A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms—January 2020 to September 2023
Abstract
1. Introduction
2. Results and Discussion
2.1. AMP Combination
2.2. Delivery System
2.3. AMP Resistance
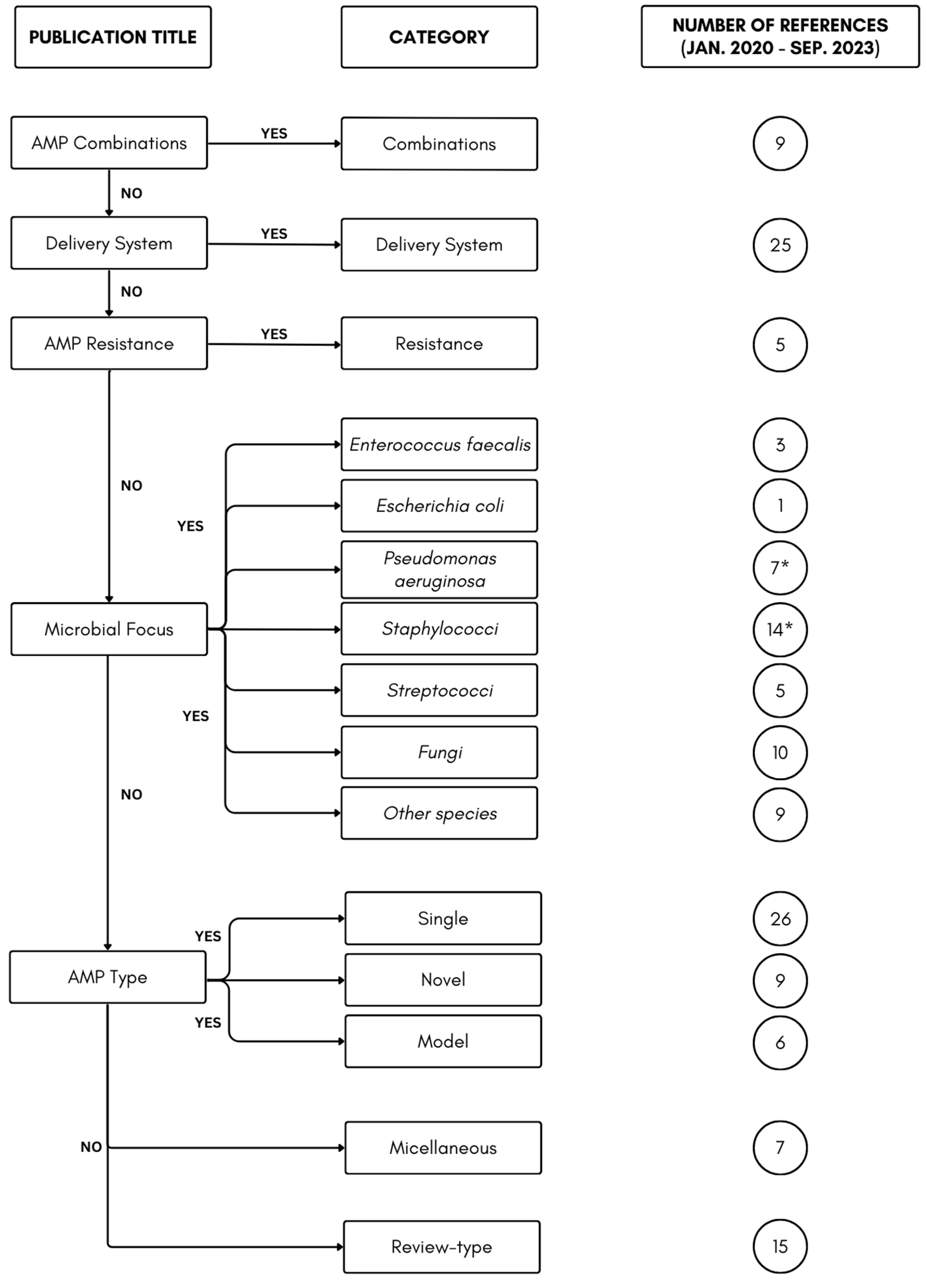
2.4. Microbial Focus
2.5. AMP Type
2.5.1. Single AMP Type
2.5.2. ‘Novel’ AMP Types
2.5.3. AMP Models
2.6. Miscellaneous
2.7. Review-Type
3. Conclusions
4. Future Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmolle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.; Jin, L.J. Microbial Persisters and Host: Recent Advances and Future Perspectives. Crit. Rev. Microbiol. 2023, 49, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Condinho, M.; Carvalho, B.; Cruz, A.; Pinto, S.N.; Arraiano, C.M.; Pobre, V. The Role of RNA Regulators, Quorum Sensing and C-Di-Gmp in Bacterial Biofilm Formation. FEBS Open Bio 2023, 13, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Mhade, S.; Kaushik, K.S. Tools of the Trade: Image Analysis Programs for Confocal Laser-Scanning Microscopy Studies of Biofilms and Considerations for Their Use by Experimental Researchers. ACS Omega 2023, 8, 20163–20177. [Google Scholar] [CrossRef] [PubMed]
- Algburi, A.; Comito, N.; Kashtanov, D.; Dicks, L.M.T.; Chikindas, M.L. Control of Biofilm Formation: Antibiotics and Beyond. Appl. Environ. Microbiol. 2017, 83, e02508-16. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.Q.; Feng, W.G.; Wang, J.Y.; Wang, R.C.; Zhang, B.W.; Bo, L.T.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial Peptides for Combating Drug-Resistant Bacterial Infections. Drug Resist. Update 2023, 68, 100954. [Google Scholar] [CrossRef] [PubMed]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed]
- Matthyssen, T.; Li, W.Y.; Holden, J.A.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. The Potential of Modified and Multimeric Antimicrobial Peptide Materials as Superbug Killers. Front. Chem. 2020, 9, 795433. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.P.; Guimaraes, M.S.; Rabelo, J.; Belen, L.H.; Perecin, C.J.; Farías, J.G.; Santos, J.H.P.M.; Rangel-Yagui, C.O. Recent Advances in the Design of Antimicrobial Peptide Conjugates. J. Mater. Chem. B 2022, 10, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhu, Y.; Zhu, N.; Liu, T.; Gou, S.; Zhang, F.; Yao, J.; Xie, J.; Ni, J. Synthesis and Anti-Pseudomonal Activity of New S-Ala Modified Analogues of the Antimicrobial Peptide Anoplin. Int. J. Med. Microbiol. 2020, 310, 151433. [Google Scholar] [CrossRef] [PubMed]
- Kalsy, M.; Tonk, M.; Hardt, M.; Dobrindt, U.; Zdybicka-Barabas, A.; Cytrynska, M.; Vilcinskas, A.; Mukherjee, K. The Insect Antimicrobial Peptide Cecropin a Disrupts Uropathogenic Escherichia coli Biofilms. NPJ Bio Microb. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Carvalho, G.; Maquera-Huacho, P.M.; Silva Pontes, C.; Annunzio, S.R.; Fontana Mendonca, C.R.; Nara de Souza Rastelli, A.; de Oliveira, K.T.; Teughels, W.; Chorilli, M.; Leal Zandim-Barcelos, D.; et al. Chlorin-E6 Conjugated to the Antimicrobial Peptide LL-37 Loaded Nanoemulsion Enhances Photodynamic Therapy against Multi-Species Biofilms Related to Periodontitis. Photodiagn. Photodyn. Ther. 2023, 43, 103725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, Y.; Feng, Z.; Li, Z.; Jiang, X.; Han, S.; Washio, J.; Takahashi, N.; Zhang, L. Combined Treatment with Fluoride and Antimicrobial Peptide Gh12 Efficiently Controls Caries in Vitro and in Vivo. Caries Res. 2022, 56, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhai, X.; Cheng, Y.; Zhang, R.; Wang, W.; Hou, H. Starch/Pbat Blown Antimicrobial Films Based on the Synergistic Effects of Two Commercial Antimicrobial Peptides. Int. J. Biol. Macromol. 2022, 204, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Chatupheeraphat, C.; Peamchai, J.; Luk-In, S.; Eiamphungporn, W. Synergistic Effect and Antibiofilm Activity of the Antimicrobial Peptide K11 with Conventional Antibiotics against Multidrug-Resistant and Extensively Drug-Resistant Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2023, 13, 1153868. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Zhang, X.; Li, Z.; Yuan, J.; Shen, F.; Zhang, S. Synergistic Effect and Antibiofilm Activity of an Antimicrobial Peptide with Traditional Antibiotics against Multi-Drug Resistant Bacteria. Microb. Pathog. 2021, 158, 105056. [Google Scholar] [CrossRef] [PubMed]
- Seena, S.; Ferrao, R.; Pala, M.; Roelants, S.; Soetaert, W.; Stevens, C.V.; Ferreira, L.; Rai, A. Acidic Sophorolipid and Antimicrobial Peptide Based Formulation as Antimicrobial and Antibiofilm Agents. Biomater. Adv. 2023, 146, 213299. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, D.; Pizzo, E.; Cafaro, V.; Notomista, E.; De Lise, F.; Bosso, A.; Gaglione, R.; Merlino, F.; Novellino, E.; Ungaro, F.; et al. Antimicrobial Peptide Temporin-L Complexed with Anionic Cyclodextrins Results in a Potent and Safe Agent against Sessile Bacteria. Int. J. Pharm. 2020, 584, 119437. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, E.; Nikapitiya, C.; De Zoysa, M.; Whang, I. Antimicrobial Peptide Octominin-Encapsulated Chitosan Nanoparticles Enhanced Antifungal and Antibacterial Activities. Int. J. Mol. Sci. 2022, 23, 15882. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Tzanov, T. Simultaneous Ultrasound-Assisted Hybrid Polyzwitterion/Antimicrobial Peptide Nanoparticles Synthesis and Deposition on Silicone Urinary Catheters for Prevention of Biofilm-Associated Infections. Nanomaterials 2021, 11, 3143. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Deng, T.; Lin, F.C.; Zhang, B.; Zink, J.I. Supramolecular Assemblies of Heterogeneous Mesoporous Silica Nanoparticles to Co-Deliver Antimicrobial Peptides and Antibiotics for Synergistic Eradication of Pathogenic Biofilms. ACS Nano 2020, 14, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; van Gent, M.E.; de Waal, A.M.; van Doodewaerd, B.R.; Bos, E.; Koning, R.I.; Cordfunke, R.A.; Drijfhout, J.W.; Nibbering, P.H. Physical and Functional Characterization of Plga Nanoparticles Containing the Antimicrobial Peptide Saap-148. Int. J. Mol. Sci. 2023, 24, 2867. [Google Scholar] [CrossRef] [PubMed]
- Parandhaman, T.; Choudhary, P.; Ramalingam, B.; Schmidt, M.; Janardhanam, S.; Das, S.K. Antibacterial and Antibiofouling Activities of Antimicrobial Peptide-Functionalized Graphene-Silver Nanocomposites for the Inhibition and Disruption of Staphylococcus aureus Biofilms. ACS Biomater. Sci. Eng. 2021, 7, 5899–5917. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Wang, H.; Zhu, M.; Feng, W.; Liang, G. Enhanced Antibacterial and Anti-Biofilm Activities of Antimicrobial Peptides Modified Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 4831–4846. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.L.; Cheng, K.; Li, Y.; Zhong, Z.T.; Hou, X.L.; Song, L.B.; Zhang, F.; Wang, J.-H.; Zhao, Y.-D.; Xu, Q.-R. The Eradication of Biofilm for Therapy of Bacterial Infected Chronic Wound Based on Ph-Responsive Micelle of Antimicrobial Peptide Derived Biodegradable Microneedle Patch. Chem. Eng. J. 2023, 462, 142222. [Google Scholar] [CrossRef]
- Hemmingsen, L.M.; Giordani, B.; Paulsen, M.H.; Vanic, Z.; Flaten, G.E.; Vitali, B.; Basnet, P.; Bayer, A.; Strøm, M.B.; Škalko-Basnet, N. Tailored Anti-Biofilm Activity—Liposomal Delivery for Mimic of Small Antimicrobial Peptide. Biomater. Adv. 2023, 145, 213238. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Ko, C.; Hyeon, H.; Jang, M.K.; Lee, D. Imaging and Targeted Antibacterial Therapy Using Chimeric Antimicrobial Peptide Micelles. ACS Appl. Mater. Interfaces 2020, 12, 54306–54315. [Google Scholar] [CrossRef] [PubMed]
- Fasiku, V.O.; Omolo, C.A.; Kiruri, L.W.; Devnarain, N.; Faya, M.; Mocktar, C.; Govender, T. A Hyaluronic Acid-Based Nanogel for the Co-Delivery of Nitric Oxide (No) and a Novel Antimicrobial Peptide (Amp) against Bacterial Biofilms. Int. J. Biol. Macromol. 2022, 206, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Andrabi, S.M.; Shahriar, S.M.S.; Wong, S.L.; Wang, G.; Xie, J. Triggered Release of Antimicrobial Peptide from Microneedle Patches for Treatment of Wound Biofilms. J. Control. Release 2023, 356, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Maystrenko, A.; Feng, Y.; Akhtar, N.; Li, J. The Addition of a Synthetic Lps-Targeting Domain Improves Serum Stability While Maintaining Antimicrobial, Antibiofilm, and Cell Stimulating Properties of an Antimicrobial Peptide. Biomolecules 2020, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, S.; Zhang, Y.; Wu, D.; Yang, X. The Dual Delivery of Growth Factors and Antimicrobial Peptide by Plga/Go Composite Biofilms to Promote Skin-Wound Healing. New J. Chem. 2020, 44, 1463–1476. [Google Scholar] [CrossRef]
- Su, Y.; Mainardi, V.L.; Wang, H.; McCarthy, A.; Zhang, Y.S.; Chen, S.; John, J.V.; Wong, S.L.; Hollins, R.R.; Wang, G.; et al. Dissolvable Microneedles Coupled with Nanofiber Dressings Eradicate Biofilms via Effectively Delivering a Database-Designed Antimicrobial Peptide. ACS Nano 2020, 14, 11775–11786. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, S.; Dou, W.; Sun, J.; Wang, Y.; Fu, M.; Chu, W.; Liu, G. Mitigation of Eh36 Ship Steel Biocorrosion Using an Antimicrobial Peptide as a Green Biocide Enhancer. Bioelectrochemistry 2023, 154, 108526. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Singha, P.; Douglass, M.; Estes, L.; Garren, M.; Griffin, L.; Kumar, A.; Handa, H. A Synergistic New Approach toward Enhanced Antibacterial Efficacy Via Antimicrobial Peptide Immobilization on a Nitric Oxide-Releasing Surface. ACS Appl. Mater. Interfaces 2021, 13, 43892–43903. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Ibanez-Fonseca, A.; Aparicio, C.; Rodriguez-Cabello, J.C. Antibiofilm Coatings Based on Protein-Engineered Polymers and Antimicrobial Peptides for Preventing Implant-Associated Infections. Biomater. Sci. 2020, 8, 2866–2877. [Google Scholar] [CrossRef] [PubMed]
- Pihl, M.; Galli, S.; Jimbo, R.; Andersson, M. Osseointegration and Antibacterial Effect of an Antimicrobial Peptide Releasing Mesoporous Titania Implant. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.T.L.; Gelamo, R.V.; Mateus Santos Obata, M.; Andrade Silva, L.E.; Silva, M.V.D.; Oliveira, C.J.F.; Silva, B.P.D.; Aoki, I.V.; Moreto, J.A.; Slade, N.B.L. Exploring the Functionalization of Ti-6al-4v Alloy with the Novel Antimicrobial Peptide Jichis-2 via Plasma Polymerization. Biofouling 2023, 39, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.; Chopra, P.; Mehta, M. Natural Prokaryotic Antimicrobial Peptide Coated Titanium Discs Prevent Staphylococcus auerus Growth and Biofilm Formation—Implications on Peri-Implant Infections. Mater. Today Proc. 2022, 50, 673–678. [Google Scholar] [CrossRef]
- De Luca, M.; Gaglione, R.; Della Ventura, B.; Cesaro, A.; Di Girolamo, R.; Velotta, R.; Arciello, A. Loading of Polydimethylsiloxane with a Human Apob-Derived Antimicrobial Peptide to Prevent Bacterial Infections. Int. J. Mol. Sci. 2022, 23, 5219. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hou, E.; Cheng, W.; Yan, X.; Zhang, T.; Li, S.; Yao, H.; Liu, J.; Guo, Y. Membrane-Targeting Neolignan-Antimicrobial Peptide Mimic Conjugates to Combat Methicillin-Resistant Staphylococcus aureus (MRSA) Infections. J. Med. Chem. 2022, 65, 16879–16892. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, T.; Liu, Y.; Zhou, J.; Shi, S.; Wang, Y.; Zhao, Z.; Yan, Z.; Liao, C.; Wang, C. Enhancing the Antibacterial Activity of Antimicrobial Peptide Pmap-37(F34-R) by Cholesterol Modification. BMC Vet. Res. 2020, 16, 419. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Bessa, L.J.; Correia, P.; Fernandes, I.; Ferraz, R.; Gameiro, P.; Teixeira, C.; Gomes, P. “Clicking” an Ionic Liquid to a Potent Antimicrobial Peptide: On the Route towards Improved Stability. Int. J. Mol. Sci. 2020, 21, 6174. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, V.; Kosaristanova, L.; Dolezelikova, K.; Adam, V.; Pumera, M. Microrobots with Antimicrobial Peptide Nanoarchitectonics for the Eradication of Antibiotic-Resistant Biofilms. Adv. Funct. Mat. 2022, 32, 2112935. [Google Scholar] [CrossRef]
- Dean, S.N.; Milton, M.E.; Cavanagh, J.; van Hoek, M.L. Francisella novicida Two-Component System Response Regulator Bfpr Modulates Iglc Gene Expression, Antimicrobial Peptide Resistance, and Biofilm Production. Front. Cell. Infect. Microbiol. 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kaspar, J.R.; Rojas-Carreno, G.; Walker, A.R.; Burne, R.A. A Single System Detects and Protects the Beneficial Oral Bacterium Streptococcus sp. A12 from a Spectrum of Antimicrobial Peptides. Mol. Microbiol. 2021, 116, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Visnovsky, S.B.; Cruz, C.D.; Fletcher, G.C.; van Vliet, A.H.M.; Hedderley, D.; Butler, R.; Flint, S.; Palmer, J.; Pitman, A.R. Inactivation of the Gene Encoding the Cationic Antimicrobial Peptide Resistance Factor Mprf Increases Biofilm Formation but Reduces Invasiveness of Listeria monocytogenes. J. Appl. Microbiol. 2021, 130, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Hofer, R.N.; Lin, A.; House, B.C.; Purvis, C.N.; Harris, B.J.; Symes, S.J.K.; Giles, D.K. Exogenous Polyunsaturated Fatty Acids (Pufas) Influence Permeability, Antimicrobial Peptide Resistance, Biofilm Formation and Membrane Phospholipid Structure in an a-Layer and Non-a-Layer Strain of Aeromonas salmonicida. J. Fish Dis. 2023, 46, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Fullen, A.R.; Gutierrez-Ferman, J.L.; Yount, K.S.; Love, C.F.; Choi, H.G.; Vargas, M.A.; Raju, D.; Corps, K.N.; Howell, P.L.; Dubey, P.; et al. Bps Polysaccharide of Bordetella pertussis Resists Antimicrobial Peptides by Functioning as a Dual Surface Shield and Decoy and Converts Escherichia coli into a Respiratory Pathogen. PLoS Pathog. 2022, 18, e1010764. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Chen, X.; Jiang, W.; Jiang, X.; Zeng, Y.; Li, X.; Feng, Z.; Luo, J.; Zhang, L. Antimicrobial Peptide Gh12 as Root Canal Irrigant Inhibits Biofilm and Virulence of Enterococcus faecalis. Int. Endod. J. 2020, 53, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Mergoni, G.; Manfredi, M.; Bertani, P.; Ciociola, T.; Conti, S.; Giovati, L. Activity of Two Antimicrobial Peptides against Enterococcus faecalis in a Model of Biofilm-Mediated Endodontic Infection. Antibiotics 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Rouchon, C.N.; Harris, J.; Zubair-Nizami, Z.; Weinstein, A.J.; Roky, M.; Frank, K.L. The Cationic Antimicrobial Peptide Activity of Lysozyme Reduces Viable Enterococcus faecalis Cells in Biofilms. Antimicrob. Agents Chemother. 2022, 66, e0233921. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Hua, K.F.; Yu, Y.H.; Cheng, Y.H.; Cheng, T.T.; Huang, Y.K.; Chang, H.W.; Chen, W.J. Antibacterial and Antibiofilm Activities of Novel Antimicrobial Peptides against Multidrug-Resistant Enterotoxigenic Escherichia coli. Int. J. Mol. Sci. 2021, 22, 3926. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Ch, S.; Hong, S.J.; Biswas, S.; Roy, S. Antimicrobial Peptide S100a12 (Calgranulin C) Inhibits Growth, Biofilm Formation, Pyoverdine Secretion and Suppresses Type Vi Secretion System in Pseudomonas aeruginosa. Microb. Pathog. 2022, 169, 105654. [Google Scholar] [CrossRef] [PubMed]
- Parducho, K.R.; Beadell, B.; Ybarra, T.K.; Bush, M.; Escalera, E.; Trejos, A.T.; Chieng, A.; Mendez, M.; Anderson, C.; Park, H.; et al. The Antimicrobial Peptide Human Beta-Defensin 2 Inhibits Biofilm Production of Pseudomonas aeruginosa without Compromising Metabolic Activity. Front. Immunol. 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Savio, V.; Stelitano, D.; Baroni, A.; Donnarumma, G. The Intestinal Biofilm of Pseudomonas aeruginosa and Staphylococcus aureus Is Inhibited by Antimicrobial Peptides Hbd-2 and Hbd-3. Appl. Sci. 2021, 11, 6595. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, P.; Wang, S.; Li, X.; Peng, L.; Fang, R.; Xiong, J.; Li, H.; Mei, C.; Gao, J.; et al. Pseudomonas aeruginosa Biofilm Dispersion by the Mouse Antimicrobial Peptide Cramp. Vet. Res. 2022, 53, 80. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Activity of Antimicrobial Peptides and Ciprofloxacin against Pseudomonas aeruginosa Biofilms. Molecules 2020, 25, 3843. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; He, Y.; Liu, K.; Zhang, F.; Zhang, H.; Lu, Y.; Yang, C.; Wang, Z.; Fareed, M.S.; et al. An Optimized Analog of Antimicrobial Peptide Jelleine-1 Shows Enhanced Antimicrobial Activity against Multidrug Resistant P. aeruginosa and Negligible Toxicity in Vitro and in Vivo. Eur. J. Med. Chem. 2021, 219, 113433. [Google Scholar] [CrossRef]
- Portelinha, J.; Angeles-Boza, A.M. The Antimicrobial Peptide Gad-1 Clears Pseudomonas aeruginosa Biofilms under Cystic Fibrosis Conditions. Chembiochem 2021, 22, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, S.; Wu, L.; Wang, Z.; Mu, Y.; Zhang, R.; Dong, C.; Zhou, B.; Zhao, B.; Zheng, J.; et al. A Novel in Silico Antimicrobial Peptide Dp7 Combats Mdr Pseudomonas aeruginosa and Related Biofilm Infections. J. Antimicrob. Chemother. 2020, 75, 3248–3259. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Yigin, A.; Demir, C. Efficacy of Antimicrobial Peptide LL-37 against Biofilm Forming Staphylococcus aureus Strains Obtained from Chronic Wound Infections. Microb. Pathog. 2022, 162, 105368. [Google Scholar] [CrossRef] [PubMed]
- Jiale, Z.; Jian, J.; Xinyi, T.; Haoji, X.; Xueqin, H.; Xiao, W. Design of a Novel Antimicrobial Peptide 1018m Targeted Ppgpp to Inhibit MRSA Biofilm Formation. AMB Express 2021, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Yang, K.; Guo, R.; Zhu, X.; Shi, Y.; Huang, A. Antibiofilm Mechanism of a Novel Milk-Derived Antimicrobial Peptide against Staphylococcus aureus by Downregulating Agr Quorum Sensing System. J. Appl. Microbiol. 2022, 133, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, Y.; Zhao, X.; Liu, S.; Xia, X.; Zhang, S.; Wang, Y.; Zhang, H.; Xu, Y.; Chen, S.; et al. The Antimicrobial Peptide Mpx Can Kill Staphylococcus aureus, Reduce Biofilm Formation, and Effectively Treat Bacterial Skin Infections in Mice. Front. Vet. Sci. 2022, 9, 819921. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.A.; Pinto, S.N.; Silva-Herdade, A.S.; Cavaco, M.; Neves, V.; Tavares, L.; Oliveira, M.; Andreu, D.; Coutinho, A.; Castanho, M.A.R.B.; et al. Quantitative Imaging of the Action of Vcpp2319, an Antimicrobial Peptide from a Viral Scaffold, against Staphylococcus aureus Biofilms of a Clinical Isolate. ACS Infect. Dis. 2023, 9, 1889–1900. [Google Scholar] [CrossRef]
- Fei, F.; Wang, T.; Jiang, Y.; Chen, X.; Ma, C.; Zhou, M.; Wu, Q.; Cao, P.; Duan, J.; Chen, T.; et al. A Frog-Derived Antimicrobial Peptide as a Potential Anti-Biofilm Agent in Combating Staphylococcus aureus Skin Infection. J. Cell. Mol. Med. 2023, 27, 1565–1579. [Google Scholar] [CrossRef]
- Casciaro, B.; Loffredo, M.R.; Cappiello, F.; Fabiano, G.; Torrini, L.; Mangoni, M.L. The Antimicrobial Peptide Temporin G: Anti-Biofilm, Anti-Persister Activities, and Potentiator Effect of Tobramycin Efficacy against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 9410. [Google Scholar] [CrossRef]
- Li, R.; Hao, P.; Yin, K.; Xu, Q.; Ren, S.; Zhao, Y.; Zhang, L.; Zhang, B. Activities of a Broad-Spectrum Antimicrobial Peptide Analogue Samp-A4-C8 and Its Combat against Pneumonia in Staphylococcus aureus-Infected Mice. J. Pept. Sci. 2023, 29, e3497. [Google Scholar] [CrossRef] [PubMed]
- Delpech, G.; Ceci, M.; Lissarrague, S.; Garcia Allende, L.; Baldaccini, B.; Sparo, M. In Vitro Activity of the Antimicrobial Peptide Ap7121 against the Human Methicillin-Resistant Biofilm Producers Staphylococcus aureus and Staphylococcus epidermidis. Biofouling 2020, 36, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Colagiorgi, A.; Festa, R.; Di Ciccio, P.A.; Gogliettino, M.; Balestrieri, M.; Palmieri, G.; Anastasio, A.; Ianieri, A. Rapid Biofilm Eradication of the Antimicrobial Peptide 1018-K6 against Staphylococcus aureus: A New Potential Tool to Fight Bacterial Biofilms. Food Control 2020, 107, 106815. [Google Scholar] [CrossRef]
- Ciandrini, E.; Morroni, G.; Cirioni, O.; Kamysz, W.; Kamysz, E.; Brescini, L.; Baffone, W.; Campana, R. Synergistic Combinations of Antimicrobial Peptides against Biofilms of Methicillin-Resistant Staphylococcus aureus (MRSA) on Polystyrene and Medical Devices. J. Glob. Antimicrob. Resist. 2020, 21, 203–210. [Google Scholar] [CrossRef]
- Seo, S.; Jung, J.; Kim, C.Y.; Kang, H.; Lee, I.H. Antimicrobial Peptides Encounter Resistance of Aureolysin During Their Action on Staphylococcus aureus Biofilm. Biotechnol. Bioprocess. Eng. 2021, 26, 216–222. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Meng, H.; Lv, H.; Liu, Y.; Liu, J.; Wang, H.; He, L.; Qin, J.; Wang, Y.; et al. Staphylococcus epidermidis Contributes to Healthy Maturation of the Nasal Microbiome by Stimulating Antimicrobial Peptide Production. Cell. Host Microbe 2020, 27, 68–78.e65. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, R.; Zhang, M.; Huang, R.; Fan, C.; Zhou, S.; Qiu, J.; He, J. In Vitro Antibacterial Activity of a Novel Acid-Activated Antimicrobial Peptide against Streptococcus mutans. Curr. Protein Pept. Sci. 2023, 25, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Sabadini, C.; Alvarez, S.; Barrera, N.P.; Schuh, C.; Aguayo, S. Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides against Oral Streptococci. Int. J. Nanomed. 2021, 16, 4891–4900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qu, Y.; Liu, J.; Mai, S.; Gu, L. A Universal Adhesive Incorporating Antimicrobial Peptide Nisin: Effects on Streptococcus mutans and Saliva-Derived Multispecies Biofilms. Odontology 2020, 108, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Luo, J.; Lyu, X.; Chen, Y.; Zhang, L. Selective Antibacterial Activity of a Novel Lactotransferrin-Derived Antimicrobial Peptide Lf-1 against Streptococcus mutans. Arch. Oral Biol. 2022, 139, 105446. [Google Scholar] [CrossRef]
- Boswell, M.T.; Cockeran, R. Effect of Antimicrobial Peptides on Planktonic Growth, Biofilm Formation and Biofilm-Derived Bacterial Viability of Streptococcus pneumoniae. S. Afr. J. Infect. Dis. 2021, 36, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Mo, X.M.; Xu, S.N.; Tang, H.N.; Zhou, Y.H.; Li, L.; Zhou, H.D. A Novel Antimicrobial Peptide Derived from Human Bpifa1 Protein Protects against Candida albicans Infection. Innate Immun. 2022, 28, 67–78. [Google Scholar] [CrossRef]
- Wei, P.W.; Song, C.R.; Wang, X.; Chen, M.; Yang, Y.X.; Wang, C.; Hu, Z.Q.; Liu, H.M.; Wang, B. A Potential Milk Preservative—Phormicin C-Ns, Sorbic Acid-Modified Housefly Antimicrobial Peptide, Inhibits Candida albicans Hypha and Biofilm Formation. LWT-Food Sci. Technol. 2022, 168, 113883. [Google Scholar] [CrossRef]
- Aguiar, F.L.L.; Santos, N.C.; de Paula Cavalcante, C.S.; Andreu, D.; Baptista, G.R.; Goncalves, S. Antibiofilm Activity on Candida albicans and Mechanism of Action on Biomembrane Models of the Antimicrobial Peptide Ctn[15-34]. Int. J. Mol. Sci. 2020, 21, 8339. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Dias, J.; de Souza Silva, C.; de Araujo, A.R.; Souza, J.M.T.; de Holanda Veloso Junior, P.H.; Cabral, W.F.; da Gloria da Silva, M.; Eaton, P.; de Souza de Almeida Leite, J.R.; Nicola, A.M.; et al. Mechanisms of Action of Antimicrobial Peptides Toap2 and Ndbp-5.7 against Candida albicans Planktonic and Biofilm Cells. Sci. Rep. 2020, 10, 10327. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Yin, K.; Xu, Q.; Ren, S.; Wang, X.; Wang, Z.; Yi, Y. Fatty Acid Modification of Antimicrobial Peptide Cga-N9 and the Combats against Candida albicans Infection. Biochem. Pharmacol. 2023, 211, 115535. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tian, Z.; Zhou, L.; Zhu, L.; Sun, C.; Huang, M.; Peng, J.; Guo, G. In Vitro Antifungal Activity of a Novel Antimicrobial Peptide Amp-17 against Planktonic Cells and Biofilms of Cryptococcus neoformans. Infect. Drug Resist. 2022, 15, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, X.; Jiao, Z.; Peng, J.; Zhou, L.; Yang, L.; Huang, M.; Tian, C.; Guo, G. The Antimicrobial Peptide Amp-17 Derived from Musca domestica Inhibits Biofilm Formation and Eradicates Mature Biofilm in Candida albicans. Antibiotics 2022, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Raber, H.F.; Sejfijaj, J.; Kissmann, A.K.; Wittgens, A.; Gonzalez-Garcia, M.; Alba, A.; Vázquez, A.A.; Vicente, F.E.M.; Erviti, J.P.; Kubiczek, D.; et al. Antimicrobial Peptides Pom-1 and Pom-2 from Pomacea poeyana Are Active against Candida auris, C. parapsilosis and C. albicans Biofilms. Pathogens 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Singulani, J.L.; Oliveira, L.T.; Ramos, M.D.; Fregonezi, N.F.; Gomes, P.C.; Galeane, M.C.; Palma, M.S.; Fusco Almeida, A.M.; Mendes Giannini, M.J.S. The Antimicrobial Peptide Mk58911-Nh2 Acts on Planktonic, Biofilm, and Intramacrophage Cells of Cryptococcus neoformans. Antimicrob. Agents Chemother. 2021, 65, e0090421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, F.J.; Lee, C.H. Effect of Antifungal Agents, Lysozyme and Human Antimicrobial Peptide LL-37 on Clinical Candida Isolates with High Biofilm Production. J. Med. Microbiol. 2021, 70, 001283. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Z.; Mao, C.; Guo, G.; Zeng, Z.; Fei, Y.; Wan, S.; Peng, J.; Wu, J. Antimicrobial Peptide Cec4 Eradicates the Bacteria of Clinical Carbapenem-Resistant Acinetobacter baumannii Biofilm. Front. Microbiol. 2020, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.J.; Liao, Y.D.; Hsu, C.C.; Huang, T.Y.; Chuang, Y.C.; Chen, J.W.; Kuo, Y.M.; Chia, J.S. Identification of Potential Therapeutic Antimicrobial Peptides against Acinetobacter baumannii in a Mouse Model of Pneumonia. Sci. Rep. 2021, 11, 7318. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Misawa, T.; Goto, C.; Demizu, Y.; Hara-Kudo, Y.; Kikuchi, Y. The Effects of Magainin 2-Derived and Rationally Designed Antimicrobial Peptides on Mycoplasma pneumoniae. PLoS ONE 2022, 17, e0261893. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Wang, M.; Li, Y.Q.; Hong, B.; Kang, D.; Ma, Y.; Wang, J.F. Two Novel Antimicrobial Peptides against Vegetative Cells, Spores and Biofilm of Bacillus cereus. Food Control 2023, 149, 109688. [Google Scholar] [CrossRef]
- Mangmee, S.; Reamtong, O.; Kalambaheti, T.; Roytrakul, S.; Sonthayanon, P. Antimicrobial Peptide Modifications against Clinically Isolated Antibiotic-Resistant Salmonella. Molecules 2021, 26, 4654. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J.; Xiao, X.; Zheng, J.; Lai, S.; Yang, L.; Li, J.; Liu, C.; Yang, Y.; Mu, Y. Antibacterial and Antibiofilm Activities of Novel Antimicrobial Peptide Dp7 against the Periodontal Pathogen Porphyromonas gingivalis. J. Appl. Microbiol. 2022, 133, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, X.; Zhu, C.; Zhao, Y.; Liu, S.; Xia, X.; Liu, X.; Zhang, H.; Xu, Y.; Hang, B.; et al. The Antimicrobial Peptide Mpx Kills Actinobacillus pleuropneumoniae and Reduces Its Pathogenicity in Mice. Vet. Microbiol. 2020, 243, 108634. [Google Scholar] [CrossRef] [PubMed]
- Tanhaeian, A.; Mirzaii, M.; Pirkhezranian, Z.; Sekhavati, M.H. Generation of an Engineered Food-Grade Lactococcus lactis Strain for Production of an Antimicrobial Peptide: In Vitro and in Silico Evaluation. BMC Biotechnol. 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Mhade, S.; Panse, S.; Tendulkar, G.; Awate, R.; Narasimhan, Y.; Kadam, S.; Yennamalli, R.M.; Kaushik, K.S. AMPing up the Search: A Structural and Functional Repository of Antimicrobial Peptides for Biofilm Studies, and a Case Study of Its Application to Corynebacterium striatum, an Emerging Pathogen. Front. Cell. Infect. Microbiol. 2021, 11, 803774. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cao, X.; Qian, J.; Liu, Z.; Wang, X.; Su, Q.; Wang, Y.; Xie, R.; Li, X. Evaluation of Antimicrobial Peptide LL-37 for Treatment of Staphylococcus aureus Biofilm on Titanium Plate. Medicine 2021, 100, e27426. [Google Scholar] [CrossRef] [PubMed]
- Wuersching, S.N.; Huth, K.C.; Hickel, R.; Kollmuss, M. Targeting Antibiotic Tolerance in Anaerobic Biofilms Associated with Oral Diseases: Human Antimicrobial Peptides LL-37 and Lactoferricin Enhance the Antibiotic Efficacy of Amoxicillin, Clindamycin and Metronidazole. Anaerobe 2021, 71, 102439. [Google Scholar] [CrossRef] [PubMed]
- Riool, M.; de Breij, A.; Kwakman, P.H.S.; Schonkeren-Ravensbergen, E.; de Boer, L.; Cordfunke, R.A.; Malanovic, N.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Thrombocidin-1-Derived Antimicrobial Peptide Tc19 Combats Superficial Multi-Drug Resistant Bacterial Wound Infections. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183282. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; You, D.G.; Kim, H.K.; Sohn, J.W.; Kim, M.J.; Park, J.K.; Lee, G.Y.; Yoo, Y.D. Romo1-Derived Antimicrobial Peptide Is a New Antimicrobial Agent against Multidrug-Resistant Bacteria in a Murine Model of Sepsis. mBio 2020, 11, 101128. [Google Scholar] [CrossRef] [PubMed]
- Van Moll, L.; De Smet, J.; Paas, A.; Tegtmeier, D.; Vilcinskas, A.; Cos, P.; van Campenhout, L. In Vitro Evaluation of Antimicrobial Peptides from the Black Soldier Fly (Hermetia illucens) against a Selection of Human Pathogens. Microbiol. Spectr. 2022, 10, e0166421. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, L.; Gloy, L.; Langfeldt, D.; Pinnow, N.; Weiland-Bräuer, N.; Schmitz, R.A. Antimicrobial Peptides Originating from Expression Libraries of Aurelia aurita and Mnemiopsis leidyi Prevent Biofilm Formation of Opportunistic Pathogens. Microorganisms 2023, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Sivakamavalli, J.; Arthur James, R.; Park, K.; Kwak, I.S.; Vaseeharan, B. Purification of WAP Domain-Containing Antimicrobial Peptides from Green Tiger Shrimp Peaneaus semisulcatus. Microb. Pathog. 2020, 140, 103920. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, D.C.; Edirisinghe, S.L.; Nikapitiya, C.; Whang, I.; De Zoysa, M. The Antimicrobial Peptide Octopromycin Suppresses Biofilm Formation and Quorum Sensing in Acinetobacter baumannii. Antibiotics 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Safronova, V.N.; Panteleev, P.V.; Sukhanov, S.V.; Toropygin, I.Y.; Bolosov, I.A.; Ovchinnikova, T.V. Mechanism of Action and Therapeutic Potential of the Beta-Hairpin Antimicrobial Peptide Capitellacin from the Marine Polychaeta Capitella teleta. Mar. Drugs 2022, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gu, X.; Song, D.; Zhang, P.; Zhang, N.; Chen, W.; Ji, S.; Qi, Y.; Ma, S. Rational Design and Synthesis of Oreoch-2 Analogues as Efficient Broad-Spectrum Antimicrobial Peptides. Bioorg. Chem. 2022, 119, 105583. [Google Scholar] [CrossRef]
- Prior, B.S.; Lange, M.D.; Salger, S.A.; Reading, B.J.; Peatman, E.; Beck, B.H. The Effect of Piscidin Antimicrobial Peptides on the Formation of Gram-Negative Bacterial Biofilms. J. Fish Dis. 2022, 45, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ajish, C.; Yang, S.; Kumar, S.D.; Lee, C.W.; Kim, D.M.; Cho, S.J.; Shin, S.Y. Cell Selectivity and Antibiofilm and Anti-Inflammatory Activities and Antibacterial Mechanism of Symmetric-End Antimicrobial Peptide Centered on D-Pro-Pro. Biochem. Biophys. Res. Commun. 2023, 666, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Masihzadeh, S.; Amin, M.; Farshadzadeh, Z. In Vitro and in Vivo Antibiofilm Activity of the Synthetic Antimicrobial Peptide Wlbu2 against Multiple Drug Resistant Pseudomonas aeruginosa Strains. BMC Microbiol. 2023, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lu, C.; Zheng, M.; Zhou, W.; Song, F.; Chen, W.; Yao, F.; Liu, D.; Cai, J. Unnatural Amino-Acid-Based Star-Shaped Poly(L-Ornithine)S as Emerging Long-Term and Biofilm-Disrupting Antimicrobial Peptides to Treat Pseudomonas aeruginosa-Infected Burn Wounds. Adv. Healthc. Mater. 2020, 9, e2000647. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, J.; Hart, R.J.; Soldano, A.; Chen, C.H.; Guha, S.; Hoffmann, J.P.; Hall, K.M.; Sun, L.; Nelson, B.J.; Lu, T.K.; et al. Optimization of Host Cell-Compatible, Antimicrobial Peptides Effective against Biofilms and Clinical Isolates of Drug-Resistant Bacteria. ACS Infect. Dis. 2023, 9, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Du, Y.; Li, X.; Yao, C. Lipoic Acid Modified Antimicrobial Peptide with Enhanced Antimicrobial Properties. Bioorg. Med. Chem. 2020, 28, 115682. [Google Scholar] [CrossRef]
- Lakshmaiah Narayana, J.; Golla, R.; Mishra, B.; Wang, X.; Lushnikova, T.; Zhang, Y.; Verma, A.; Kumar, V.; Xie, J.; Wang, G. Short and Robust Anti-Infective Lipopeptides Engineered Based on the Minimal Antimicrobial Peptide Kr12 of Human LL-37. ACS Infect. Dis. 2021, 7, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.L.; Mei, M.L.; Chu, C.H. Remineralising Dentine Caries Using an Artificial Antimicrobial Peptide: An in Vitro Study. J. Dent. 2021, 111, 103736. [Google Scholar] [CrossRef] [PubMed]
- White, J.K.; Muhammad, T.; Alsheim, E.; Mohanty, S.; Blasi-Romero, A.; Gunasekera, S.; Stromstedt, A.A.; Ferraz, N.; Goransson, U.; Brauner, A. A Stable Cyclized Antimicrobial Peptide Derived from LL-37 with Host Immunomodulatory Effects and Activity against Uropathogens. Cell. Mol. Life Sci. 2022, 79, 411. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, M.; Oyardi, O.; Bozkurt-Guzel, C.; Savage, P.B. Antibiofilm Activities of Ceragenins and Antimicrobial Peptides against Fungal-Bacterial Mono and Multispecies Biofilms. J. Antibiot. 2020, 73, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.T.; Kuppusamy, R.; Yasir, M.; Hassan, M.M.; Alghalayini, A.; Gadde, S.; Deplazes, E.; Cranfield, C.; Willcox, M.D.P.; Black, D.S.; et al. Design, Synthesis and Biological Evaluation of Biphenylglyoxamide-Based Small Molecular Antimicrobial Peptide Mimics as Antibacterial Agents. Int. J. Mol. Sci. 2020, 21, 6789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, Y.; Luo, J.; Chen, X.; Zeng, Y.; Li, X.; Feng, Z.; Zhang, L. Antimicrobial Peptide GH12 Prevents Dental Caries by Regulating Dental Plaque Microbiota. Appl. Environ. Microbiol. 2020, 86, e00527-20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Luo, J.; Wang, Y.; Chen, X.; Jiang, X.; Feng, Z.; Zhang, L. The Ph-Responsive Property of Antimicrobial Peptide GH12 Enhances Its Anticaries Effects at Acidic Ph. Caries Res. 2021, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, T.; Hargraves, S.; Georgieva, M.; Widmann, C.; Jacquier, N. The Antimicrobial Peptide Tat-Rasgap317-326 Inhibits the Formation and Expansion of Bacterial Biofilms in Vitro. J. Glob. Antimicrob. Resist. 2021, 25, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.S.; Ramalho, S.R.; Sandim, G.C.; Parisotto, E.B.; Orlandi Sardi, J.C.; Rodrigues Macedo, M.L. Prevention of Hospital Pathogen Biofilm Formation by Antimicrobial Peptide Kwi18. Microb. Pathog. 2022, 172, 105791. [Google Scholar] [CrossRef]
- Chen, Y.C.; Qiu, W.; Zhang, W.; Zhang, J.; Chen, R.; Chen, F.; Wang, K.J. A Novel Antimicrobial Peptide Sp-Lecin with Broad-Spectrum Antimicrobial Activity and Anti-Pseudomonas aeruginosa Infection in Zebrafish. Int. J. Mol. Sci. 2022, 24, 267. [Google Scholar] [CrossRef] [PubMed]
- Maselli, V.; Galdiero, E.; Salzano, A.M.; Scaloni, A.; Maione, A.; Falanga, A.; Naviglio, D.; Guida, M.; Di Cosmo, A.; Galdiero, S. Octopartenopin: Identification and Preliminary Characterization of a Novel Antimicrobial Peptide from the Suckers of Octopus vulgaris. Mar. Drugs 2020, 18, 380. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, F.; Chen, H.Y.; Peng, H.; Hao, H.; Wang, K.J. A Novel Antimicrobial Peptide Scyreprocin from Mud Crab Scylla paramamosain Showing Potent Antifungal and Anti-Biofilm Activity. Front. Microbiol. 2020, 11, 1589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ouyang, X.; Zhang, F.; Li, B.; Chang, L.; Yang, P.; Mao, W.; Gou, S.; Zhang, Y.; Liu, H.; et al. Structure-Activity Relationship Study of Antimicrobial Peptide Pe2 Delivered Novel Linear Derivatives with Potential of Eradicating Biofilms and Low Incidence of Drug Resistance. J. Med. Chem. 2023, 66, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Suchi, S.A.; Nam, K.B.; Kim, Y.K.; Tarek, H.; Yoo, J.C. A Novel Antimicrobial Peptide Ys12 Isolated from Bacillus velezensis Cbsys12 Exerts Anti-Biofilm Properties against Drug-Resistant Bacteria. Bioprocess. Biosyst. Eng. 2023, 46, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Masadeh, M.; Ayyad, A.; Haddad, R.; Alsaggar, M.; Alzoubi, K.; Alrabadi, N. Functional and Toxicological Evaluation of Maa-41: A Novel Rationally Designed Antimicrobial Peptide Using Hybridization and Modification Methods from LL-37 and Bmap-28. Curr. Pharm. Des. 2022, 28, 2177–2188. [Google Scholar] [CrossRef]
- Shang, L.; Wu, Y.; Wei, N.; Yang, F.; Wang, M.; Zhang, L.; Fei, C.; Liu, Y.; Xue, F.; Gu, F. Novel Arginine End-Tagging Antimicrobial Peptides to Combat Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2022, 14, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.A.; Kalajirao, S.; Alin Bose, J.; Palanimuthu, V.R.; Rajeshkumar, R. Insilico Design, Evaluation of Novel Antimicrobial Peptide Ss-Bf-36 Targeting Biofilms and Multidrug-Resistant Organisms. NeuroQuantology 2022, 20, 1655–1660. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, R.; Zhang, J.; Chen, F.; Wang, K.J. A Novel Antimicrobial Peptide Spampcin56-86 from Scylla paramamosain Exerting Rapid Bactericidal and Anti-Biofilm Activity in Vitro and Anti-Infection in Vivo. Int. J. Mol. Sci. 2022, 23, 13316. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.A.; Bayramov, D.F.; Patel, E.A.; Miao, J. Novel Antimicrobial Peptides Formulated in Chitosan Matrices Are Effective against Biofilms of Multidrug-Resistant Wound Pathogens. Mil. Med. 2020, 185 (Suppl. S1), 637–643. [Google Scholar] [CrossRef] [PubMed]
- Di Fermo, P.; Ciociola, T.; Di Lodovico, S.; D’Ercole, S.; Petrini, M.; Giovati, L.; Conti, S.; Di Giulio, M.; Cellini, L. Antimicrobial Peptide L18r Displays a Modulating Action against Inter-Kingdom Biofilms in the Lubbock Chronic Wound Biofilm Model. Microorganisms 2021, 9, 1779. [Google Scholar] [CrossRef] [PubMed]
- Melichercik, P.; Kotaska, K.; Jahoda, D.; Landor, I.; Cerovsky, V. Antimicrobial Peptide in Polymethylmethacrylate Bone Cement as a Prophylaxis of Infectious Complications in Orthopedics-an Experiment in a Murine Model. Folia Microbiol. 2022, 67, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Polizzotto, A.; Flores, N.; Semorile, L.; Maffia, P.C. Antibacterial, Anti-Biofilm and in Vivo Activities of the Antimicrobial Peptides P5 and P6.2. Microb. Pathog. 2020, 139, 103886. [Google Scholar] [CrossRef] [PubMed]
- Stillger, L.; Viau, L.; Holtmann, D.; Muller, D. Antibiofilm Assay for Antimicrobial Peptides Combating the Sulfate-Reducing Bacteria Desulfovibrio vulgaris. Microbiologyopen 2023, 12, e1376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, M.H.; Chen, Y.; Ouyang, J.H.; Wang, Y.; Yang, H.X.; Luo, X.J.; Zhang, D.D.; Lu, Y.; Yu, H.N.; et al. Antimicrobial, Anti-Biofilm Properties of Three Naturally Occurring Antimicrobial Peptides against Spoilage Bacteria, and Their Synergistic Effect with Chemical Preservatives in Food Storage. Food Control 2021, 123, 107729. [Google Scholar] [CrossRef]
- Stillger, L.; Viau, L.; Kamm, L.; Holtmann, D.; Muller, D. Optimization of Antimicrobial Peptides for the Application against Biocorrosive Bacteria. Appl. Microbiol. Biotechnol. 2023, 107, 4041–4049. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Das, J.; Agrawal, N.R.; Kushwaha, G.S.; Ghosh, M.; Son, Y.O. Marine Antimicrobial Peptides-Based Strategies for Tackling Bacterial Biofilm and Biofouling Challenges. Molecules 2022, 27, 7546. [Google Scholar] [CrossRef] [PubMed]
- Wieland, T.; Assmann, J.; Bethe, A.; Fidelak, C.; Gmoser, H.; Jansen, T.; Kotthaus, K.; Lubke-Becker, A.; Wieler, L.H.; Urban, G.A. A Real-Time Thermal Sensor System for Quantifying the Inhibitory Effect of Antimicrobial Peptides on Bacterial Adhesion and Biofilm Formation. Sensors 2021, 21, 2771. [Google Scholar] [CrossRef] [PubMed]
- Fenner, A. Antimicrobial Peptide Derived from Moths Can Eradicate Upec Biofilms and Could Offer a Novel Therapeutic Option. Nat. Rev. Urol. 2020, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Avatapalli, S.; Narasimhan, Y.; Kaushik, K.S.; Yennamalli, R.M. ‘Targeting’ the Search: An Upgraded Structural and Functional Repository of Antimicrobial Peptides for Biofilm Studies (B-Amp V2.0) with a Focus on Biofilm Protein Targets. Front. Cell. Infect. Microbiol. 2022, 12, 1020391. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, F.; Leier, A.; Xiang, D.; Shen, H.H.; Marquez Lago, T.T.; Li, J.; Yu, D.J.; Song, J. Comprehensive Assessment of Machine Learning-Based Methods for Predicting Antimicrobial Peptides. Brief. Bioinform. 2021, 22, bbab083. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Wang, Y.F.; Jiang, W.T.; Zhang, L.L. Effects of Antimicrobial Peptide Gh12 on the Morphology and Composition of Cariogenic Three-Species Biofilm. West. China J. Stomatol. 2021, 39, 188–194. [Google Scholar] [CrossRef]
- de Pontes, J.T.C.; Borges, A.B.T.; Roque-Borda, C.A.; Pavan, F.R. Antimicrobial Peptides as an Alternative for the Eradication of Bacterial Biofilms of Multi-Drug Resistant Bacteria. Pharmaceutics 2022, 14, 642. [Google Scholar] [CrossRef]
- Ben Hur, D.; Kapach, G.; Wani, N.A.; Kiper, E.; Ashkenazi, M.; Smollan, G.; Keller, N.; Efrati, O.; Shai, Y. Antimicrobial Peptides against Multidrug-Resistant Pseudomonas aeruginosa Biofilm from Cystic Fibrosis Patients. J. Med. Chem. 2022, 65, 9050–9062. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, P.; Liu, X.; Wang, Y.; Wei, H.; Zhang, J.; Yu, L.; Yan, X.; He, Z. Dealing with Mdr Bacteria and Biofilm in the Post-Antibiotic Era: Application of Antimicrobial Peptides-Based Nano-Formulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112318. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Swetha, T.K.; Pandian, S.K. Chapter 20—Antimicrobial Peptides as a Potent Therapeutic Regimen to Quench Biofilm-Mediated Antimicrobial Resistance. In Microbial and Natural Macromolecules; Academic Press: Cambridge, MA, USA, 2021; pp. 531–570. [Google Scholar] [CrossRef]
- Pervin, Z.; Hassan, M.M. Synergistic Therapeutic Actions of Antimicrobial Peptides to Treat Multidrug-Resistant Bacterial Infection. Rev. Res. Med. Microbiol. 2021, 32, 83–89. [Google Scholar] [CrossRef]
- Starr, C.G.; Ghimire, J.; Guha, S.; Hoffmann, J.P.; Wang, Y.; Sun, L.; Landreneau, B.N.; Kolansky, Z.D.; Kilanowski-Doroh, I.M.; Sammarco, M.C.; et al. Synthetic Molecular Evolution of Host Cell-Compatible, Antimicrobial Peptides Effective against Drug-Resistant, Biofilm-Forming Bacteria. Proc. Natl. Acad. Sci. USA 2020, 117, 8437–8448. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Ghosh, A.K.; Mandal, S.M. Coevolution of Resistance against Antimicrobial Peptides. Microb. Drug Resist. 2020, 26, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Beito, B.; Oliveira, M.; Martins, M.T.; Gallas, B.; Salmain, M.; Boujday, S.; Humblot, V. Strategies for Antimicrobial Peptides Immobilization on Surfaces to Prevent Biofilm Growth on Biomedical Devices. Antibiotics 2022, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.; Jain, V.K.; Iyengar, K.P. Antimicrobial Peptides (AMP) in Biofilm Induced Orthopaedic Device-Related Infections. J. Clin. Orthop. Trauma 2022, 25, 101780. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Mishra, P.; Prasad, R.; Poluri, K.M. Dissecting the Therapeutic Potency of Antimicrobial Peptides against Microbial Biofilms. Curr. Protein Pept. Sci. 2021, 22, 336–360. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef] [PubMed]
- Vieira-da-Silva, B.; Castanho, M. The Structure and Matrix Dynamics of Bacterial Biofilms as Revealed by Antimicrobial Peptides’ Diffusion. J. Pept. Sci. 2023, 29, e3470. [Google Scholar] [CrossRef] [PubMed]
- Naaz, T.; Lahiri, D.; Pandit, S.; Nag, M.; Gupta, P.K.; Al-Dayan, N.; Rai, N.; Chaubey, K.K.; Gupta, A.K. Antimicrobial Peptides against Microbial Biofilms: Efficacy, Challenges, and Future Prospect. Int. J. Pept. Res. Ther. 2023, 29, 48. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, A.; Verma, V.K. Strategies Adopted by Gastric Pathogen Helicobacter pylori for a Mature Biofilm Formation: Antimicrobial Peptides as a Visionary Treatment. Microbiol. Res. 2023, 273, 127417. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, S.; Balakrishnan, S.; Ranjith, S.; Husain, F.; Sathyan, A.; Peter, A.S.; Prahalathan, C.; Kumarasamy, A. Bacteriocin-a Potential Antimicrobial Peptide Towards Disrupting and Preventing Biofilm Formation in the Clinical and Environmental Locales. Environ. Sci. Pollut. Res. Int. 2020, 27, 44922–44936. [Google Scholar] [CrossRef] [PubMed]
| Organism | Number |
|---|---|
| Staphylococcus aureus/MRSA | 13 |
| Pseudomonas aeruginosa | 8 |
| Candida albicans/spp. | 8 |
| Streptococcus pneumonaie/mutans/oral | 5 |
| Enterococcus faecalis | 3 |
| Escherichia coli | 1 |
| Acinetobacter baumannii | 2 |
| Cryptococcus neoformans | 2 |
| Staphylococcus epidermidis | 2 |
| Actinobacillus pleuropneumoniae | 1 |
| Bacillus cereus | 1 |
| Corynebacterium striatum | 1 |
| Lactococcus lactis | 1 |
| Mycoplasma pneumoniae | 1 |
| Porphyromonas gingivalis | 1 |
| Salmonella serovars | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontanot, A.; Ellinger, I.; Unger, W.W.J.; Hays, J.P. A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms—January 2020 to September 2023. Antibiotics 2024, 13, 343. https://doi.org/10.3390/antibiotics13040343
Fontanot A, Ellinger I, Unger WWJ, Hays JP. A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms—January 2020 to September 2023. Antibiotics. 2024; 13(4):343. https://doi.org/10.3390/antibiotics13040343
Chicago/Turabian StyleFontanot, Alessio, Isabella Ellinger, Wendy W. J. Unger, and John P. Hays. 2024. "A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms—January 2020 to September 2023" Antibiotics 13, no. 4: 343. https://doi.org/10.3390/antibiotics13040343
APA StyleFontanot, A., Ellinger, I., Unger, W. W. J., & Hays, J. P. (2024). A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms—January 2020 to September 2023. Antibiotics, 13(4), 343. https://doi.org/10.3390/antibiotics13040343









