa, P. Virulence-associated genotypes of Helicobacter pylori: Do they explain the African enigma? Am. J. Gastroenterol. 2002, 97, 2839–2842. [Google Scholar] [CrossRef]
- Camargo, M.C.; Burk, R.F.; Bravo, L.E.; Piazuelo, M.B.; Hill, K.E.; Fontham, E.T.; Motley, A.K.; Yepez, M.C.; Mora, Y.; Schneider, B.G.; et al. Plasma selenium measurements in subjects from areas with contrasting gastric cancer risks in Colombia. Arch. Med. Res. 2008, 39, 443–451. [Google Scholar] [CrossRef]
- Mannion, A.; Dzink-Fox, J.; Shen, Z.; Piazuelo, M.B.; Wilson, K.T.; Correa, P.; Peek, R.M.; Camargo, M.C.; Fox, J.G. Helicobacter pylori antimicrobial resistance and gene variants in high-and low-gastric-cancer-risk populations. J. Clin. Microbiol. 2021, 59, e03203-20. [Google Scholar] [CrossRef]
- Otero, W.; Gómez, M.; Otero, L.; Trespalacios, A. Helicobacter pylori:¿ cómo se trata en el 2018? Rev. Gastroenterol. Perú 2018, 38, 54–63. [Google Scholar]
- Graham, D.Y.; Moss, S.F. Antimicrobial Susceptibility Testing for Helicobacter pylori Is Now Widely Available: When, How, Why. Am. J. Gastroenterol. 2022, 117, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC (v0.11.7). Barbraham Institute. 2018. Available online: https://github.com/s-andrews/FastQC/releases (accessed on 19 April 2023).
- Krueger, F. Trim Galore: A Wrapper Tool around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files, with Some Extra Functionality for MspI-Digested RRBS-Type (Reduced Representation Bisufite-Seq) Libraries. 2012. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore (accessed on 25 April 2023).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 7, 10–12. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Hays, C.; Burucoa, C.; Lehours, P.; Tran, C.T.; Leleu, A.; Raymond, J. Molecular characterization of Helicobacter pylori resistance to rifamycins. Helicobacter 2018, 23, e12451. [Google Scholar] [CrossRef] [PubMed]
- Heep, M.; Rieger, U.; Beck, D.; Lehn, N. Mutations in the beginning of the rpoB gene can induce resistance to rifamycins in both Helicobacter pylori and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2000, 44, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xu, H.; Zhang, C.; Shao, S.; Li, L.; Wang, H.; Wang, H.; Qiu, G. Mutations in Helicobacter pylori porD and oorD genes may contribute to furazolidone resistance. Croat. Med. J. 2006, 47, 410–415. [Google Scholar] [PubMed]
- Jolley, K.M.; Martin, C.J. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ivica, L.; Peer, B. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 293–296. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).