Comparative Efficacy of Continuous Ceftazidime Infusion vs. Intermittent Bolus against In Vitro Ceftazidime-Susceptible and -Resistant Pseudomonas aeruginosa Biofilm
Abstract
1. Introduction
2. Results
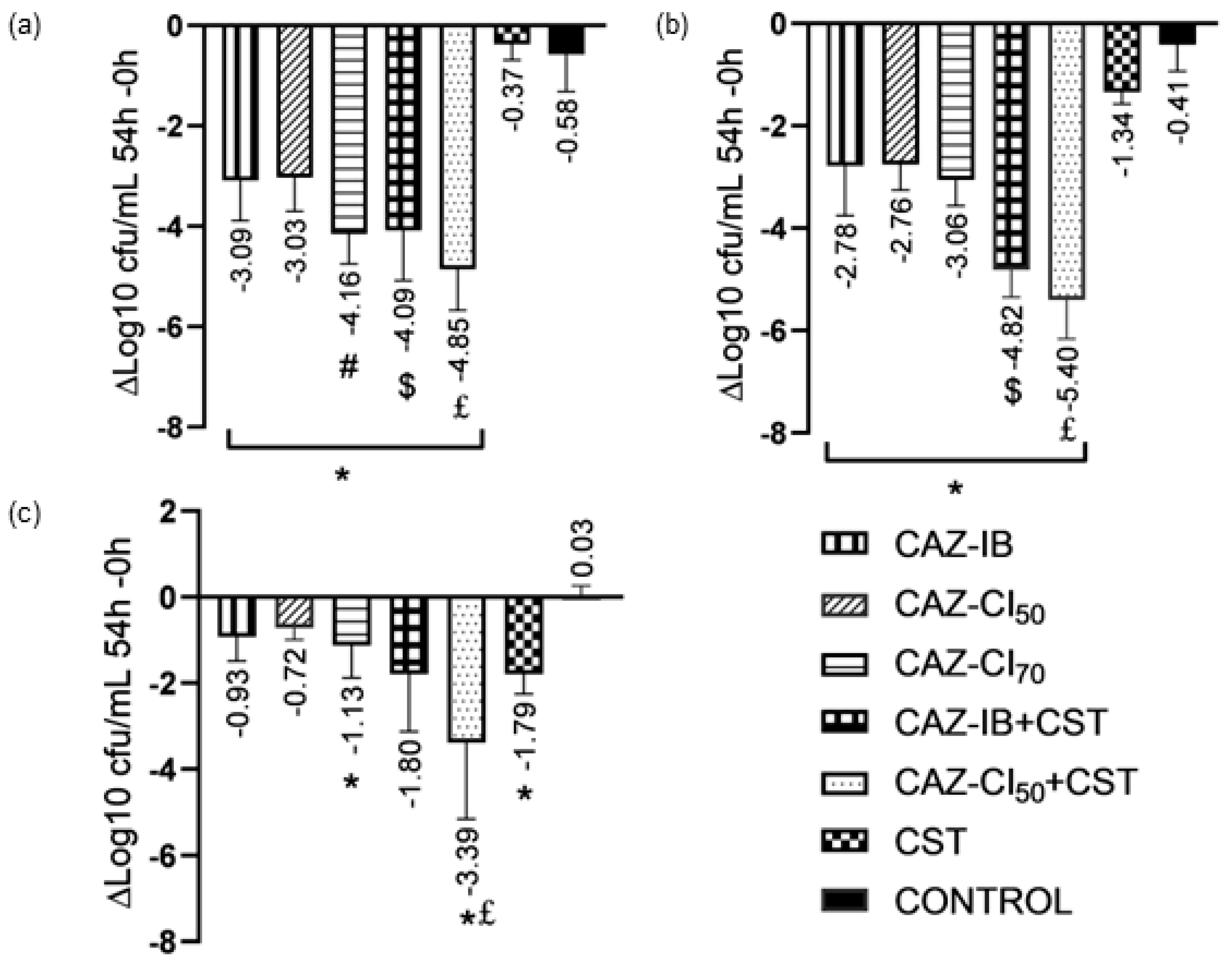
2.1. PK/PD Analyses of CAZ Monotherapies
2.2. Combination Therapy with Colistin
2.3. Confocal Laser Scanning Microscopy
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates and Antimicrobial Agents
4.2. Determination of Minimum Inhibitory Concentrations, Minimum Biofilm Inhibitory Concentrations, and Eradication Concentrations
4.3. In Vitro Pharmacokinetic/Pharmacodynamic Biofilm Model
4.4. Pharmacokinetic/Pharmacodynamic Analysis
4.5. Confocal Laser Scanning Microscopy
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronyms
| CAZ | ceftazidime |
| CBR | Center for Disease Control and Prevention Biofilm Reactor |
| CDC | Center for Disease Control and Prevention |
| CFU | colony-forming unit |
| CI | continuous infusion |
| CST | colistin |
| fAUC0–24h | free area under the curve from 0 to 24 h |
| fCmax | peak concentration |
| fCss | steady-state concentration |
| f%T | the percentage of time |
| GNB | Gram-negative bacilli |
| IB | intermittent bolus |
| MBEC | minimum biofilm eradication concentration |
| MBIC | minimum biofilm inhibitory concentration |
| MIC | minimum inhibitory concentration |
| PD | pharmacodynamic |
| PK | pharmacokinetic |
| t ½ | elimination half-life |
References
- del Pozo, J.L.; Patel, R. The Challenge of Treating Biofilm-Associated Bacterial Infections. Clin. Pharmacol. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic Resistance of Bacteria in Biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–10; quiz 11–12. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodríguez-Pardo, D.; Sorlí, L.; Fresco, G.; Dolores del Toro, M.; Guío, L.; Sánchez-Rivas, E.; et al. Time Trends in the Aetiology of Prosthetic Joint Infections: A Multicentre Cohort Study. Clin. Microbiol. Infect. 2016, 22, 732.E1-8. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- del Barrio-Tofi, E.; Zamorano, L.; Cortes-Lara, S.; Ló pez-Causapé, C.; Sá nchez-Diener, I.; Cabot, G.; Bou, G.; Martínez-Martínez, L.; Oliver, A. Spanish Nationwide Survey on Pseudomonas Aeruginosa Antimicrobial Resistance Mechanisms and Epidemiology. J. Antimicrob. Chemother. 2019, 74, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pardo, D.; Pigrau, C.; Lora-Tamayo, J.; Soriano, A.; del Toro, M.D.; Cobo, J.; Palomino, J.; Euba, G.; Riera, M.; Sánchez-Somolinos, M.; et al. Gram-Negative Prosthetic Joint Infection: Outcome of a Debridement, Antibiotics and Implant Retention Approach. A Large Multicentre Study. Clin. Microbiol. Infect. 2014, 20, O911–O919. [Google Scholar] [CrossRef]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Høiby, N. In Vivo Pharmacokinetics/Pharmacodynamics of Colistin and Imipenem in Pseudomonas Aeruginosa Biofilm Infection. Antimicrob. Agents Chemother. 2012, 56, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Hengzhuang, W.; Ciofu, O.; Yang, L.; Wu, H.; Song, Z.; Oliver, A.; Høiby, N. High β-Lactamase Levels Change the Pharmacodynamics of β-Lactam Antibiotics in Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Gómez-Junyent, J.; Murillo, O.; Yu, H.H.; Azad, M.A.K.; Wickremasinghe, H.; Rigo-Bonnin, R.; Benavent, E.; Ariza, J.; Li, J. In Vitro Pharmacokinetics/Pharmacodynamics of Continuous Ceftazidime Infusion Alone and in Combination with Colistin against Pseudomonas Aeruginosa Biofilm. Int. J. Antimicrob. Agents 2021, 57, 106246. [Google Scholar] [CrossRef]
- Gómez-Junyent, J.; Rigo-Bonnin, R.; Benavent, E.; Soldevila, L.; Padullés, A.; Cabo, X.; Tubau, F.; Ariza, J.; Murillo, O. Efficacy and Therapeutic Drug Monitoring of Continuous Beta-Lactam Infusion for Osteoarticular Infections Caused by Fluoroquinolone-Resistant Pseudomonas Aeruginosa: A Prospective Cohort Study. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Grossi, O.; Asseray, N.; Bourigault, C.; Corvec, S.; Valette, M.; Navas, D.; Happi-Djeukou, L.; Touchais, S.; Bémer, P.; Boutoille, D.; et al. Gram-Negative Prosthetic Joint Infections Managed According to a Multidisciplinary Standardized Approach: Risk Factors for Failure and Outcome with and without Fluoroquinolones. J. Antimicrob. Chemother. 2016, 71, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Goeres, D.M.; Loetterle, L.R.; Hamilton, M.A.; Murga, R.; Kirby, D.W.; Donlan, R.M. Statistical Assessment of a Laboratory Method for Growing Biofilms. Microbiology 2005, 151, 757–762. [Google Scholar] [CrossRef]
- Benavent, E.; Ulldemolins, M.; El Haj, C.; Rigo-Bonnin, R.; Yu, H.; Wang, L.; Wickremasinghe, H.; Ariza, J.; Murillo, O. Efficacy of Meropenem Extended Infusion vs Intermittent Bolus Monotherapy and in Combination with Colistin against Pseudomonas Aeruginosa Biofilm. Int. J. Antimicrob. Agents 2023, 62, 106856. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.V.; Kuti, J.L. Pharmacodynamic Thresholds for Beta-Lactam Antibiotics: A Story of Mouse Versus Man. Front. Pharmacol. 2022, 18, 833189. [Google Scholar] [CrossRef]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Høiby, N. Pharmacokinetics/Pharmacodynamics of Colistin and Imipenem on Mucoid and Nonmucoid Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 2011, 55, 4469–4474. [Google Scholar] [CrossRef] [PubMed]
- Cappelletty, D.M.; Kang, S.L.; Palmer, S.M.; Rybak, M.J. Pharmacodynamics of Ceftazidime Administered as Continuous Infusion or Intermittent Bolus Alone and in Combination with Single Daily-Dose Amikacin against Pseudomonas Aeruginosa in an in Vitro Infection Model. Antimicrob. Agents Chemother. 1995, 39, 1797–1801. [Google Scholar] [CrossRef]
- Mouton, J.W.; Den Hollander, J.G. Killing of Pseudomonas Aeruginosa during Continuous and Intermittent Infusion of Ceftazidime in an in Vitro Pharmacokinetic Model. Antimicrob. Agents Chemother. 1994, 38, 931–936. [Google Scholar] [CrossRef]
- Schentag, J.J.; Nix, D.E.; Adelman, M.H. Mathematical Examination of Dual Individualization Principles (I): Relationships between AUC above MIC and Area under the Inhibitory Curve for Cefmenoxime, Ciprofloxacin, and Tobramycin. DICP 1991, 25, 1050–1057. [Google Scholar] [CrossRef]
- Goss, T.F.; Forrest, A.; Nix, D.E.; Ballow, C.H.; Birmingham, M.C.; Cumbo, T.J.; Schentag, J.J. Mathematical Examination of Dual Individualization Principles (II): The Rate of Bacterial Eradication at the Same Area under the Inhibitory Curve Is More Rapid for Ciprofloxacin than for Cefmenoxime. Ann. Pharmacother. 1994, 28, 863–868. [Google Scholar] [CrossRef]
- Schentag, J.J.; Gilliland, K.K.; Paladino, J.A. What Have We Learned from Pharmacokinetic and Pharmacodynamic Theories? Clin. Infect. Dis. 2001, 32 (Suppl. S1), S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Pybus, C.A.; Felder-Scott, C.; Obuekwe, V.; Greenberg, D.E. Cefiderocol Retains Antibiofilm Activity in Multidrug-Resistant Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2021, 65, e01194-20. [Google Scholar] [CrossRef] [PubMed]
- Papalini, C.; Sabbatini, S.; Monari, C.; Mencacci, A.; Francisci, D.; Perito, S.; Pasticci, M.B. In Vitro Antibacterial Activity of Ceftazidime/Avibactam in Combination against Planktonic and Biofilm Carbapenemase-Producing Klebsiella Pneumoniae Isolated from Blood. J. Glob. Antimicrob. Resist. 2020, 23, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Velez Perez, A.L.; Schmidt-Malan, S.M.; Kohner, P.C.; Karau, M.J.; Greenwood-Quaintance, K.E.; Patel, R. In Vitro Activity of Ceftolozane/Tazobactam against Clinical Isolates of Pseudomonas Aeruginosa in the Planktonic and Biofilm States. Diagn. Microbiol. Infect. Dis. 2016, 85, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Pamp, S.J.; Gjermansen, M.; Johansen, H.K.; Tolker-Nielsen, T. Tolerance to the Antimicrobial Peptide Colistin in Pseudomonas Aeruginosa Biofilms Is Linked to Metabolically Active Cells, and Depends on the Pmr and MexAB-OprM Genes. Mol. Microbiol. 2008, 68, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.R.; Cockerill, F.R.; Bradford, P.A.; Eliopoulos, G.M.; Hindler, J.A.; Jenkins, S.G.; Lewis, J.S.; Limbargo, B.; Miller, L.A.; Nicolau, D.P.; et al. M07-A10—Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Leroy, A.; Leguy, F.; Borsa, F.; Spencer, G.R.; Fillastre, J.P.; Humbert, G. Pharmacokinetics of Ceftazidime in Normal and Uremic Subjects. Antimicrob. Agents Chemother. 1984, 25, 638–642. [Google Scholar] [CrossRef]
- Grayson, M.L.; Cosgrove, S.E.; Crowe, S.; Hope, W.; McCarthy, J.S.; Mills, J.; Mouton, J.W.; Paterson, D.L. Kucers’ The Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs, 7th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-49-874795-0. [Google Scholar]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population Pharmacokinetics of Colistin Methanesulfonate and Formed Colistin in Critically Ill Patients from a Multicenter Study Provide Dosing Suggestions for Various Categories of Patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef]
- Peterson, L.R.; Shanholtzer, C.J. Tests for Bactericidal Effects of Antimicrobial Agents: Technical Performance and Clinical Relevance. Clin. Microbiol. Rev. 1992, 5, 420–432. [Google Scholar] [CrossRef]

| Strain | MIC (mg/L) | MBIC (mg/L) | MBEC (mg/L) | |||
|---|---|---|---|---|---|---|
| CAZ | CST | CAZ | CST | CAZ | CST | |
| HUB-PAS | 1 | 1 | 4 | 16–8 | >512 | >512 |
| PAO1 | 2 | 0.5 | 8 | 32 | >512 | >512 |
| HUB-XDR | 32 | 1 | 64 | 16 | >512 | >512 |
| f%T > MIC (%) | fAUC0–24h/MIC | fCmax/MIC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | HUB-PAS | PAO1 | HUB-XDR | HUB-PAS | PAO1 | HUB-XDR | HUB-PAS | PAO1 | HUB-XDR | |
| CAZ regimen | ||||||||||
| IB | 100 | 100 | 48.5 | 1078.34 ± 136.75 | 539.17 ± 68.38 | 33.69 ± 4.27 | 132.16 ± 5.49 | 66.08 ± 2.74 | 4.13 ± 0.17 | |
| CI50 | 100 | 100 | 100 | 1157.10 ± 61.75 | 578.55 ± 30.87 | 36.15 ± 1.92 | 51.45 ± 6.3 | 25.72 ± 3.15 | 1.6 ± 0.19 | |
| CI70 | 100 | 100 | 100 | 1594.59 ± 96.43 | 797.29 ± 48.22 | 49.83 ± 3.01 | 67.75 ± 4.15 | 33.87 ± 2.07 | 2.11 ± 0.19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Haj, C.; Agustí, E.; Benavent, E.; Soldevila-Boixader, L.; Rigo-Bonnin, R.; Tubau, F.; Torrejón, B.; Esteban, J.; Murillo, O. Comparative Efficacy of Continuous Ceftazidime Infusion vs. Intermittent Bolus against In Vitro Ceftazidime-Susceptible and -Resistant Pseudomonas aeruginosa Biofilm. Antibiotics 2024, 13, 344. https://doi.org/10.3390/antibiotics13040344
El Haj C, Agustí E, Benavent E, Soldevila-Boixader L, Rigo-Bonnin R, Tubau F, Torrejón B, Esteban J, Murillo O. Comparative Efficacy of Continuous Ceftazidime Infusion vs. Intermittent Bolus against In Vitro Ceftazidime-Susceptible and -Resistant Pseudomonas aeruginosa Biofilm. Antibiotics. 2024; 13(4):344. https://doi.org/10.3390/antibiotics13040344
Chicago/Turabian StyleEl Haj, Cristina, Eugènia Agustí, Eva Benavent, Laura Soldevila-Boixader, Raül Rigo-Bonnin, Fe Tubau, Benjamín Torrejón, Jaime Esteban, and Oscar Murillo. 2024. "Comparative Efficacy of Continuous Ceftazidime Infusion vs. Intermittent Bolus against In Vitro Ceftazidime-Susceptible and -Resistant Pseudomonas aeruginosa Biofilm" Antibiotics 13, no. 4: 344. https://doi.org/10.3390/antibiotics13040344
APA StyleEl Haj, C., Agustí, E., Benavent, E., Soldevila-Boixader, L., Rigo-Bonnin, R., Tubau, F., Torrejón, B., Esteban, J., & Murillo, O. (2024). Comparative Efficacy of Continuous Ceftazidime Infusion vs. Intermittent Bolus against In Vitro Ceftazidime-Susceptible and -Resistant Pseudomonas aeruginosa Biofilm. Antibiotics, 13(4), 344. https://doi.org/10.3390/antibiotics13040344










