Low Oxygen Concentration Reduces Neisseria gonorrhoeae Susceptibility to Resazurin
Abstract
1. Introduction
2. Results
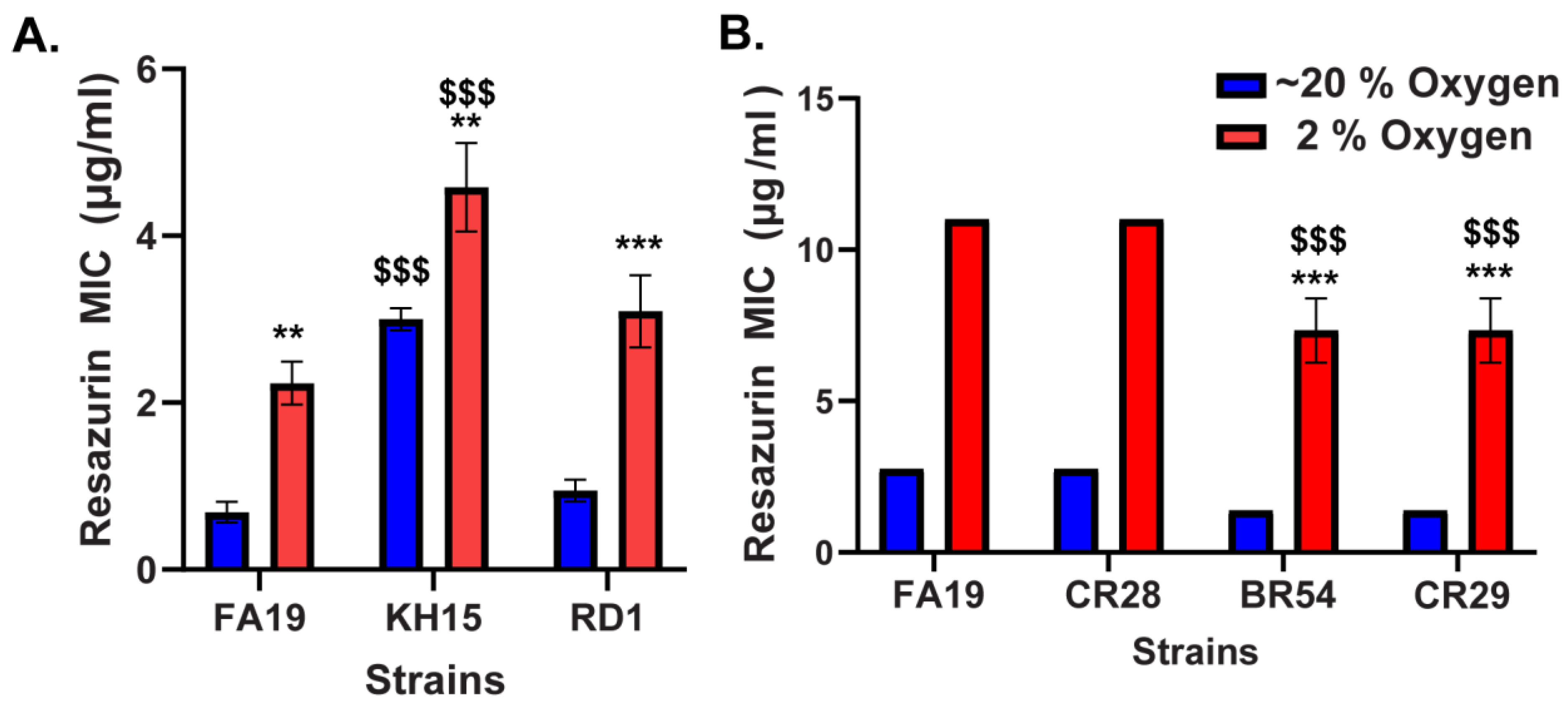
2.1. Reduced Susceptibility of N. gonorrhoeae to Resazurin at Low Oxygen
2.2. MtrCDE Efflux Pump Contributes to the Reduced Susceptibility of N. gonorrhoeae to Resazurin at Low Oxygen
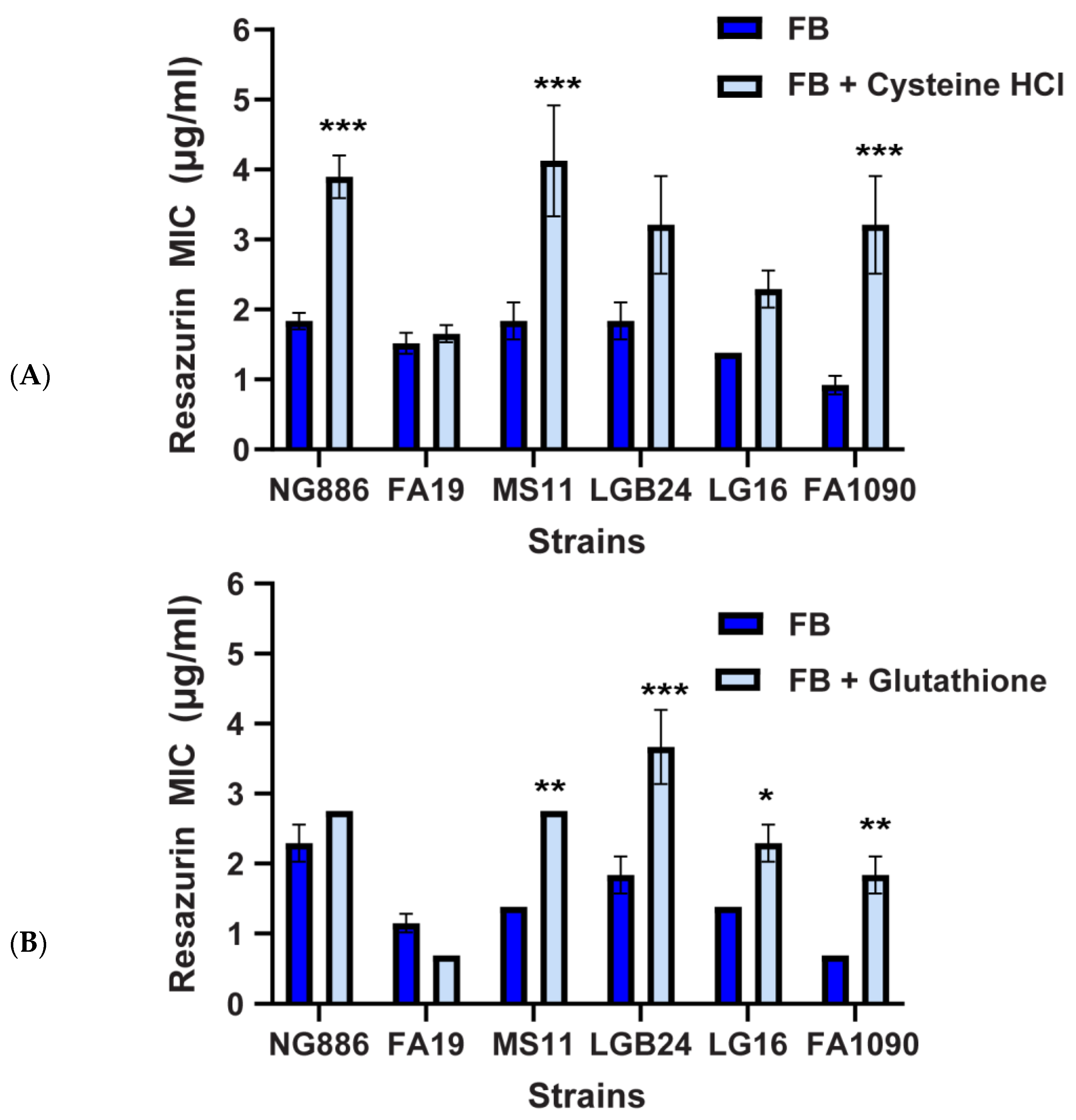
2.3. Oxidative Stress Plays a Role in the Enhanced Susceptibility of N. gonorrhoeae to Resazurin at Atmospheric Oxygen
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Reagents
4.2. Antibiotic Susceptibility Testing
4.3. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226–240. [Google Scholar] [CrossRef]
- Tsevat, D.G.; Wiesenfeld, H.C.; Parks, C.; Peipert, J.F. Sexually transmitted diseases and infertility. Am. J. Obstet. Gynecol. 2017, 216, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.R.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Seifert, H.S.; Hook, E.W., 3rd; Hawkes, S.; Ndowa, F.; Dillon, J.R. Gonorrhoea. Nat. Rev. Dis. Primers 2019, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Shafer, W.M. Antibiotic resistance in Neisseria gonorrhoeae: Origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 2011, 1230, E19–E28. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed]
- St Cyr, S.; Barbee, L.; Workowski, K.A.; Bachmann, L.H.; Pham, C.; Schlanger, K.; Torrone, E.; Weinstock, H.; Kersh, E.N.; Thorpe, P. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M. Current and future antimicrobial treatment of gonorrhoea—The rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect. Dis. 2015, 15, 364. [Google Scholar] [CrossRef]
- Bolan, G.A.; Sparling, P.F.; Wasserheit, J.N. The emerging threat of untreatable gonococcal infection. N. Engl. J. Med. 2012, 366, 485–487. [Google Scholar] [CrossRef]
- Hill, S.A.; Masters, T.L.; Wachter, J. Gonorrhea—An evolving disease of the new millennium. Microb. Cell 2016, 3, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Adamson, P.C.; Klausner, J.D. Epidemiology, Treatments, and Vaccine Development for Antimicrobial-Resistant Neisseria gonorrhoeae: Current Strategies and Future Directions. Drugs 2021, 81, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Lahra, M.M.; Escher, M.; Eremin, S.; Cole, M.J.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.R.; Galas, M.; et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017–18: A retrospective observational study. Lancet Microbe 2021, 2, e627–e636. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.M.; Connolly, K.L.; Jerse, A.E.; Detrick, M.S.; Horzempa, J. Antibacterial activity of resazurin-based compounds against Neisseria gonorrhoeae in vitro and in vivo. Int. J. Antimicrob. Agents 2016, 48, 367–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmitt, D.M.; O’Dee, D.M.; Cowan, B.N.; Birch, J.W.; Mazzella, L.K.; Nau, G.J.; Horzempa, J. The use of resazurin as a novel antimicrobial agent against Francisella tularensis. Front. Cell. Infect. Microbiol. 2013, 3, 93. [Google Scholar] [CrossRef] [PubMed]
- Souder, K.; Beatty, E.J.; McGovern, S.C.; Whaby, M.; Young, E.; Pancake, J.; Weekley, D.; Rice, J.; Primerano, D.A.; Denvir, J.; et al. Role of dipA and pilD in Francisella tularensis Susceptibility to Resazurin. Antibiotics 2021, 10, 992. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Jagannathan, L.; Cuddapah, S.; Costa, M. Oxidative stress under ambient and physiological oxygen tension in tissue culture. Curr. Pharmacol. Rep. 2016, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Laskar, N.; Kadouri, D.E. Evaluating the Effect of Oxygen Concentrations on Antibiotic Sensitivity, Growth, and Biofilm Formation of Human Pathogens. Microbiol. Insights 2016, 9, 37–46. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Schaible, B.; Taylor, C.T.; Schaffer, K. Hypoxia increases antibiotic resistance in Pseudomonas aeruginosa through altering the composition of multidrug efflux pumps. Antimicrob. Agents Chemother. 2012, 56, 2114–2118. [Google Scholar] [CrossRef] [PubMed]
- Borriello, G.; Werner, E.; Roe, F.; Kim, A.M.; Ehrlich, G.D.; Stewart, P.S. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 2004, 48, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Schaible, B.; Crifo, B.; Schaffer, K.; Taylor, C.T. The putative bacterial oxygen sensor Pseudomonas prolyl hydroxylase (PPHD) suppresses antibiotic resistance and pathogenicity in Pseudomonas aeruginosa. J. Biol. Chem. 2020, 295, 1195–1201. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Hsing, L.C.; Villet, R.; Bolduc, G.R.; Estabrooks, Z.; Taguezem, G.F.; Hooper, D.C. Reduced aeration affects the expression of the NorB efflux pump of Staphylococcus aureus by posttranslational modification of MgrA. J. Bacteriol. 2012, 194, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Horiyama, T.; Zhang, Y.; Li, X.; Nishino, K.; Yan, A. The multidrug efflux pump MdtEF protects against nitrosative damage during the anaerobic respiration in Escherichia coli. J. Biol. Chem. 2011, 286, 26576–26584. [Google Scholar] [CrossRef] [PubMed]
- Shafer, W.M.; Yu, E.W.; Rouquette-Loughlin, C.; Golparian, D.; Jerse, A.E.; Unemo, M. Efflux Pumps in Neisseria gonorrhoeae: Contributions to Antimicrobial Resistance and Virulence. In Efflux-Mediated Antimicrobial Resistance in Bacteria: Mechanisms, Regulation and Clinical Implications; Li, X.-Z., Elkins, C.A., Zgurskaya, H.I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 439–469. [Google Scholar]
- Chitsaz, M.; Booth, L.; Blyth, M.T.; O’Mara, M.L.; Brown, M.H. Multidrug Resistance in Neisseria gonorrhoeae: Identification of Functionally Important Residues in the MtrD Efflux Protein. mBio 2019, 10, e02277-19. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Moseng, M.A.; Reimche, J.L.; Holley, C.L.; Dhulipala, V.; Su, C.C.; Shafer, W.M.; Yu, E.W. Cryo-EM Structures of a Gonococcal Multidrug Efflux Pump Illuminate a Mechanism of Drug Recognition and Resistance. mBio 2020, 11, e00996-20. [Google Scholar] [CrossRef] [PubMed]
- Rouquette-Loughlin, C.; Dunham, S.A.; Kuhn, M.; Balthazar, J.T.; Shafer, W.M. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J. Bacteriol. 2003, 185, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2021, 12, 821535. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.; Imlay, J.A. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J. Bacteriol. 2006, 188, 6326–6334. [Google Scholar] [CrossRef]
- Erikstein, B.S.; Hagland, H.R.; Nikolaisen, J.; Kulawiec, M.; Singh, K.K.; Gjertsen, B.T.; Tronstad, K.J. Cellular stress induced by resazurin leads to autophagy and cell death via production of reactive oxygen species and mitochondrial impairment. J. Cell. Biochem. 2010, 111, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Prutz, W.A.; Butler, J.; Land, E.J. Photocatalytic and free radical interactions of the heterocyclic N-oxide resazurin with NADH, GSH, and Dopa. Arch. Biochem. Biophys. 1996, 327, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rymovicz, A.U.; Souza, R.D.; Gursky, L.C.; Rosa, R.T.; Trevilatto, P.C.; Groppo, F.C.; Rosa, E.A. Screening of reducing agents for anaerobic growth of Candida albicans SC5314. J. Microbiol. Methods 2011, 84, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Doern, G.V.; Brecher, S.M. The Clinical Predictive Value (or Lack Thereof) of the Results of In Vitro Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2011, 49, S11–S14. [Google Scholar] [CrossRef]
- Heithoff, D.M.; Barnes, V.L.; Mahan, S.P.; Fried, J.C.; Fitzgibbons, L.N.; House, J.K.; Mahan, M.J. Re-evaluation of FDA-approved antibiotics with increased diagnostic accuracy for assessment of antimicrobial resistance. Cell Rep. Med. 2023, 4, 101023. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, J. Clinical limitations of in vitro testing of microorganism susceptibility. Am. J. Hosp. Pharm. 1987, 44, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Gould, I.M. Towards a common susceptibility testing method? J. Antimicrob. Chemother. 2000, 45, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef]
- Zeitlinger, M.A.; Derendorf, H.; Mouton, J.W.; Cars, O.; Craig, W.A.; Andes, D.; Theuretzbacher, U. Protein binding: Do we ever learn? Antimicrob. Agents Chemother. 2011, 55, 3067–3074. [Google Scholar] [CrossRef]
- Hill, D.R.; Brunner, M.E.; Schmitz, D.C.; Davis, C.C.; Flood, J.A.; Schlievert, P.M.; Wang-Weigand, S.Z.; Osborn, T.W. In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J. Appl. Physiol. 2005, 99, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Delahay, R.M.; Robertson, B.D.; Balthazar, J.T.; Shafer, W.M.; Ison, C.A. Involvement of the gonococcal MtrE protein in the resistance of Neisseria gonorrhoeae to toxic hydrophobic agents. Microbiology 1997, 143 Pt 7, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Shafer, W.M.; Qu, X.; Waring, A.J.; Lehrer, R.I. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA 1998, 95, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Hagman, K.E.; Pan, W.; Spratt, B.G.; Balthazar, J.T.; Judd, R.C.; Shafer, W.M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 1995, 141 Pt 3, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Cannon, J.G.; Jerse, A.E.; Charniga, L.M.; Isbey, S.F.; Whicker, L.G. Human Experimentation with Neisseria gonorrhoeae: Rationale, Methods, and Implications for the Biology of Infection and Vaccine Development. J. Infect. Dis. 1994, 169, 532–537. [Google Scholar] [CrossRef] [PubMed]
- McKnew, D.L.; Lynn, F.; Zenilman, J.M.; Bash, M.C. Porin Variation among Clinical Isolates of Neisseria gonorrhoeae over a 10-Year Period, as Determined by Por Variable Region Typing. J. Infect. Dis. 2003, 187, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Cern, A.; Connolly, K.L.; Jerse, A.E.; Barenholz, Y. In Vitro Susceptibility of Neisseria gonorrhoeae Strains to Mupirocin, an Antibiotic Reformulated for Parenteral Administration in Nanoliposomes. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonocci. J. Exp. Med. 1972, 136, 1258–1271. [Google Scholar] [CrossRef]
- Mickelsen, P.A.; Sparling, P.F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 1981, 33, 555–564. [Google Scholar] [CrossRef]
- Garvin, L.E.; Bash, M.C.; Keys, C.; Warner, D.M.; Ram, S.; Shafer, W.M.; Jerse, A.E. Phenotypic and Genotypic Analyses of Neisseria gonorrhoeae Isolates That Express Frequently Recovered PorB PIA Variable Region Types Suggest that Certain P1a Porin Sequences Confer a Selective Advantage for Urogenital Tract Infection. Infect. Immun. 2008, 76, 3700–3709. [Google Scholar] [CrossRef] [PubMed]
- Hagman, K.E.; Shafer, W.M. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J. Bacteriol. 1995, 177, 4162–4165. [Google Scholar] [CrossRef] [PubMed]

| Strain | Description | Source or Reference |
|---|---|---|
| FA1090 | Isolated from patient with disseminated gonococcal infection. Resistant to streptomycin. | Cohen et al. [47] |
| LGB-24 | Isolated from urogenital tract. Resistant to tetracycline and penicillin; not a β-lactamase producer. | McKnew et al. [48] |
| NG886 | Penicillin, tetracycline, and fluoroquinolone-resistant strain. | Cern et al. [49] |
| MS11 | Isolated from a case of cervicitis. Overexpresses the MtrCDE multidrug efflux pump. Resistant to azithromycin and penicillin. | Swanson et al. [50] |
| FA19 | Isolated from patient with disseminated gonococcal infection. | Mickelsen et al. [51] |
| LG-16 | Isolated from urogenital tract. Resistant to penicillin, tetracycline, and azithromycin; β-lactamase producer. | Garvin et al. [52] |
| KH15 | -T at MtrR binding site (mtr-79), overexpresses MtrCDE efflux pump | Hagman et al. [53] |
| RD1 | FA19 mtrE::Km | Delahay et al. [43] |
| BR54 | FA19 mtrD-54 | Rouquette-Loughlin et al. [29] |
| CR28 | FA19 norM::Km | Rouquette-Loughlin et al. [29] |
| CR29 | BR54 norM::Km | Rouquette-Loughlin et al. [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rice, J.; Gibson, J.; Young, E.; Souder, K.; Cunningham, K.; Schmitt, D.M. Low Oxygen Concentration Reduces Neisseria gonorrhoeae Susceptibility to Resazurin. Antibiotics 2024, 13, 395. https://doi.org/10.3390/antibiotics13050395
Rice J, Gibson J, Young E, Souder K, Cunningham K, Schmitt DM. Low Oxygen Concentration Reduces Neisseria gonorrhoeae Susceptibility to Resazurin. Antibiotics. 2024; 13(5):395. https://doi.org/10.3390/antibiotics13050395
Chicago/Turabian StyleRice, Justin, Jordan Gibson, Emily Young, Kendall Souder, Kailee Cunningham, and Deanna M. Schmitt. 2024. "Low Oxygen Concentration Reduces Neisseria gonorrhoeae Susceptibility to Resazurin" Antibiotics 13, no. 5: 395. https://doi.org/10.3390/antibiotics13050395
APA StyleRice, J., Gibson, J., Young, E., Souder, K., Cunningham, K., & Schmitt, D. M. (2024). Low Oxygen Concentration Reduces Neisseria gonorrhoeae Susceptibility to Resazurin. Antibiotics, 13(5), 395. https://doi.org/10.3390/antibiotics13050395






