A Mini-Review of Anti-Listerial Compounds from Marine Actinobacteria (1990–2023)
Abstract
1. Introduction
2. Background
2.1. Foodborne Listeriosis
2.1.1. Clinical Features
2.1.2. Listeriosis Outbreaks
2.1.3. Chemotherapeutic Treatment for Human Listeriosis
2.1.4. Resistance to Quinolones and Fluoroquinolones
2.1.5. Resistance to Penicillins and Cephalosporins
2.1.6. Resistance to Aminoglycosides
2.1.7. Resistance to Tetracyclines
2.1.8. Resistance to Trimethoprim
2.1.9. Resistance to Chloramphenicol
2.1.10. Resistance to Macrolides
2.2. Drivers of Antimicrobial Resistance
2.3. Actinobacteria as Potential Sources of Anti-Listerial Compounds
2.3.1. Streptomycineae
2.3.2. Micromonosporineae
2.3.3. Corynebacterineae
2.3.4. Streptosporangineae
2.4. Marine Environment as a Source of Microbes Harbouring Novel Bioactive Metabolites
3. Anti-Listerial Compounds from Marine Actinobacteria
4. Challenges and Opportunities Associated with Antimicrobials from Marine Actinobacteria
| Marine Actinomycete Strain | Year of Isolation | Country | Method Used for Antibacterial Activity Assay | Test Strain | Reference |
|---|---|---|---|---|---|
| Pseudonocardia carboxydivorans VO36-3 | 2013 | Chile | Cross-streak method | L. monocytogenes 07PF0776 | [141] |
| Salinoactinospora qingdaoensis VN6-2 | |||||
| Microbacterium profundi VP2-3 | |||||
| Arthrobacter phenanthrenivorans VO30-3 | |||||
| Aeromicrobium alkaliterrae V040-3 | |||||
| Gordonia bronchialis VO29-3 | |||||
| Isoptericola halotolerans VP3-3 | |||||
| Streptomyces janthinus VS4-2 | |||||
| Streptomyces albogriseolus VH47-3 | |||||
| Streptomyces sp. H-KF8 | 2013 | Chile | Cross-streak method | L. monocytogenes 07PF0776 | [144] |
| Streptomyces sp. H-KF8 | 2013 | Chile | Cross-streak method | L. monocytogenes 07PF0776 | [143] |
| Rhodococcus H-CA8F | |||||
| Micrococcus H-CD9b | |||||
| Kocuria H-KB6 | |||||
| Curtobacterium H-ED12 | |||||
| Curtobacterium H-BE10 | |||||
| Corynebacterium H-EH3 | |||||
| Brachybacterium H-CG1 | |||||
| Brachybacterium H-CD1 | |||||
| Arthrobacter H-CA8b | |||||
| Actinomycete 111 | 2013 | Iran | Cross-streak method | L. monocytogenes ATCC 1298 | [142] |
| Actinomycete 112 | |||||
| Actinomycete 115 | |||||
| Actinomycete 117 | |||||
| Actinomycete 127 | |||||
| Actinomycete 131 | |||||
| Actinomycete 135 | |||||
| Actinomycete 141 | |||||
| Actinomycete 275 | |||||
| Tsukamurella strandjordii 101-1518 | 2015 | Northern Iceland | Agar diffusion assay and cross-streak method | L. monocytogenes | [145] |
| Marine Actinomycete Strain | Year of Isolation | Country of Isolation | Crude Extract | Pathogen Target | Reference |
|---|---|---|---|---|---|
| Streptomyces cyaneofuscatus M-157 | 2013 | Spain | Ethyl acetate extract | L. monocytogenes 72964 * | [148] |
| Streptomyces cyaneofuscatus M-169 | |||||
| Streptomyces cyaneofuscatus M-192 | |||||
| Streptomyces cyaneofuscatus M-207 | |||||
| Streptomyces cyaneofuscatus M-220 | |||||
| Streptomyces cyaneofuscatus M-231 | |||||
| Streptomyces sp. Sp1 | 2018 | Egypt | n-butanol extract | L. monocytogenes 19115 | [150] |
| Nocardiopsis alba PB-1 | 2020 | India | Ethyl acetate extract | L. monocytogenes ATCC 19112 | [149] |
| Nocardiopsis alba PB-3 | |||||
| N. dassonvillei SOD(B)ST2SA2 | 2021 | South Africa | Chloroform extract | L. monocytogenes KGEO161 | [85] |
| L. monocytogenes ILemanAP345 | |||||
| L. monocytogenes ILemanEO299 | |||||
| L. monocytogenes ILemanER317 | |||||
| L. monocytogenes ILestanBR361 | |||||
| L. monocytogenes ILestanBR363 | |||||
| L. monocytogenes ILestanGP395 | |||||
| L. monocytogenes ILestanGP400 | |||||
| L. monocytogenes ATCC 15313 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matle, I.; Mbatha, K.R.; Lentsoane, O.; Magwedere, K.; Morey, L.; Madoroba, E. Occurrence, serotypes, and characteristics of Listeria monocytogenes in meat and meat products in South Africa between 2014 and 2016. J. Food Saf. 2019, 39, e12629. [Google Scholar] [CrossRef]
- De Noordhout, C.M.; Devleesschauwer, B.; Angulo, F.J.; Verbeke, G.; Haagsma, J.; Kirk, M.; Havelaar, A.; Speybroeck, N. The global burden of listeriosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 13 February 2024).
- Smith, A.M.; Tau, N.P.; Smouse, S.L.; Allam, M.; Ismail, A.; Ramalwa, N.R.; Disenyeng, B.; Ngomane, M.; Thomas, J. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: Laboratory activities and experiences associated with whole-genome sequencing analysis of isolates. Foodborne Pathog. Dis. 2019, 16, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, T.; Ntuli, V.; Neetoo, S.H.; Habib, I.; Njage, P.M.K.; Parry-Hanson Kunadu, A.; Andoh, A.H.; Coorey, R.; Buys, E.M. Listeria monocytogenes at the food–human interface: A review of risk factors influencing transmission and consumer exposure in Africa. Int. J. Food Sci. Technol. 2023, 58, 4114–4126. [Google Scholar] [CrossRef]
- Kaptchouang Tchatchouang, C.-D.; Fri, J.; De Santi, M.; Brandi, G.; Schiavano, G.F.; Amagliani, G.; Ateba, C.N. Listeriosis outbreak in South Africa: A comparative analysis with previously reported cases worldwide. Microorganisms 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet. Res. 2020, 87, 1–20. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Hanes, R.M.; Huang, Z. Investigation of antimicrobial resistance genes in Listeria monocytogenes from 2010 through to 2021. Int. J. Environ. Res. Public Health 2022, 19, 5506. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance#:~:text=As%20a%20result%20of%20drug,severe%20illness%2C%20disability%20and%20death (accessed on 11 March 2024).
- Paul, S.I.; Majumdar, B.C.; Ehsan, R.; Hasan, M.; Baidya, A.; Bakky, M.A.H. Bioprospecting potential of marine microbial natural bioactive compounds. Appl. Biotechnol. Rep. 2021, 8, 96–108. [Google Scholar]
- Van Bergeijk, D.A.; Terlouw, B.R.; Medema, M.H.; van Wezel, G.P. Ecology and genomics of actinobacteria: New concepts for natural product discovery. Nat. Rev. Microbiol. 2020, 18, 546–558. [Google Scholar] [CrossRef]
- Girão, M.; Ribeiro, I.; Carvalho, M.d.F. Actinobacteria from marine environments: A unique source of natural products. In Natural Products from Actinomycetes: Diversity, Ecology and Drug Discovery; Rai, R.V., Bai, J.A., Eds.; Springer: Singapore, 2022; pp. 1–45. [Google Scholar]
- Sarkar, G.; Suthindhiran, K. Diversity and biotechnological potential of marine actinomycetes from India. Indian J. Microbiol. 2022, 62, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Aalbersberg, W. Marine actinomycetes: An ongoing source of novel bioactive metabolites. Microbiol. Res. 2012, 167, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Lacey, H.J.; Rutledge, P.J. Recently discovered secondary metabolites from Streptomyces species. Molecules 2022, 27, 887. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Listeria (Listeriosis). Available online: https://www.cdc.gov/listeria/faq.html#:~:text=Yes.,we%20call%20the%20infection%20invasive (accessed on 13 February 2023).
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef] [PubMed]
- Osek, J.; Wieczorek, K. Listeria monocytogenes—How this pathogen uses its virulence mechanisms to infect the hosts. Pathogens 2022, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Ramos Cela, M.; Medina Martínez, A.J.; Vera Martín, I. Infection of Listeria monocytogenes Associated to the Central Nervous System: Cell Pathogenesis, Diagnosis and Risk Factors; Archivos de Medicina Universitaria: Granada, Spain, 2022. [Google Scholar]
- Donovan, S. Listeriosis: A rare but deadly disease. Clin. Microbiol. Newsl. 2015, 37, 135–140. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, X.; Liu, S.; Zhao, Y.; Yang, X. An update review on Listeria infection in pregnancy. Infect. Drug Resist. 2021, 14, 1967–1978. [Google Scholar] [CrossRef]
- Olson-Chen, C. Neurologic infections in pregnancy. In Neurological Diseases and Pregnancy: A Coordinated CARE model for Best Management; Ciafaloni, E., Bushnell, C., Thornburg, L.L., Ciafaloni, E., Thornburg, L.L., Bushnell, C.D., Eds.; Oxford University Press: New York, NY, USA, 2018; pp. 95–104. [Google Scholar]
- Farber, J.M.; Peterkin, P.I.; Carter, A.O.; Varughese, P.V.; Ashton, F.E.; Ewan, E.P. Neonatal listeriosis due to cross-infection confirmed by isoenzyme typing and DNA fingerprinting. J. Infect. Dis. 1991, 163, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Tesini, B.L. Neonatal Listeriosis. Available online: https://www.msdmanuals.com/professional/pediatrics/infections-in-neonates/neonatal-listeriosis (accessed on 13 February 2024).
- Schlech III, W.F.; Lavigne, P.M.; Bortolussi, R.A.; Allen, A.C.; Haldane, E.V.; Wort, A.J.; Hightower, A.W.; Johnson, S.E.; King, S.H.; Nicholls, E.S. Epidemic listeriosis–evidence for transmission by food. N. Engl. J. Med. 1983, 308, 203–206. [Google Scholar] [CrossRef]
- Fleming, D.W.; Cochi, S.L.; MacDonald, K.L.; Brondum, J.; Hayes, P.S.; Plikaytis, B.D.; Holmes, M.B.; Audurier, A.; Broome, C.V.; Reingold, A.L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N. Engl. J. Med. 1985, 312, 404–407. [Google Scholar] [CrossRef]
- Büla, C.J.; Bille, J.; Glauser, M.P. An epidemic of food-borne listeriosis in western Switzerland: Description of 57 cases involving adults. Clin. Infect. Dis. 1995, 20, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S. Detection, Subtyping and Control of Listeria monocytogenes in Food Processing Environments; Swinburne University of Technology: Hawthorn, VIC, Australia, 2015. [Google Scholar]
- Buchanan, R.L.; Gorris, L.G.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Warmate, D.; Onarinde, B.A. Food safety incidents in the red meat industry: A review of foodborne disease outbreaks linked to the consumption of red meat and its products, 1991 to 2021. Int. J. Food Microbiol. 2023, 398, 110240. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Listeria (Listeriosis)–Listeria Outbreaks. Available online: https://www.cdc.gov/listeria/outbreaks/index.html (accessed on 14 February 2024).
- Zhang, Y. Antimicrobial Resistance of Listeria monocytogenes and Enterococcus faecium from Food and Animal Sources; University of Maryland: College Park, MD, USA, 2005. [Google Scholar]
- Kananub, S.; Lertsakkongkul, P.; Aryatawong, P.; Horhirunkhajohn, W.; Pinniam, N.; Krajanglikit, P.; Sonthong, K.; Kasemsuwan, S. Listeria contamination in milk-processing chain and proficiency in Listeria monocytogenes decontamination of small-scale milk retailers. J. Food Qual. 2024, 2024, 6263938. [Google Scholar] [CrossRef]
- Thomas, J.; Govender, N.; McCarthy, K.M.; Erasmus, L.K.; Doyle, T.J.; Allam, M.; Ismail, A.; Ramalwa, N.; Sekwadi, P.; Ntshoe, G.; et al. Outbreak of listeriosis in South Africa associated with processed meat. N. Engl. J. Med. 2020, 382, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.S.; Dunne, E.F.; Graves, L.; Wiedmann, M.; Patrick, M.; Hunter, S.; Salehi, E.; Mostashari, F.; Craig, A.; Mshar, P.; et al. Nationwide outbreak of listeriosis due to contaminated meat. Epidemiol. Infect. 2006, 134, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Pichler, J.; Much, P.; Kasper, S.; Fretz, R.; Auer, B.; Kathan, J.; Mann, M.; Huhulescu, S.; Ruppitsch, W.; Pietzka, A. An outbreak of febrile gastroenteritis associated with jellied pork contaminated with Listeria monocytogenes. Wien. Klin. Wochenschr. 2009, 121, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Larsson, J.T.; Lisby, M.; Müller, L.; Madsen, S.B.; Engberg, J.; Bangsborg, J.; Ethelberg, S.; Kemp, M. Outbreak of listeriosis caused by infected beef meat from a meals-on-wheels delivery in Denmark 2009. Clin. Microbiol. Infect. 2011, 17, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Awofisayo-Okuyelu, A.; Arunachalam, N.; Dallman, T.; Grant, K.; Aird, H.; McLauchlin, J.; Painset, A.; Amar, C. An outbreak of human listeriosis in England between 2010 and 2012 associated with the consumption of pork pies. J. Food Prot. 2016, 79, 732–740. [Google Scholar] [CrossRef]
- Rivas, L.; Dupont, P.Y.; Wilson, M.; Rohleder, M.; Gilpin, B. An outbreak of multiple genotypes of Listeria monocytogenes in New Zealand linked to contaminated ready-to-eat meats—A retrospective analysis using whole-genome sequencing. Lett. Appl. Microbiol. 2019, 69, 392–398. [Google Scholar] [CrossRef]
- Okpo, E.; Leith, J.; Smith-Palmer, A.; Bell, J.; Parks, D.; Browning, F.; Byers, L.; Corrigan, H.; Webster, D.; Karcher, A.M.; et al. An outbreak of an unusual strain of Listeria monocytogenes infection in North-East Scotland. J. Infect. Public Health 2015, 8, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Dahl, V.; Sundqvist, L.; Hedenström, I.; Löfdahl, M.; Alm, E.; Ringberg, H.; Lindblad, M.; Wallensten, A.; Thisted Lambertz, S.; Jernberg, C. A nationwide outbreak of listeriosis associated with cold-cuts, Sweden 2013–2014. Infect. Ecol. Epidemiol. 2017, 7, 1324232. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, R.; Halbedel, S.; Adler, M.; Becker, N.; Allerberger, F.; Holzer, A.; Boone, I.; Falkenhorst, G.; Kleta, S.; Al Dahouk, S. Nationwide outbreak of invasive listeriosis associated with consumption of meat products in health care facilities, Germany, 2014–2019. Clin. Microbiol. Infect. 2021, 27, 1035.e1–1035.e5. [Google Scholar] [CrossRef] [PubMed]
- Duranti, A.; Sabbatucci, M.; Blasi, G.; Acciari, V.A.; Ancora, M.; Bella, A.; Busani, L.; Centorame, P.; Cammà, C.; Conti, F. A severe outbreak of listeriosis in central Italy with a rare pulsotype associated with processed pork products. J. Med. Microbiol. 2018, 67, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Maurella, C.; Gallina, S.; Ru, G.; Adriano, D.; Bellio, A.; Bianchi, D.M.; Chiavacci, L.; Crescio, M.I.; Croce, M.; D’Errico, V.; et al. Outbreak of febrile gastroenteritis caused by Listeria monocytogenes 1/2a in sliced cold beef ham, Italy, May 2016. Eurosurveillance 2018, 23, 17-00155. [Google Scholar] [CrossRef] [PubMed]
- Althaus, D.; Jermini, M.; Giannini, P.; Martinetti, G.; Reinholz, D.; Nüesch-Inderbinen, M.; Lehner, A.; Stephan, R. Local outbreak of Listeria monocytogenes serotype 4b sequence type 6 due to contaminated meat pâté. Foodborne Pathog. Dis. 2017, 14, 219–222. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control; European Food Safety Authority. Rapid Outbreak Assessment: Multi-Country Outbreak of Listeria monocytogenes Sequence Type 6 Infections Linked to Ready-to-Eat Meat Products. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-outbreak-assessment-multi-country-outbreak-listeria-monocytogenes-sequence (accessed on 11 February 2024).
- Luque-Sastre, L.; Arroyo, C.; Fox, E.M.; McMahon, B.J.; Bai, L.; Li, F.; Fanning, S. Antimicrobial resistance in Listeria species. Microbiol. Spectr. 2018, 6, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Sarr, M.; Alou, M.T.; Padane, A.; Diouf, F.S.; Beye, M.; Sokhna, C.; Fenollar, F.; Mboup, S.; Raoult, D.; Million, M. A review of the literature of Listeria monocytogenes in Africa highlights breast milk as an overlooked human source. Front. Microbiol. 2023, 14, 1213953. [Google Scholar] [CrossRef] [PubMed]
- Hof, H.; Nichterlein, T.; Kretschmar, M. Management of listeriosis. Clin. Microbiol. Rev. 1997, 10, 345–357. [Google Scholar] [CrossRef]
- Lamont, R.F.; Sobel, J.; Mazaki-Tovi, S.; Kusanovic, J.P.; Vaisbuch, E.; Kim, S.K.; Uldbjerg, N.; Romero, R. Listeriosis in human pregnancy: A systematic review. J. Perinat. Med. 2011, 39, 227–236. [Google Scholar] [CrossRef]
- Keet, R. Categorization of Listeria monocytogenes from Food, Environmental, and Clinical Origin in the Western Cape (South Africa); Stellenbosch University: Western Cape, South Africa, 2020. [Google Scholar]
- Keet, R.; Rip, D. Listeria monocytogenes isolates from Western Cape, South Africa exhibit resistance to multiple antibiotics and contradicts certain global resistance patterns. AIMS Microbiol. 2021, 7, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Janakiraman, V. Listeriosis in pregnancy: Diagnosis, treatment, and prevention. Rev. Obstet. Gynecol. 2008, 1, 179–185. [Google Scholar] [PubMed]
- Dos Reis, J.O.; Vieira, B.S.; Cunha Neto, A.; Castro, V.S.; Figueiredo, E.E.S. Antimicrobial resistance of Listeria monocytogenes from animal foods to first- and second-line drugs in the treatment of listeriosis from 2008 to 2021: A systematic review and meta-analysis. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 1351983. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Akazawa, K. Treatment of Listeria monocytogenes bacteremia with oral levofloxacin in an immunocompromised patient. IDCases 2023, 31, e01680. [Google Scholar] [CrossRef]
- Kayode, A.J.; Okoh, A.I. Antibiotic resistance profile of Listeria monocytogenes recovered from ready-to-eat foods surveyed in South Africa. J. Food Prot. 2022, 85, 1807–1814. [Google Scholar] [CrossRef]
- Da Rocha, L.S.; Gunathilaka, G.U.; Zhang, Y. Antimicrobial-resistant Listeria species from retail meat in metro Detroit. J. Food Prot. 2012, 75, 2136–2141. [Google Scholar] [CrossRef]
- Sosnowski, M.; Lachtara, B.; Wieczorek, K.; Osek, J. Antimicrobial resistance and genotypic characteristics of Listeria monocytogenes isolated from food in Poland. Int. J. Food Microbiol. 2019, 289, 1–6. [Google Scholar] [CrossRef]
- Elsayed, M.E.; Abd El-Hamid, M.I.; El-Gedawy, A.; Bendary, M.M.; RM, E.L.; Alhomrani, M.; Alamri, A.S.; Alghamdi, S.A.; Arnout, M.; Binjawhar, D.N.; et al. New insights into Listeria monocytogenes antimicrobial resistance, virulence attributes and their prospective correlation. Antibiotics 2022, 11, 1447. [Google Scholar] [CrossRef]
- Caruso, M.; Fraccalvieri, R.; Pasquali, F.; Santagada, G.; Latorre, L.M.; Difato, L.M.; Miccolupo, A.; Normanno, G.; Parisi, A. Antimicrobial susceptibility and multilocus sequence typing of Listeria monocytogenes isolated over 11 years from food, humans, and the environment in Italy. Foodborne Pathog. Dis. 2020, 17, 284–294. [Google Scholar] [CrossRef]
- Hailu, W.; Helmy, Y.A.; Carney-Knisely, G.; Kauffman, M.; Fraga, D.; Rajashekara, G. Prevalence and antimicrobial resistance profiles of foodborne pathogens isolated from dairy cattle and poultry manure amended farms in Northeastern Ohio, the United States. Antibiotics 2021, 10, 1450. [Google Scholar] [CrossRef]
- Lachtara, B.; Wieczorek, K.; Osek, J. Antimicrobial resistance of Listeria monocytogenes serogroups IIa and IVb from food and food-production environments in Poland. J. Vet. Res. 2023, 67, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Poyart-Salmeron, C.; Carlier, C.; Trieu-Cuot, P.; Courtieu, A.L.; Courvalin, P. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 1990, 335, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, E.; Courvalin, P. Antibiotic resistance in Listeria spp. Antimicrob. Agents Chemother. 1999, 43, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.M.; Sherwood, L.M.; Woolverton, C.J. Prescott’s Microbiology, 8th ed.; McGraw-Hill: New York, NY, USA, 2011; pp. 832–838. [Google Scholar]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological effects of quinolones: A family of broad-spectrum antimicrobial agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef] [PubMed]
- Lyon, S.A.; Berrang, M.E.; Fedorka-Cray, P.J.; Fletcher, D.L.; Meinersmann, R.J. Antimicrobial resistance of Listeria monocytogenes isolated from a poultry further processing plant. Foodborne Pathog. Dis. 2008, 5, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Morvan, A.; Moubareck, C.; Leclercq, A.; Hervé-Bazin, M.; Bremont, S.; Lecuit, M.; Courvalin, P.; Le Monnier, A. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob. Agents Chemother. 2010, 54, 2728–2731. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.I.; Sabala, R.F. Potential public health hazards related to consumption of poultry contaminated with antibiotic resistant Listeria monocytogenes in Egypt. BMC Microbiol. 2024, 24, 41. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Arnold, C.; Threlfall, E.J. Rapid detection of gyrA and parC mutations in quinolone-resistant Salmonella enterica using Pyrosequencing® technology. J. Microbiol. Methods 2007, 68, 163–171. [Google Scholar] [CrossRef]
- Mata, M.; Baquero, F.; Perez-Diaz, J. A multidrug efflux transporter in Listeria monocytogenes. FEMS Microbiol. Lett. 2000, 187, 185–188. [Google Scholar] [CrossRef]
- Godreuil, S.; Galimand, M.; Gerbaud, G.; Jacquet, C.; Courvalin, P. Efflux pump Lde is associated with fluoroquinolone resistance in Listeria monocytogenes. Antimicrob. Agents Chemother. 2003, 47, 704–708. [Google Scholar] [CrossRef]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; De Schaetzen, M.-A.; Van Huffel, X.; Imberechts, H.; Dierick, K. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- Guerin, F.; Galimand, M.; Tuambilangana, F.; Courvalin, P.; Cattoir, V. Overexpression of the novel MATE fluoroquinolone efflux pump FepA in Listeria monocytogenes is driven by inactivation of its local repressor FepR. PLoS ONE 2014, 9, e106340. [Google Scholar] [CrossRef] [PubMed]
- Courvalin, P. Antimicrobial drug resistance: “prediction is very difficult, especially about the future”. Emerg. Infect. Dis. 2005, 11, 1503. [Google Scholar] [CrossRef] [PubMed]
- Cernicchi, G.; Felicetti, T.; Sabatini, S. Microbial efflux pump inhibitors: A journey around quinoline and indole derivatives. Molecules 2021, 26, 6996. [Google Scholar] [CrossRef] [PubMed]
- Troxler, R.; Von Graevenitz, A.; Funke, G.; Wiedemann, B.; Stock, I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin. Microbiol. Infect. 2000, 6, 525–535. [Google Scholar]
- Turner, J.; Muraoka, A.; Bedenbaugh, M.; Childress, B.; Pernot, L.; Wiencek, M.; Peterson, Y.K. The chemical relationship among beta-lactam antibiotics and potential impacts on reactivity and decomposition. Front. Microbiol. 2022, 13, 807955. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk-Balska, A.; Markiewicz, Z. The intrinsic cephalosporin resistome of Listeria monocytogenes in the context of stress response, gene regulation, pathogenesis and therapeutics. J. Appl. Microbiol. 2016, 120, 251–265. [Google Scholar] [CrossRef]
- Mpundu, P.; Muma, J.B.; Mukubesa, A.N.; Kainga, H.; Mudenda, S.; Bumbangi, F.N.; Muleya, W.; Katemangwe, P.; Munyeme, M. Antibiotic resistance patterns of Listeria species isolated from broiler abattoirs in Lusaka, Zambia. Antibiotics 2022, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Von Wintersdorff, C.J.; Penders, J.; Van Niekerk, J.M.; Mills, N.D.; Majumder, S.; Van Alphen, L.B.; Savelkoul, P.H.; Wolffs, P.F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Zakrzewski, A.J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Antimicrobial resistance and virulence characterization of Listeria monocytogenes strains isolated from food and food processing environments. Pathogens 2022, 11, 1099. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Ngema, S.S.; Khumalo, S.H.; Ojo, M.C.; Pooe, O.J.; Malilehe, T.S.; Basson, A.K.; Madoroba, E. Evaluation of antimicrobial activity by marine Nocardiopsis dassonvillei against foodborne Listeria monocytogenes and Shiga toxin-producing Escherichia coli. Microorganisms 2023, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Wałecka-Zacharska, E.; Chen, J.C.; Katarzyna, K.-P.; Devlieghere, F.; Van Meervenne, E.; Osek, J.; Wieczorek, K.; Bania, J. Listeria monocytogenes–an examination of food chain factors potentially contributing to antimicrobial resistance. Food Microbiol. 2016, 54, 178–189. [Google Scholar] [CrossRef]
- Srinivasan, V.; Nam, H.; Nguyen, L.; Tamilselvam, B.; Murinda, S.; Oliver, S. Prevalence of antimicrobial resistance genes in Listeria monocytogenes isolated from dairy farms. Foodborne Pathog. Dis. 2005, 2, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.O.; Hussien, A.A.; Youseef, A.G.; Younis, W.K.; Mubarak, A.G. Prevalence, antibiotic resistance, and phylogenetic analysis of Listeria monocytogenes isolated from various sources in Egypt: Fish, vegetables, and humans. Iraqi J. Vet. Sci. 2024, 38, 15–27. [Google Scholar] [CrossRef]
- Shaw, K.; Rather, P.; Hare, R.; Miller, G. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 1993, 57, 138–163. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Leclercq, A.; Vales, G.; Tessaud-Rita, N.; Bracq-Dieye, H.; Thouvenot, P.; Madec, Y.; Charlier, C.; Lecuit, M. Phenotypic and genotypic antimicrobial resistance of Listeria monocytogenes: An observational study in France. Lancet Reg. Health Eur. 2024, 37, 100800. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, H.; Roberts, A.P.; Bedi, R.; Wilson, M.; Mullany, P. Characterization of Tn 916 S, a Tn 916-like element containing the tetracycline resistance determinant tet(S). J. Bacteriol. 2004, 186, 4395–4398. [Google Scholar] [CrossRef]
- Charpentier, E.; Courvalin, P. Emergence of the trimethoprim resistance gene dfrD in Listeria monocytogenes BM4293. Antimicrob. Agents Chemother. 1997, 41, 1134–1136. [Google Scholar] [CrossRef]
- Kayode, A.J.; Okoh, A.I. Antimicrobial-resistant Listeria monocytogenes in ready-to-eat foods: Implications for food safety and risk assessment. Foods 2023, 12, 1346. [Google Scholar] [CrossRef]
- Huovinen, P.; Sundström, L.; Swedberg, G.; Sköld, O. Trimethoprim and sulfonamide resistance. Antimicrob. Agents Chemother. 1995, 39, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Facinelli, B.; Giovanetti, E.; Varaldo, P.E. Transferable erythromycin resistance in Listeria spp. isolated from food. Appl. Environ. Microbiol. 1996, 62, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Granier, S.A.; Moubareck, C.; Colaneri, C.; Lemire, A.; Roussel, S.; Dao, T.-T.; Courvalin, P.; Brisabois, A. Antimicrobial resistance of Listeria monocytogenes isolates from food and the environment in France over a 10-year period. Appl. Environ. Microbiol. 2011, 77, 2788–2790. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Loneragan, G.H.; Scott, H.M.; Singer, R.S. Antimicrobial resistance: Challenges and perspectives. Compr. Rev. Food Sci. Food Saf. 2013, 12, 234–248. [Google Scholar] [CrossRef]
- Rostamian, M.; Kooti, S.; Mohammadi, B.; Salimi, Y.; Akya, A. A systematic review and meta-analysis of Listeria monocytogenes isolated from human and non-human sources: The antibiotic susceptibility aspect. Diagn. Microbiol. Infect. Dis. 2022, 102, 115634. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Okoh, A.I.; Lues, R. Occurrence and multidrug resistance in strains of Listeria monocytogenes recovered from the anaerobic co-digestion sludge contained in a single stage steel biodigester: Implications for antimicrobial stewardship. Microorganisms 2023, 11, 725. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Letchumanan, V.; Tan, L.T.-H.; Ser, H.-L.; Goh, B.-H.; Lee, L.-H. The rising of “modern actinobacteria” era. Prog. Microbes Mol. Biol. 2020, 3, a0000064. [Google Scholar] [CrossRef][Green Version]
- Hui, M.L.; Tan, L.T.; Letchumanan, V.; He, Y.W.; Fang, C.M.; Chan, K.G.; Law, J.W.; Lee, L.H. The extremophilic actinobacteria: From microbes to medicine. Antibiotics 2021, 10, 682. [Google Scholar] [CrossRef]
- Aly, M.M.; Bahamdain, L.A.; Aba, S.A. Unexplored extreme habitats as sources of novel and rare actinomycetes with enzyme and antimicrobial activities. J. Pharm. Biol. Sci. 2019, 14, 45–54. [Google Scholar]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.M.; Sandman, K.M.; Wood, D.H. Prescott’s Microbiology, 11th ed.; McGraw-Hill Education: New York, NY, USA, 2020; pp. 538–545. [Google Scholar]
- Li, F.; Liu, S.; Lu, Q.; Zheng, H.; Osterman, I.A.; Lukyanov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Liu, S.; Ye, J. Studies on antibacterial activity and diversity of cultivable actinobacteria isolated from mangrove soil in Futian and Maoweihai of China. Evid. Based Complement. Altern. Med. 2019, 2019, 3476567. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.S.; El-Naggar, M.E.; Allam, A.; Morsy, O.M.; Othman, S.I. Microbial natural products in drug discovery. Processes 2020, 8, 470. [Google Scholar] [CrossRef]
- Pacios-Michelena, S.; Aguilar González, C.N.; Alvarez-Perez, O.B.; Rodriguez-Herrera, R.; Chávez-González, M.; Arredondo Valdés, R.; Ascacio Valdés, J.A.; Govea Salas, M.; Ilyina, A. Application of Streptomyces antimicrobial compounds for the control of phytopathogens. Front. Sustain. Food Syst. 2021, 5, 696518. [Google Scholar] [CrossRef]
- Hifnawy, M.S.; Fouda, M.M.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; AbouZid, S.F.; Rateb, M.E.; Keller, A.; Adamek, M.; Ziemert, N.; et al. The genus Micromonospora as a model microorganism for bioactive natural product discovery. RSC Adv. 2020, 10, 20939–20959. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, M.; Khatun, M.; Rahman, H. Isolation and screening of actinomycetes from Sundarbans soil for antibacterial activity. Afr. J. Biotechnol. 2010, 9, 4615–4619. [Google Scholar]
- El-sersy, N.A.; Abou-Elela, G.M. Antagonistic effect of marine Nocardia brasiliensis against the fish pathogen Vibrio damsela: Application of Piackett-Burman experimental design to evaluate factors affecting the production of the antibacterial agent. Int. J. Oceans Oceanogr. 2006, 1, 141–150. [Google Scholar]
- Naragani, K.; Munaganti, R.K.; Mangamuri, U.K.; Vijayalakshmi, M. Optimization of culture conditions for enhanced antimicrobial activity of Rhodococcus erythropolis VLK-12 isolated from South Coast of Andhra Pradesh, India. Br. Microbiol. Res. J. 2014, 4, 63–79. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Rainey, F.A.; Ward-Rainey, N.L. Proposal for a new hierarchic classification system, actinobacteria classis nov. Int. J. Syst. Evol. Microbiol. 1997, 47, 479–491. [Google Scholar] [CrossRef]
- Bhairamkar, S.; Kadam, P.; Anjulal, H.; Joshi, A.; Chaudhari, R.; Bagul, D.; Javdekar, V.; Zinjarde, S. Comprehensive updates on the biological features and metabolic potential of the versatile extremophilic actinomycete Nocardiopsis dassonvillei. Res. Microbiol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species: Incidence, ecological roles and adaptations. Microbiol. Res. 2015, 174, 33–47. [Google Scholar] [CrossRef]
- Ali, S.M.; Siddiqui, R.; Khan, N.A. Antimicrobial discovery from natural and unusual sources. J. Pharm. Pharmacol. 2018, 70, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Sipkema, D. Marine rare actinomycetes: A promising source of structurally diverse and unique novel natural products. Mar. Drugs 2019, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Norouzi, H.; Hamedi, J.; Roohi, A. Screening of antibacterial producing actinomycetes from sediments of the Caspian Sea. Int. J. Mol. Cell. Med. 2013, 2, 64. [Google Scholar] [PubMed]
- Kamjam, M.; Sivalingam, P.; Deng, Z.; Hong, K. Deep sea actinomycetes and their secondary metabolites. Front. Microbiol. 2017, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Yaradoddi, J.S.; Kontro, M.H.; Ganachari, S.V.; Banapurmath, N.R.; Oli, A.; Katti, A.S.; Sulochana, M. Actinobacteria in marine environments. In Actinobacteria: Ecology, Diversity, Classification and Extensive Applications; Yaradoddi, J.S., Kantro, M.H., Ganachari, S.V., Eds.; Springer: Singapore, 2022; pp. 21–38. [Google Scholar]
- Zhang, L.; Xi, L.; Ruan, J.; Huang, Y. Microbacterium marinum sp. nov., isolated from deep-sea water. Syst. Appl. Microbiol. 2012, 35, 81–85. [Google Scholar] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Jaisankar, P.; Das, S.; Sarkar, K.K.; Roy, S.; Besra, S.E.; Vedasiromani, J.R.; Ghosh, D.; Sana, B.; Mukherjee, J. Production and purification of a bioactive substance inhibiting multiple drug resistant bacteria and human leukemia cells from a salt-tolerant marine actinobacterium sp. isolated from the Bay of Bengal. Biotechnol. Lett. 2006, 28, 1083–1088. [Google Scholar] [CrossRef]
- Arumugam, M.; Mitra, A.; Jaisankar, P.; Dasgupta, S.; Sen, T.; Gachhui, R.; Kumar Mukhopadhyay, U.; Mukherjee, J. Isolation of an unusual metabolite 2-allyloxyphenol from a marine actinobacterium, its biological activities and applications. Appl. Microbiol. Biotechnol. 2010, 86, 109–117. [Google Scholar] [CrossRef]
- Siddharth, S.; Rai, V.R. Isolation and characterization of bioactive compounds with antibacterial, antioxidant and enzyme inhibitory activities from marine-derived rare actinobacteria, Nocardiopsis sp. SCA21. Microb. Pathog. 2019, 137, 103775. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, L.; Schultz, P.J.; Tamayo-Castillo, G.; Dotson, G.D.; Sherman, D.H.; Tripathi, A. Adipostatins E–J, new potent antimicrobials identified as inhibitors of coenzyme-A biosynthesis. Tetrahedron Lett. 2020, 61, 151469. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.Y.; Barakat, K.M.; Aly-Eldeen, M.A.; Ghoneim, H.B.; Hamdan, A.M. Veratraldehyde as a food additive produced by the marine isolate Streptomyces diastaticus LC360811. Egypt. J. Aquat. Biol. Fish 2021, 25, 1–19. [Google Scholar] [CrossRef]
- Yi, K.-X.; Xie, Q.-Y.; Ma, Q.-Y.; Yang, L.; Dai, H.-F.; Zhao, Y.-X.; Hao, Y.-E. Diverse ansamycin derivatives from the marine-derived Streptomyces sp. ZYX-F-97 and their antibacterial activities. Fitoterapia 2024, 173, 105814. [Google Scholar] [PubMed]
- Uemura, M.; Kobayashi, K.; Sato, N.; Nagai, K.; Seki, R.; Kamio, M.; Fukuda, T.; Tsubouchi, T.; Tomoda, H.; Ohshiro, T.; et al. Haneummycin, a new 22-membered macrolide lactam antibiotic, produced by marine-derived Streptomyces sp. KM77-8. J. Antibiot. 2023, 76, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Schinke, C.; Martins, T.; Queiroz, S.C.; Melo, I.S.; Reyes, F.G. Antibacterial compounds from marine bacteria, 2010–2015. J. Nat. Prod. 2017, 80, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, Y.; Cao, S. Antimicrobial compounds from marine actinomycetes. Arch. Pharm. Res. 2020, 43, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, W.; Lu, H.; Lan, J.; Liang, K.; Cao, S. A review: Halogenated compounds from marine Actinomycetes. Molecules 2021, 26, 2754. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; She, J.; Fu, J.; Wang, J.; Ye, Y.; Yang, B.; Liu, Y.; Zhou, X.; Tao, H. Advances in natural products from the marine-sponge-associated microorganisms with antimicrobial activity in the last decade. Mar. Drugs 2023, 21, 236. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Pinto, E.; Kijjoa, A.; Pinto, M.; Sousa, E. Targeting antimicrobial drug resistance with marine natural products. Int. J. Antimicrob. Agents 2020, 56, 106005. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 16 August 2023).
- Molinos, A.C.; Abriouel, H.; López, R.L.; Omar, N.B.; Valdivia, E.; Gálvez, A. Enhanced bactericidal activity of enterocin AS-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against Listeria monocytogenes in ready-to-eat salad. Food Chem. Toxicol. 2009, 47, 2216–2223. [Google Scholar] [CrossRef]
- Martínez, B.; Rodríguez, A.; Suárez, E. Antimicrobial peptides produced by bacteria: The bacteriocins. In New Weapons to Control Bacterial Growth; Villa, T.G., Vinas, M., Eds.; Springer: Cham, Switzerland, 2016; pp. 15–38. [Google Scholar]
- Batiha, G.E.-S.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Yousefi, M. Antibacterial activity of pediocin and pediocin-producing bacteria against Listeria monocytogenes in meat products. Front. Microbiol. 2021, 12, 709959. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Lucera, A.; Conte, A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 28087. [Google Scholar]
- Claverías, F.P.; Undabarrena, A.N.; González, M.; Cámara, B.P. Culturable diversity and antimicrobial activity of actinobacteria from marine sediments in Valparaíso bay, Chile. Front. Microbiol. 2015, 6, 121535. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Shahnavaz, B.; Ghazvini, K.; Valinasab, T. Screening of actinomycetes from Lipar area of Oman Sea to investigate the antibacterial compounds. Avicenna J. Clin. Microbiol. Infect. 2015, 2, 23621. [Google Scholar] [CrossRef]
- Undabarrena, A.; Beltrametti, F.; Claverías, F.P.; Moore, E.R.; Cámara, B. Exploring the diversity and antimicrobial potential of marine actinobacteria from the comau fjord in Northern Patagonia, Chile. Front. Microbiol. 2016, 7, 210381. [Google Scholar] [CrossRef]
- Undabarrena, A.; Ugalde, J.A.; Seeger, M.; Cámara, B. Genomic data mining of the marine actinobacteria Streptomyces sp. H-KF8 unveils insights into multi-stress related genes and metabolic pathways involved in antimicrobial synthesis. PeerJ 2017, 5, e2912. [Google Scholar] [PubMed]
- Eythorsdottir, A.; Omarsdottir, S.; Einarsson, H. Antimicrobial activity of marine bacterial symbionts retrieved from shallow water hydrothermal vents. Mar. Biotechnol. 2016, 18, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.W.; Walsh, C.J.; Gomez-Sala, B.; Guijarro-García, E.; Stokes, D.; Jakobsdóttir, K.B.; Kristjánsson, K.; Burns, F.; Cotter, P.D.; Rea, M.C. The microbiome of deep-sea fish reveals new microbial species and a sparsity of antibiotic resistance genes. Gut Microbes 2021, 13, 1921924. [Google Scholar] [CrossRef]
- Uniacke-Lowe, S.; Collins, F.W.; Hill, C.; Ross, R.P. Bioactivity screening and genomic analysis reveals deep-sea fish microbiome isolates as sources of novel antimicrobials. Mar. Drugs 2023, 21, 444. [Google Scholar] [CrossRef]
- Sarmiento-Vizcaíno, A.; González, V.; Braña, A.F.; Palacios, J.J.; Otero, L.; Fernández, J.; Molina, A.; Kulik, A.; Vázquez, F.; Acuña, J.L. Pharmacological potential of phylogenetically diverse actinobacteria isolated from deep-sea coral ecosystems of the submarine Avilés Canyon in the Cantabrian Sea. Microb. Ecol. 2017, 73, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Dangar, K.G.; Singh, S.P. Antimicrobial Activities and Antibiotic Resistance of Nocardiopsis alba Isolated from the Saline Habitats of Coastal Gujarat. In Proceedings of the National Conference on Innovations in Biological Sciences (NCIBS), Rajkot, India, 10 January 2020. [Google Scholar]
- Atallah, B.M.; El-Mohsnawy, E.; El-Shouny, W.; Haroun, S. Identification and characterization of different potentially antibacterial compounds from a marine Streptomyces sp. Sp1. J. Anim. Plant Sci. 2023, 33, 166–173. [Google Scholar]
- Sharma, N.; Khajuria, V.; Gupta, S.; Kumar, C.; Sharma, A.; Lone, N.A.; Paul, S.; Meena, S.R.; Ahmed, Z.; Satti, N.K. Dereplication based strategy for rapid identification and isolation of a novel anti-inflammatory flavonoid by LCMS/MS from Colebrookea oppositifolia. ACS Omega 2021, 6, 30241–30259. [Google Scholar] [CrossRef] [PubMed]

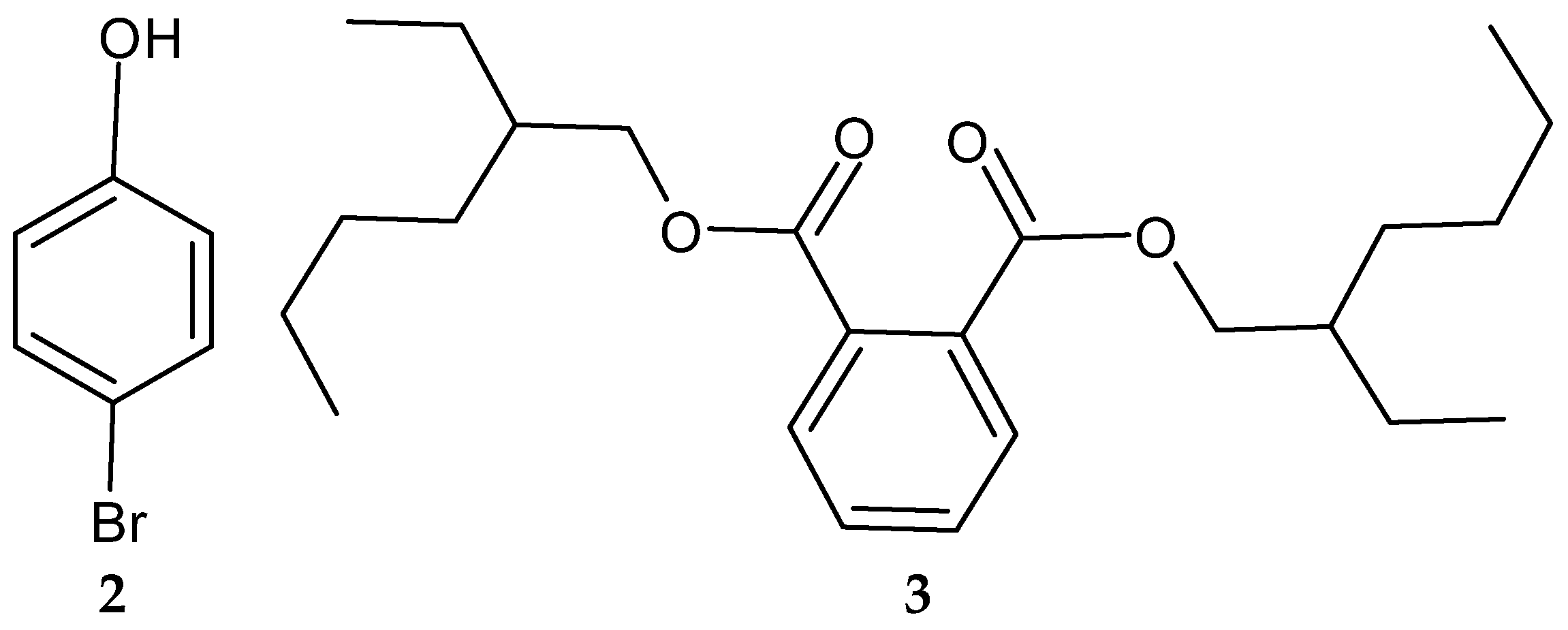

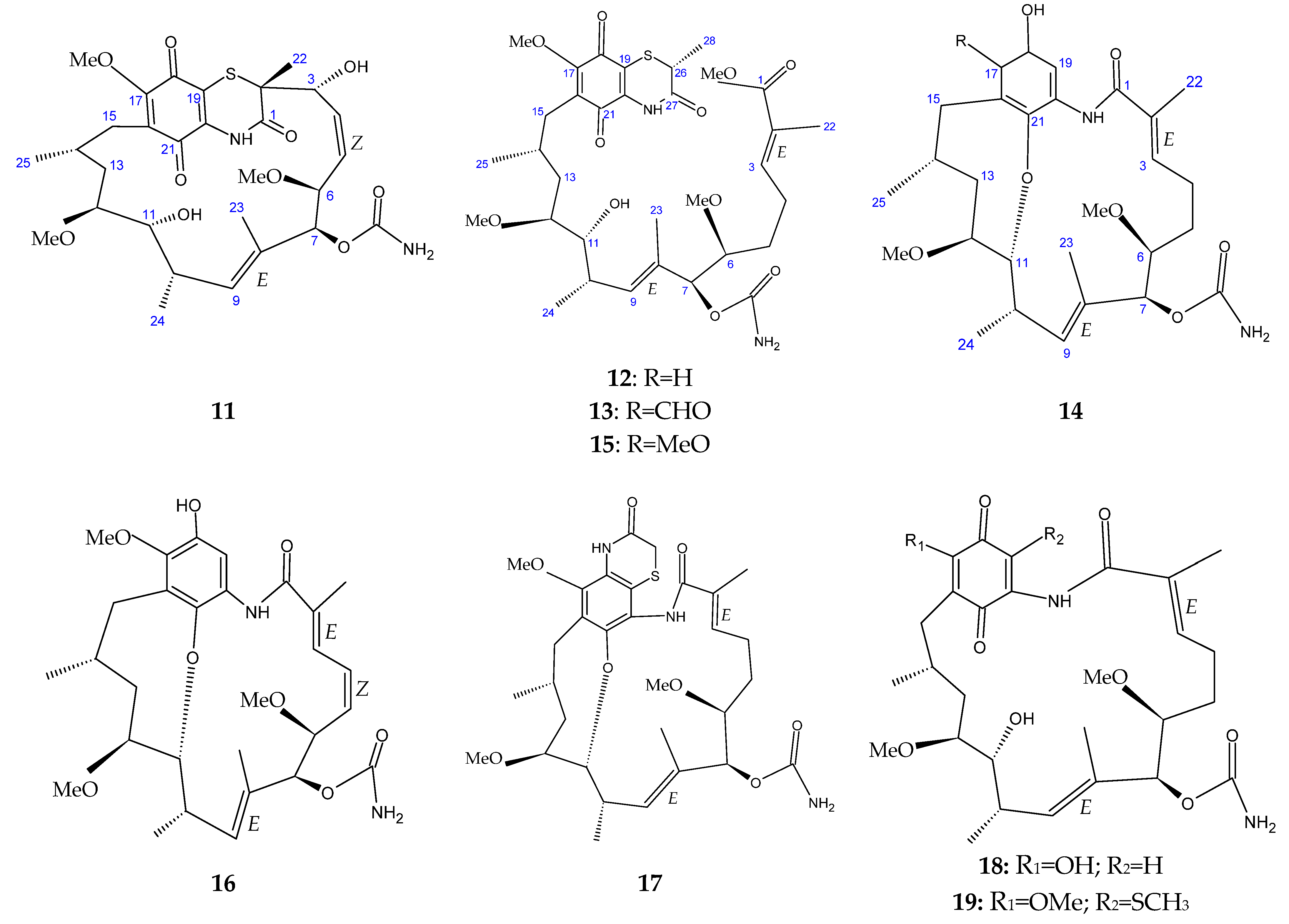

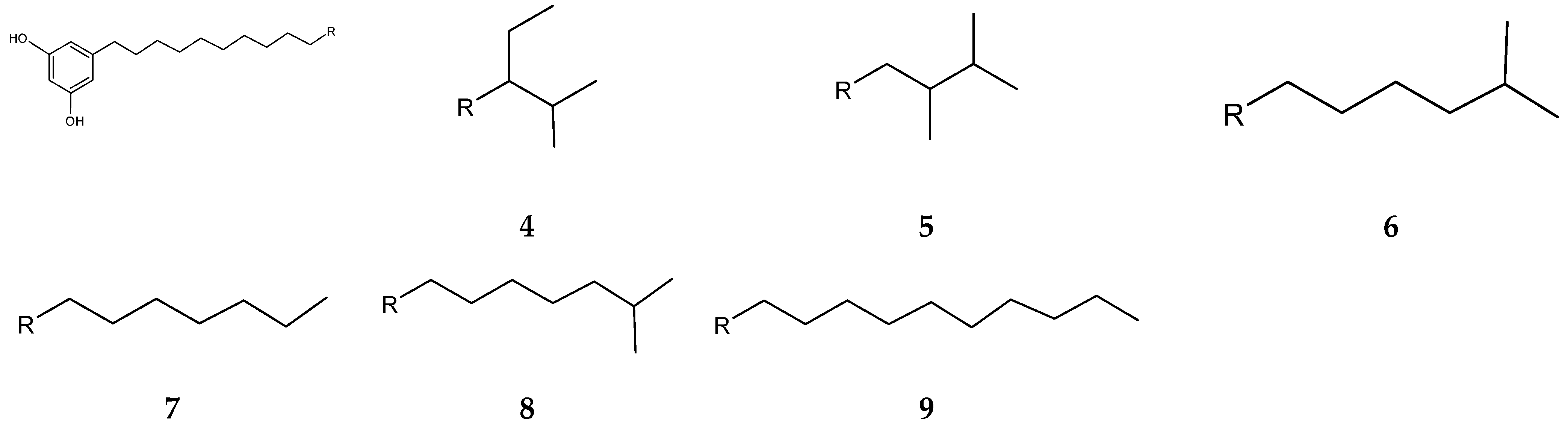
| Year | Location | No. of Cases (Death) | Type of Meat | Serotype | References |
|---|---|---|---|---|---|
| 1987–1989 | United Kingdom | 366 (ND) | Paté | 4b | [7] |
| 1900 | Australia | 9 (6) | Processed meats, paté | — | [7] |
| 1992 | France | 279 (85) | Pork tongue in jelly | 4b | [7] |
| 1993 | France | 38 (10) | Rillettes | 4b | [7] |
| 1996 | Australia | 5 (1) | Diced, cooked chicken | — | [7] |
| 1998–1999 | United States | 108 (14) | Hot dogs | 4b | [7,31,36] |
| 1999 | United States | 11 (ND) | Paté | — | [7,29] |
| 1999–2000 | France | 10 (3) | Rillettes | 4b | [7,29] |
| 1999–2000 | France | 32 (10) | Pork tongue in aspic | 4b | [7,29] |
| 2000 | United States | 30 (7) | RTE deli turkey meat | ½a | [7,29] |
| 2000 | New Zealand | 30 (ND) | RET deli meats | ½a | [7,29] |
| 2001 | United States | 16 (ND) | Deli meats | ½a | [7,29] |
| 2002 | United States | 54 (8) | RET deli turkey meat | 4b | [7,29] |
| 2006–2007 | Germany | 16 (0) | RET scalded sausage | 4b | [7,31] |
| 2008 | Australia | 13 (0) | Jellied pork | 4b | [7,37] |
| 2009 | Denmark | 8 (2) | Beef meat | — | [31,38] |
| 2011 | Switzerland | 6 (ND) | Cooked ham | ½a | [7] |
| 2012 | England | 14 (0) | Pork pie | — | [31,39] |
| 2012 | New Zealand | 4 (2) | RTE meat | — | [31,40] |
| 2013 | Scotland | 3 (0) | Steak pie | ½a | [31,41] |
| 2013–2014 | Denmark | 41 (7) | Meat products | — | [7] |
| 2014 | Sweden | 51 (0) | Cold cut ham | — | [31,42] |
| 2014 | Germany | 39 (18) | RTE sausage (pork) | — | [31,43] |
| 2015 | Italy | 35 (4) | Hog head cheese | ½a * | [31,44] |
| 2016 | Italy | 162 (0) | Cooked beef ham | ½a ** | [31,45] |
| 2016 | Switzerland | 7 (0) | Meat pâté (beef) | 4b (ST6) | [31,46] |
| 2017–2018 | South Africa | 1060 (216) | Polony | 4b (ST6) | [4,6,7] |
| 2019 | The Netherlands | 21 (3) | RTE meat products | — | [31,47] |
| Compound | Producing Strain | Biological Activity | References |
|---|---|---|---|
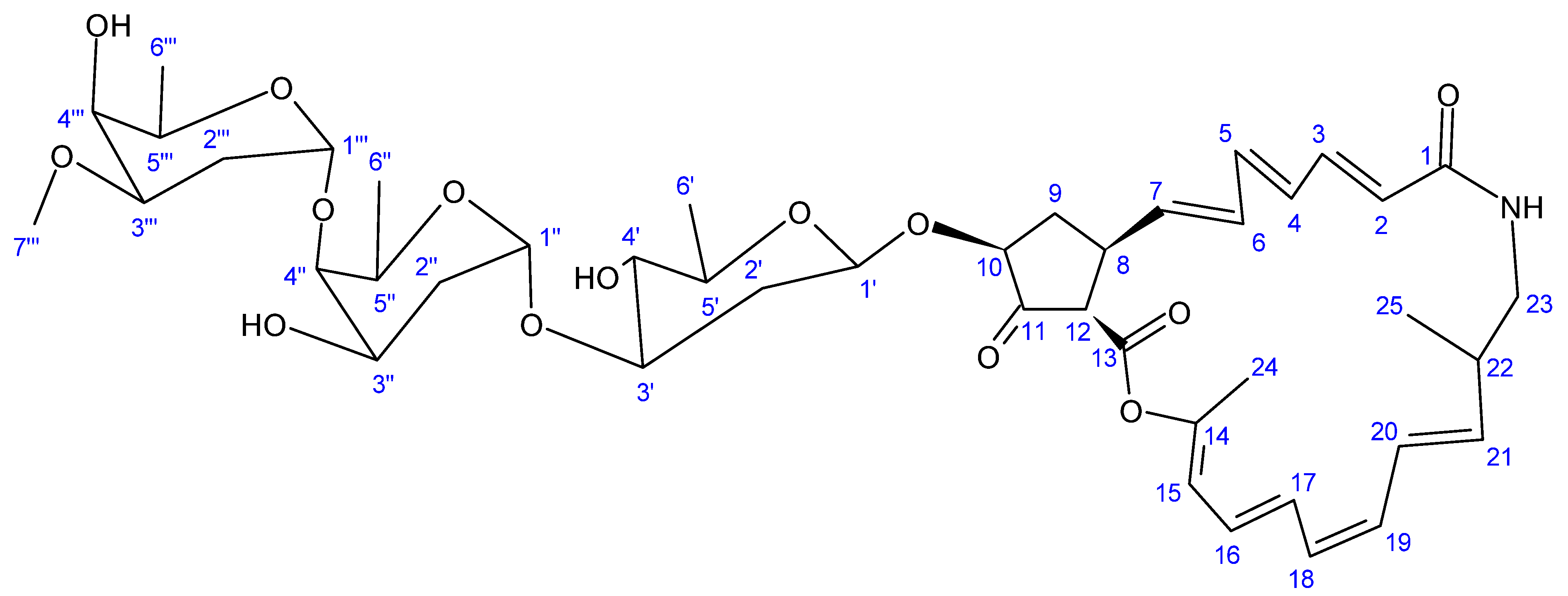
| 2-Allyoxyphenol (1) | Streptomyces MS1/7 | Antibacterial; antifungal; antioxidative; food preservative; oral disinfectant | [123,124] |
| 4-Bromophenol (2) | Nocardiopsis sp. SCA21 | Antibacterial; antioxidant; metal chelating; enzyme inhibitory activity | [125] |
| Adipostatins E–G (4–6) | Streptomyces blancoensis 20733 | Antibacterial | [126] |
| Seco-geldanamycin B (14) | Streptomyces sp. ZYX-F-97 | Antibacterial | [128] |
| 4.5-Dihydro-17-O- demethylgeldanamycin (18) | |||
| Seco-geldanamycin (22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngema, S.S.; Madoroba, E. A Mini-Review of Anti-Listerial Compounds from Marine Actinobacteria (1990–2023). Antibiotics 2024, 13, 362. https://doi.org/10.3390/antibiotics13040362
Ngema SS, Madoroba E. A Mini-Review of Anti-Listerial Compounds from Marine Actinobacteria (1990–2023). Antibiotics. 2024; 13(4):362. https://doi.org/10.3390/antibiotics13040362
Chicago/Turabian StyleNgema, Siyanda S., and Evelyn Madoroba. 2024. "A Mini-Review of Anti-Listerial Compounds from Marine Actinobacteria (1990–2023)" Antibiotics 13, no. 4: 362. https://doi.org/10.3390/antibiotics13040362
APA StyleNgema, S. S., & Madoroba, E. (2024). A Mini-Review of Anti-Listerial Compounds from Marine Actinobacteria (1990–2023). Antibiotics, 13(4), 362. https://doi.org/10.3390/antibiotics13040362