Cerebral Infectious Opportunistic Lesions in a Patient with Acute Myeloid Leukaemia: The Challenge of Diagnosis and Clinical Management
Abstract
1. Introduction
2. Case Report
2.1. Leukaemia Diagnosis, Infectious Complications of Remission Induction and Consolidation Chemo-Therapies, Diagnosis and Management of Central Nervous System Lesions
2.1.1. Case Presentation
2.1.2. Case Discussion and Literature Review
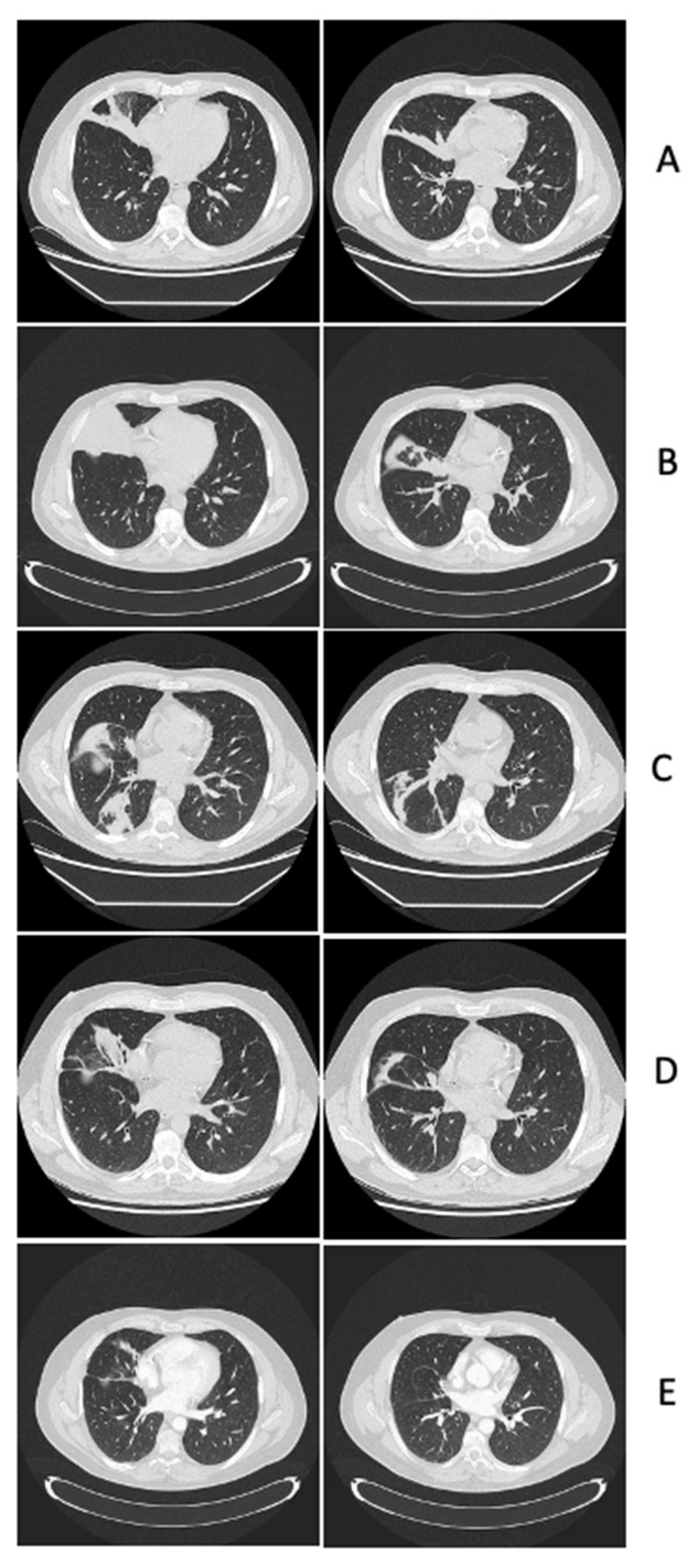
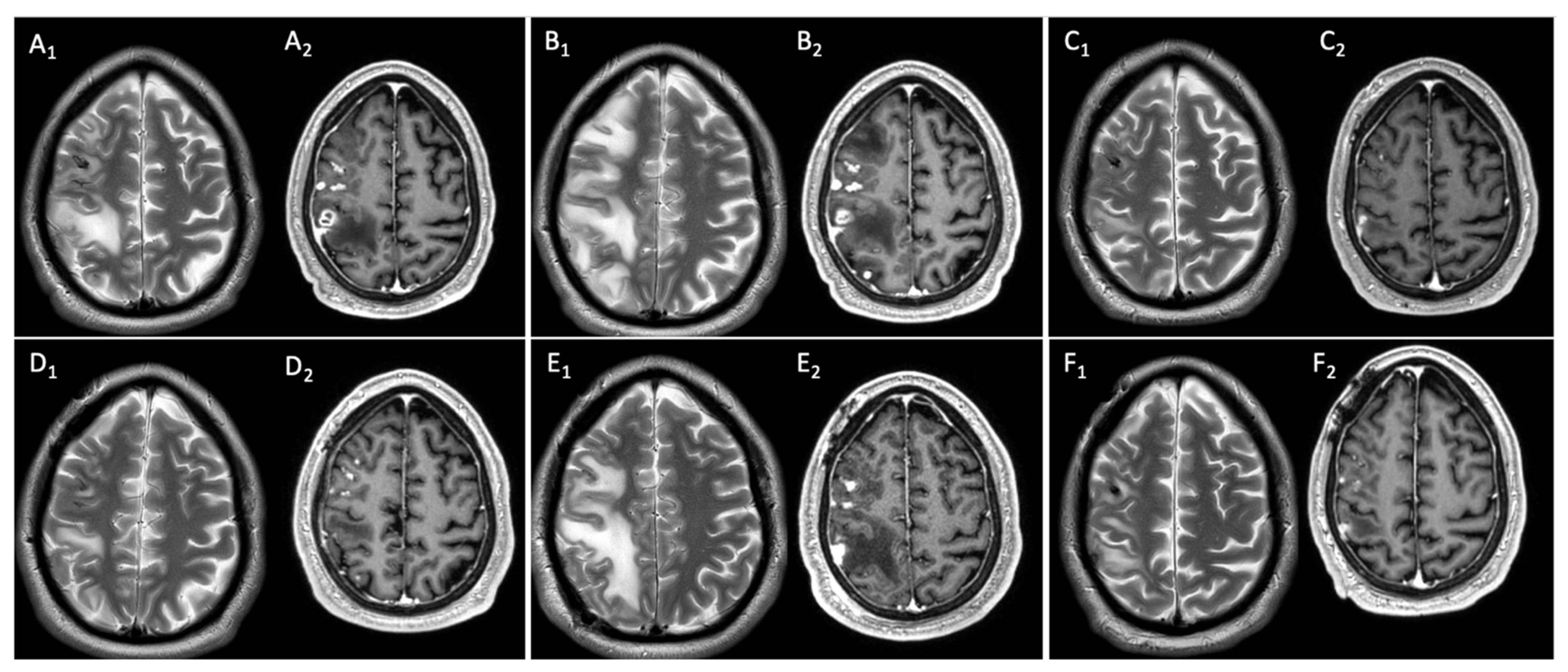
2.2. Clinical and Neuroimaging Follow-Up of Central Nervous System Infectious Lesions
2.2.1. Case Presentation
2.2.2. Case Discussion and Literature Review
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.A.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R., III. Defining Breakthrough Invasive Fungal Infection–Position Paper of the Mycoses Study Group Education and Research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM). Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Bodilsen, J.; D’Alessandris, Q.G.; Humphreys, H.; Iro, M.A.; Klein, M.; Last, K.; Montesinos, I.L.; Pagliano, P.; Sipahi, O.R.; San-Juan, R.; et al. European society of Clinical Microbiology and Infectious Diseases guidelines on diagnosis and treatment of brain abscess in children and adults. Clin. Microbiol. Infect. 2024, 30, 66–89. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [PubMed]
- Candoni, A.; Klimko, N.; Busca, A.; Di Blasi, R.; Shadrivova, O.; Cesaro, S.; Zannier, M.E.; Verga, L.; Forghieri, F.; Calore, E.; et al. Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients. Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses 2019, 62, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Economides, M.P.; Ballester, L.Y.; Kumar, V.A.; Jiang, Y.; Tarrand, J.; Prieto, V.; Torres, H.A.; Kontoyiannis, D.P. Invasive mold infections of the central nervous system in patients with hematologic cancer or stem cell transplantation (2000–2016): Uncommon, with improved survival but still deadly often. J. Infect. 2017, 75, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Cornely, O.A.; Hamed, K.; Marty, F.M.; Maertens, J.; Rahav, G.; Herbrecht, R.; Heinz, W.J. Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med. Mycol. 2020, 58, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Hoffmann, A.-H.; Kato, K.; Townsend, R.; Potchoiba, M.J.; Hope, W.W.; Andes, D.; Spickermann, J.; Schneidkraut, M.J. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob. Agents Chemother. 2017, 61, e01292-17. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Negroni, R.; Graybill, J.R.; Bustamante, B.; Pappas, P.; Chapman, S.; Hare, R.S.; Hardalo, C.J. Activity of posaconazole in the treatment of central nervous system fungal infections. J. Antimicrob. Chemother. 2005, 56, 745–755. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef]
- Averbuch, D.; De Greef, J. Nocardia Infections in Hematopoietic Cell Transplant Recipients: A Multicenter International Retrospective Study of the Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation. Clin. Infect. Dis. 2022, 75, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hershko, Y.; Levytskyi, K.; Rannon, E.; Assous, M.V.; Ken-Dror, S.; Amit, S.; Ben-Zvi, H.; Sagi, O.; Schwartz, O.; Sorek, N.; et al. Phenotypic and genotypic analysis of antimicrobial resistance in Nocardia species. J. Antimicrob. Chemother. 2023, 78, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Rodloff, A.C.; Vielkind, P.; Borgmann, T.; Stingu, C.-S. Antimicrobial Susceptibility of Clinical Oral Isolates of Actinomyces spp. Microorganisms 2022, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Scerra, S.; Coignard-Biehler, H.; Lanternier, F.; Suarez, F.; Charlier-Woerther, C.; Bougnoux, M.-E.; Gilquin, J.; Lecuit, M.; Hermine, O.; Lortholary, O. Disseminated toxoplasmosis in non-allografted patients with hematologic malignancies: Report of two cases and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Little, J.S.; Coughlin, C.; Hsieh, C.; Lanza, M.; Huang, W.Y.; Kumar, A.; Dandawate, T.; Tucker, R.; Gable, P.; Deida, A.A.V.; et al. Neuroinvasive Bacillus cereus Infection in Immunocompromised Hosts: Epidemiologic Investigation of 5 Patients With Acute Myeloid Leukemia. Open Forum Infect. Dis. 2024, 11, ofae048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, J.; Gui, R.; Li, Z.; Zu, Y.; Wang, J.; Yu, F.; Zhang, Y.; Zhao, H.; Ji, Z.; et al. Metagenomic Next Generation Sequencing in the Detection of Pathogens in Cerebrospinal Fluid of Patients after Alternative Donor Transplantation: A Feasibility Analysis. Front. Cell Infect. Microbiol. 2021, 11, 720132. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Kus, J.V.; Patel, S.N. Antimicrobial susceptibility profiles of invasive isolates of anaerobic bacteria from a large Canadian reference laboratory: 2012–2019. Anaerobe 2021, 70, 102386. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Li, N.; Zhang, Y.; Lü, X.; Liu, B. Antibiotic susceptibility and biofilm-forming ability of Veillonella strains. Anaerobe 2022, 78, 102667. [Google Scholar] [CrossRef] [PubMed]
- Lampros, A.; Caumes, E. Infection associated cerebral vasculitis. Rev. Med. Interne 2021, 42, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.; Fekkar, A.; Bonnin, S.; Shor, N.; Seilhean, D.; Plu, I.; Touitou, V.; Leblond, V.; Weiss, N.; Demeret, S.; et al. Cerebral vasculitis due to Aspergillus spp. in immunocompromised patients: Literature review. Int. J. Infect. Dis. 2022, 122, 244–251. [Google Scholar] [CrossRef]
- Gao, Y.; Qu, M.; Song, C.; Yin, L.; Zhang, M. Cerebral vasculitis caused by Talaromyces marneffei and Aspergillus niger in a HIV-positive patient: A case report and literature review. J. NeuroVirology 2022, 28, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Mearelli, F.; Occhipinti, A.; Altamura, N.; Fernandes, G.; Pivetti, G.; Chendi, E.; Spalluti, A.; Mamolo, L.; Casarsa, C.; Biolo, G. Invasive filamentous fungus infection with secondary cerebral vasculitis in a patient with no obvious immune suppression. Int. J. Infect. Dis. 2014, 19, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Berlit, P.; Kramer, R. Cerebral involvement in systemic vasculitides. Extracts from the guideline of the German neurological society. Neurol. Res. Pract. 2019, 1, 13. [Google Scholar] [CrossRef]
- Wieczorek, M.; Mariotto, S.; Ferrari, S.; Mosna, F.; Micò, M.C.; Borghero, C.; Dubbini, M.V.; Malagola, M.; Skert, C.; Andreini, A.; et al. Neurological complications in adult allogeneic hematopoietic stem cell transplant patients: Incidence, characteristics and long-term follow-up in a multicenter series. Bone Marrow Transplant. 2022, 57, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavazza, G.; Motto, C.; Regna-Gladin, C.; Travi, G.; Di Gennaro, E.; Peracchi, F.; Monti, B.; Corti, N.; Greco, R.; Minga, P.; et al. Cerebral Infectious Opportunistic Lesions in a Patient with Acute Myeloid Leukaemia: The Challenge of Diagnosis and Clinical Management. Antibiotics 2024, 13, 387. https://doi.org/10.3390/antibiotics13050387
Cavazza G, Motto C, Regna-Gladin C, Travi G, Di Gennaro E, Peracchi F, Monti B, Corti N, Greco R, Minga P, et al. Cerebral Infectious Opportunistic Lesions in a Patient with Acute Myeloid Leukaemia: The Challenge of Diagnosis and Clinical Management. Antibiotics. 2024; 13(5):387. https://doi.org/10.3390/antibiotics13050387
Chicago/Turabian StyleCavazza, Gabriele, Cristina Motto, Caroline Regna-Gladin, Giovanna Travi, Elisa Di Gennaro, Francesco Peracchi, Bianca Monti, Nicolò Corti, Rosa Greco, Periana Minga, and et al. 2024. "Cerebral Infectious Opportunistic Lesions in a Patient with Acute Myeloid Leukaemia: The Challenge of Diagnosis and Clinical Management" Antibiotics 13, no. 5: 387. https://doi.org/10.3390/antibiotics13050387
APA StyleCavazza, G., Motto, C., Regna-Gladin, C., Travi, G., Di Gennaro, E., Peracchi, F., Monti, B., Corti, N., Greco, R., Minga, P., Riva, M., Rimoldi, S., Vecchi, M., Rogati, C., Motta, D., Pazzi, A., Vismara, C., Bandiera, L., Crippa, F., ... Puoti, M. (2024). Cerebral Infectious Opportunistic Lesions in a Patient with Acute Myeloid Leukaemia: The Challenge of Diagnosis and Clinical Management. Antibiotics, 13(5), 387. https://doi.org/10.3390/antibiotics13050387






