A Study of Metabolites from Basidiomycota and Their Activities against Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results and Discussion
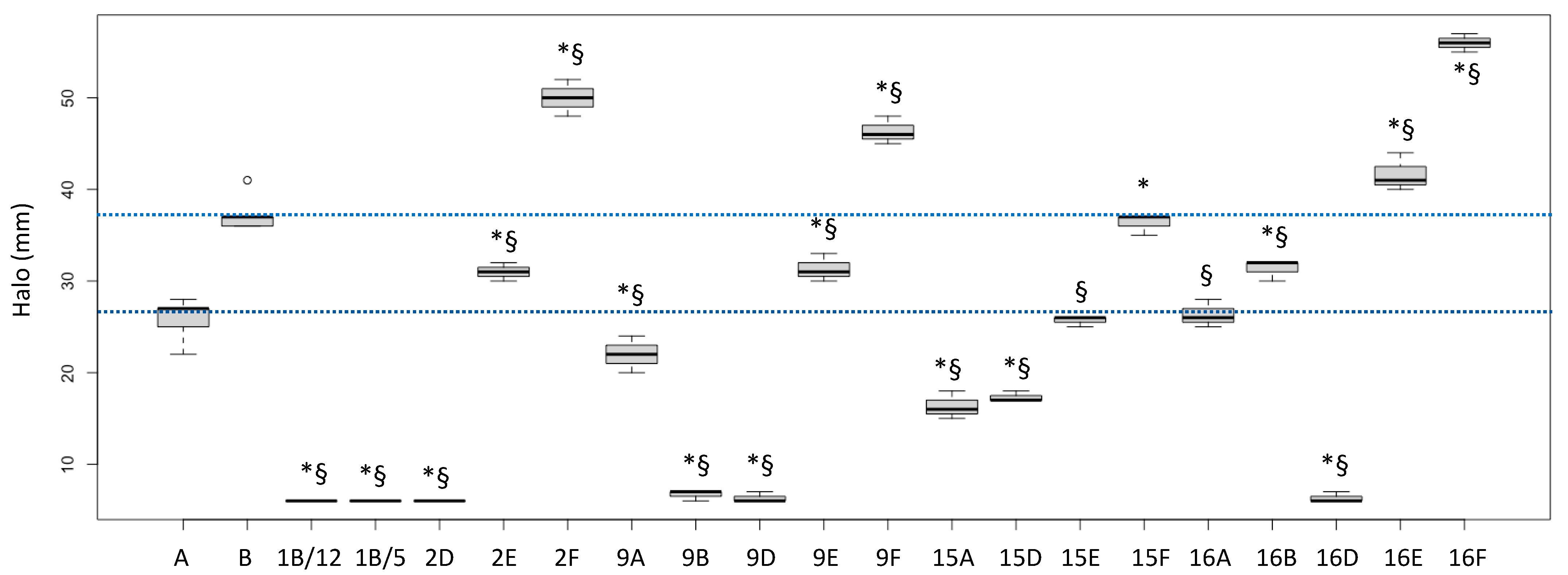
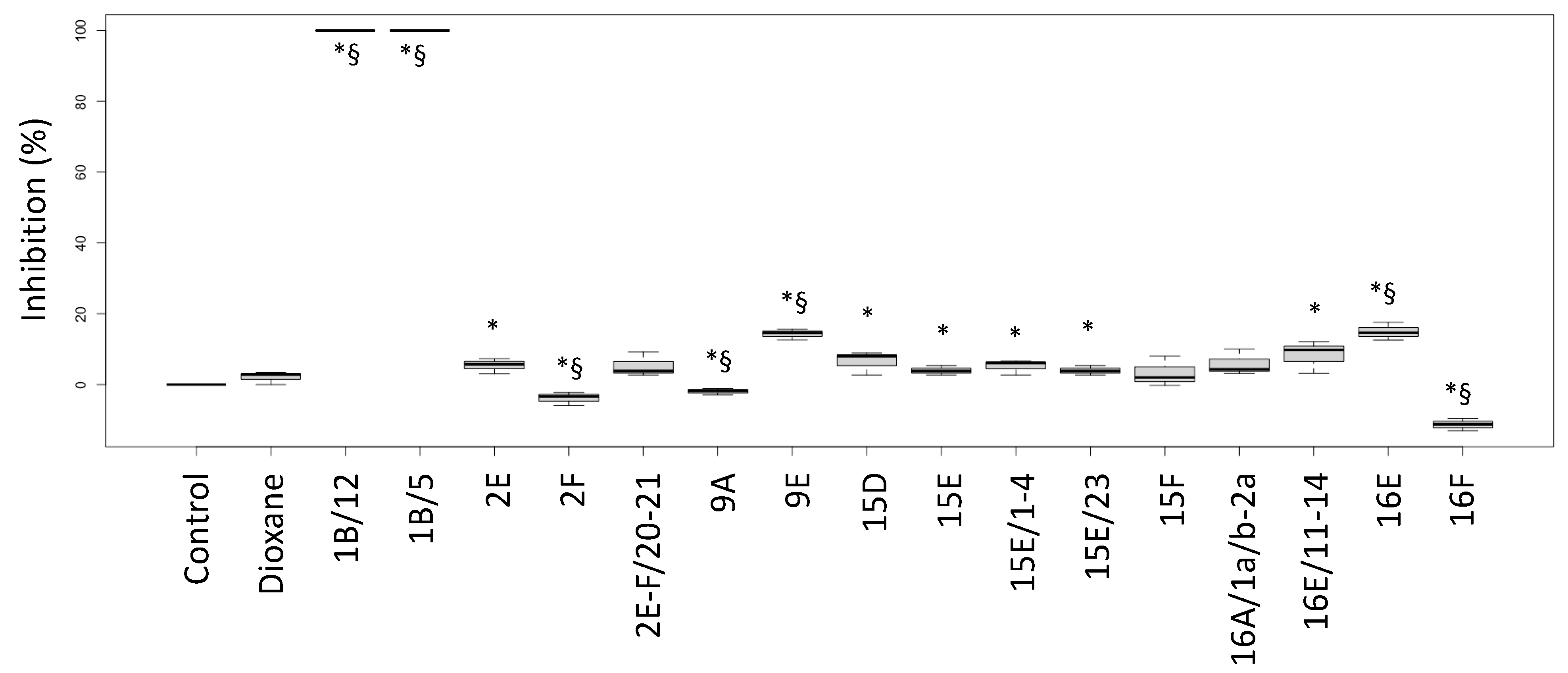
2.1. Biological Activities of Fractions
2.2. Chromatographic Separation and Chemical Characterization of Active Fractions
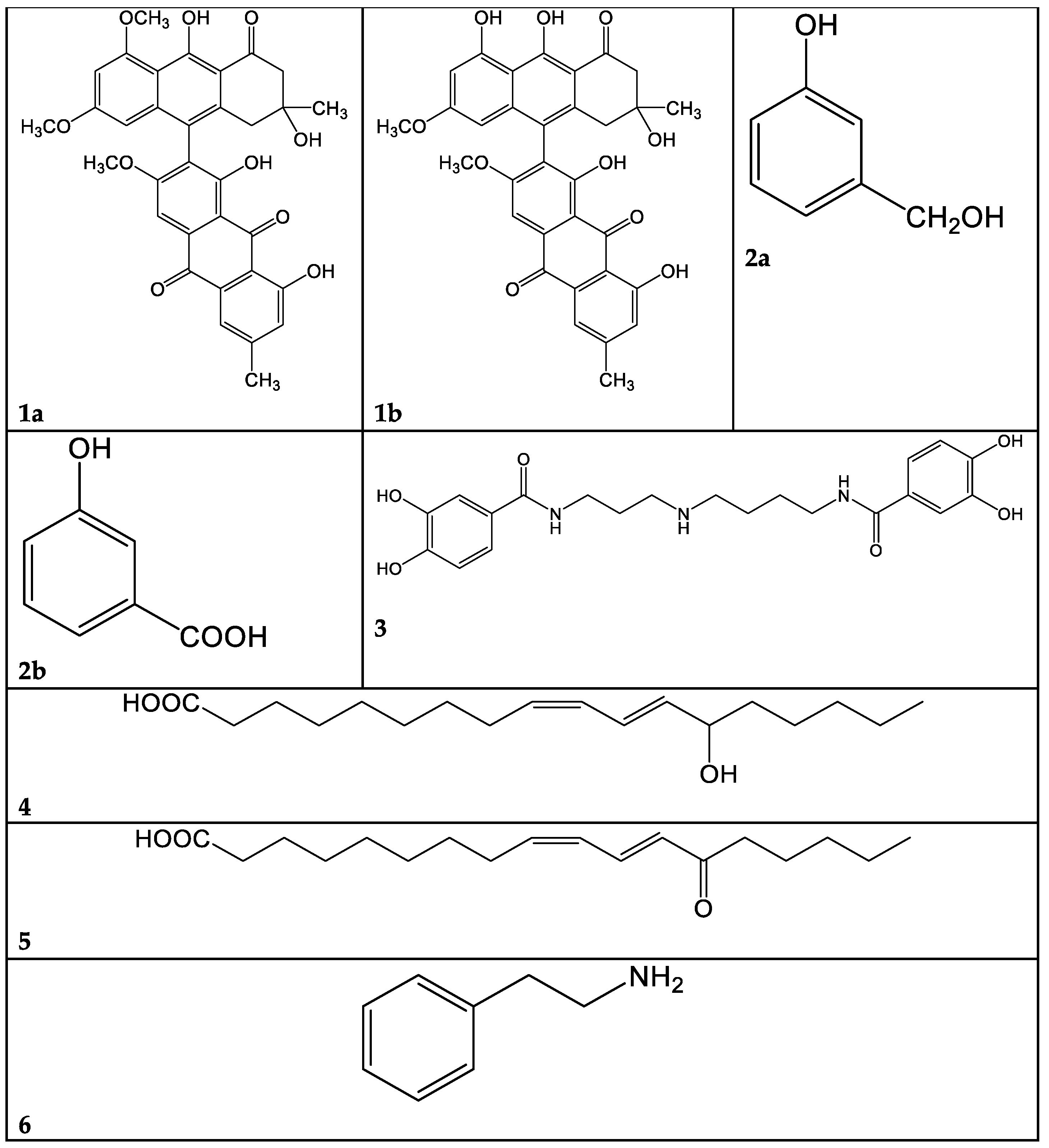
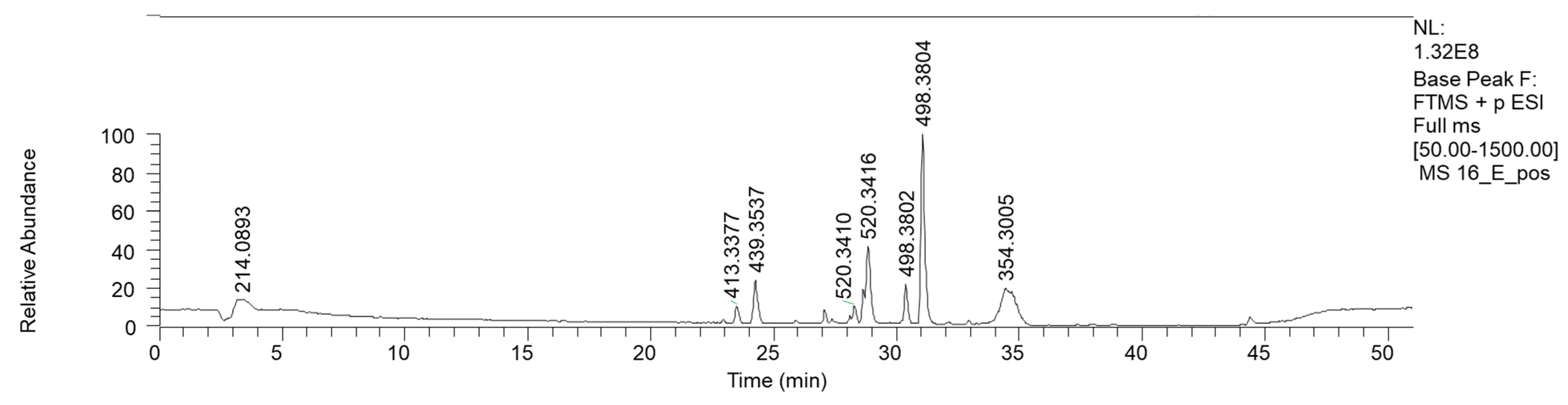
2.2.1. Cortinarius mussivus (Species 1)
2.2.2. Cortinarius caesiocanescens (Species 2)
2.2.3. Cortinarius variicolor (Species 3)
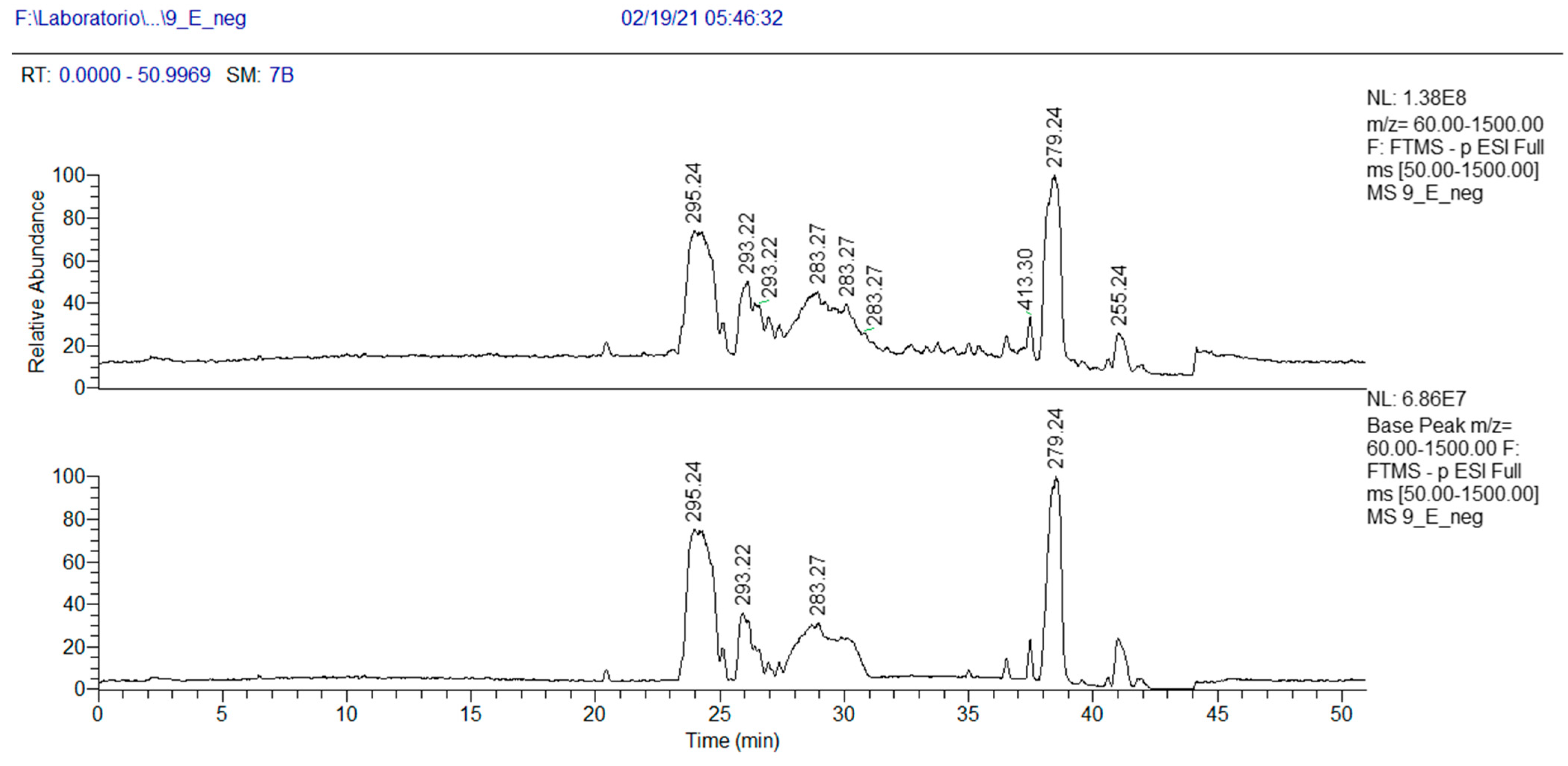
2.2.4. Ramaria parabotrytits (Species 9)
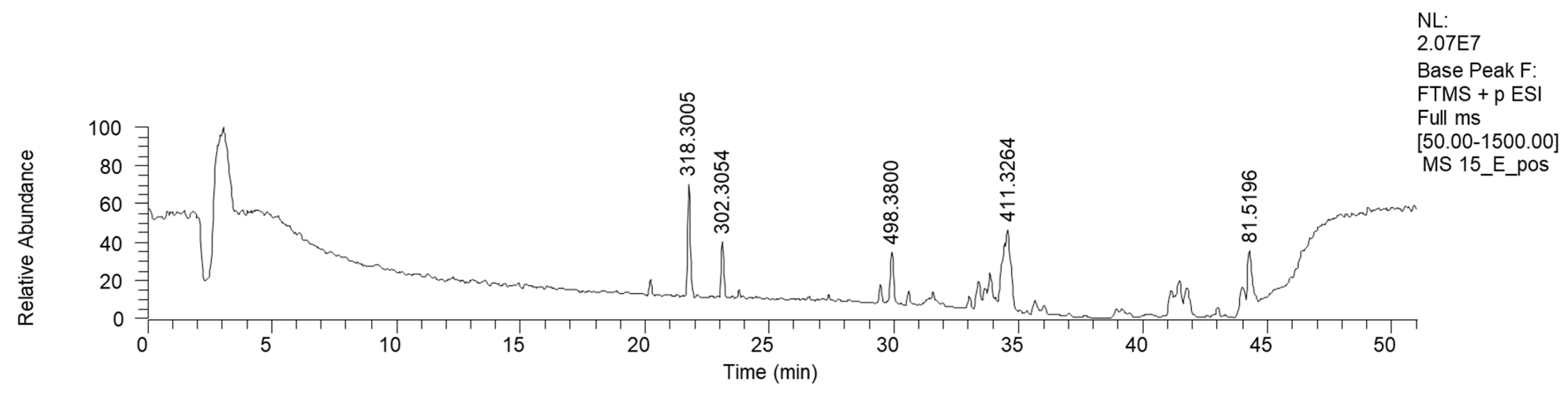
2.2.5. Mycena renati (15)
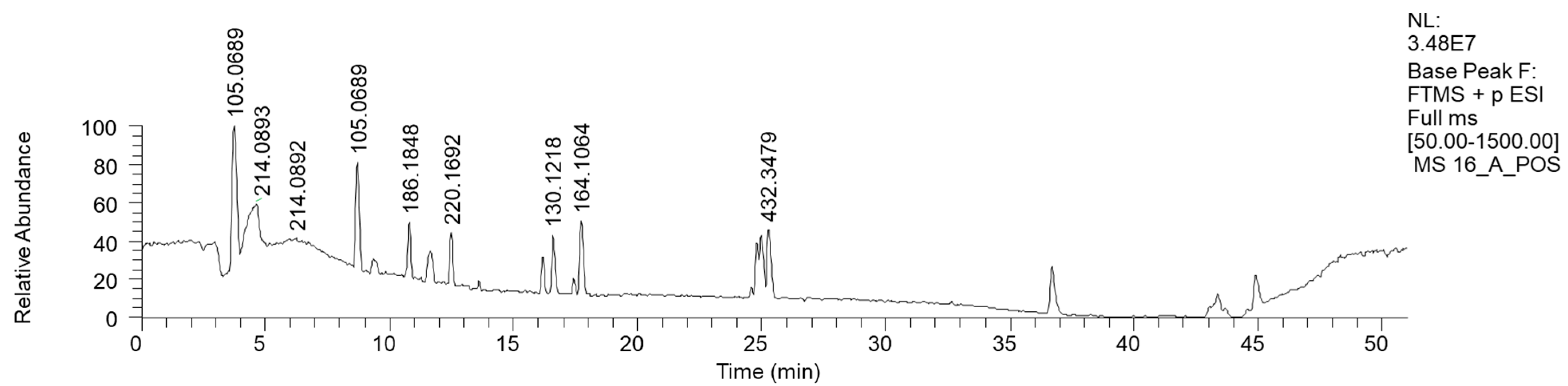
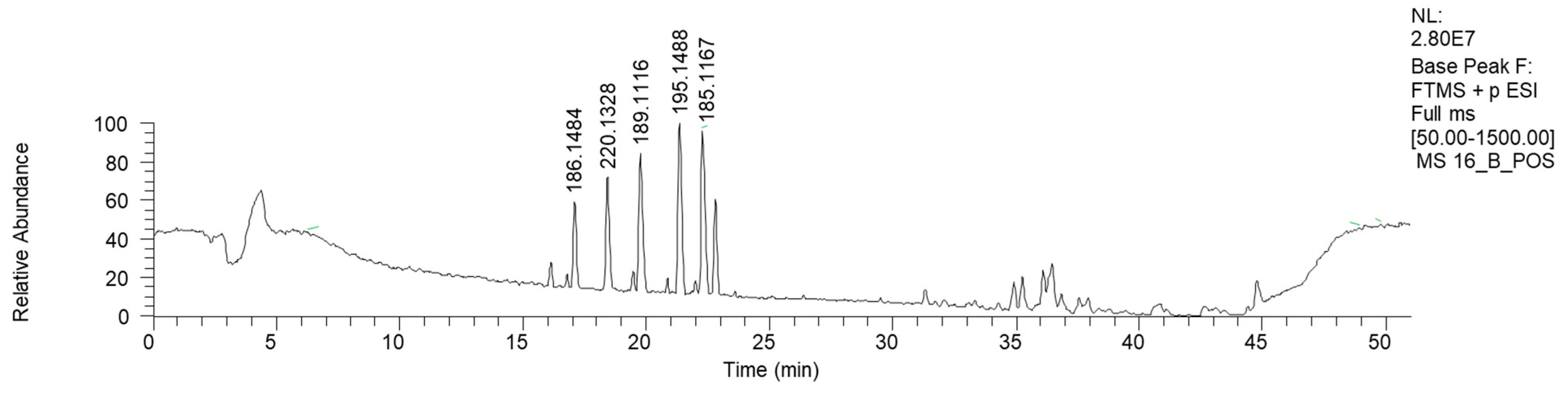
2.2.6. Mycena zephyrus (16)
3. Materials and Methods
3.1. Reagents
3.2. Analytical Sample Preparation
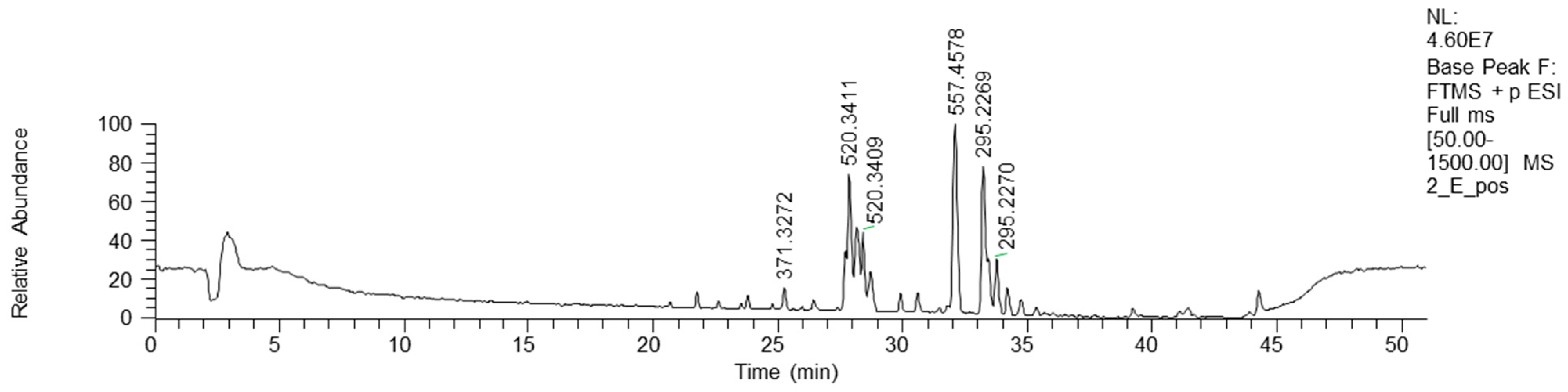
3.3. FIA and HPLC-HRMS Analysis
3.4. HPLC-HRMS Data Analysis
3.5. Preparative Liquid Chromatography
3.6. NMR Spectroscopy
3.7. Antibacterial Assays and Siderophore Production Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect Med. Chem 2014, 6, PMC.S14459. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence Factors of Pseudomonas Aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.; Forbes, A.; Perkins, A.; Davey, A.; Chess-Williams, R.; Kiefel, M.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas Aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Bivona, M.; Gamalero, E.; Bona, E.; Novello, G.; Massa, N.; Dovana, F.; Marengo, E.; Robotti, E. A Systematic Study of the Antibacterial Activity of Basidiomycota Crude Extracts. Antibiotics 2021, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Hussain, F.H.S.; Amin, H.I.M.; Bona, E.; Gamalero, E.; Giorgia, N.; Lappano, R.; Talia, M.; Maggiolini, M.; Bazzicalupo, M.; et al. Cytotoxic, Anti-Bacterial, and Wound-Healing Activity of Prenylated Phenols from the Kurdish Traditional Medicinal Plant Onobrychis Carduchorum (Fabaceae). Planta Medica Int. Open 2020, 7, e106–e113. [Google Scholar] [CrossRef]
- Bona, E.; Arrais, A.; Gema, L.; Perotti, V.; Birti, B.; Massa, N.; Novello, G.; Gamalero, E. Chemical Composition and Antimycotic Activity of Six Essential Oils (Cumin, Fennel, Manuka, Sweet Orange, Cedar and Juniper) against Different Candida spp. Nat. Prod. Res. 2019, 35, 1–6. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Pavan, M.; Novello, G.; Massa, N.; Rocchetti, A.; Berta, G.; Gamalero, E. Sensitivity of Candida Albicans to Essential Oils: Are They an Alternative to Antifungal Agents? J. Appl. Microbiol. 2016, 121, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Massa, N.; Novello, G.; Pavan, M.; Rocchetti, A.; Berta, G.; Gamalero, E. Essential Oil Antibacterial Activity against Methicillin-Resistant and -Susceptible Staphylococcus Aureus Strains. Microbiol. Res. 2019, 10, 8331. [Google Scholar] [CrossRef]
- Karwehl, S.; Stadler, M. Exploitation of Fungal Biodiversity for Discovery of Novel Antibiotics. In How to Overcome the Antibiotic Crisis; Stadler, M., Dersch, P., Eds.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2016; Volume 398, pp. 303–338. ISBN 978-3-319-49282-7. [Google Scholar]
- Alves, M.; Ferreira, I.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Klančnik, A.; Megušar, P.; Sterniša, M.; Jeršek, B.; Bucar, F.; Smole Možina, S.; Kos, J.; Sabotič, J. Aqueous Extracts of Wild Mushrooms Show Antimicrobial and Antiadhesion Activities against Bacteria and Fungi: Bioactivity of Aqueous Extracts of Wild Mushrooms. Phytother. Res. 2017, 31, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Vallavan, V.; Krishnasamy, G.; Zin, N.M.; Abdul Latif, M. A Review on Antistaphylococcal Secondary Metabolites from Basidiomycetes. Molecules 2020, 25, 5848. [Google Scholar] [CrossRef] [PubMed]
- Suay, I.; Arenal, F.; Asensio, F.J.; Basilio, A.; Cabello, M.A.; Dıez, M.T.; Garcıa, J.B.; Gorrochategui, J.; Hernandez, P.; Pelaez, F.; et al. Screening of Basidiomycetes for Antimicrobial Activities. Antonie Van Leeuwenhoek 2000, 78, 11. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Maillard, M.; Keller, J.; Hostettmann, K. Screening of European Fungi for Antibacterial, Antifungal, Larvicidal, Molluscicidal, Antioxidant and Free-Radical Scavenging Activities and Subsequent Isolation of Bioactive Compounds. Pharm. Biol. 2002, 40, 518–525. [Google Scholar] [CrossRef]
- Gill, M.; Steglich, W. Pigments of Fungi (Macromycetes). In Progress in the Chemistry of Organic Natural Products; Herz, W., Ed.; Springer: New York, NY, USA, 1987. [Google Scholar] [CrossRef]
- Masi, M.; Evidente, A. Fungal Bioactive Anthraquinones and Analogues. Toxins 2020, 12, 714. [Google Scholar] [CrossRef] [PubMed]
- Steglich, W.; Topfer-Petersen, E.; Pils, I. Pigments of Fungi. XVI. Novel Phlegmacin Derivatives from Cortinarius Percomis (Agaricales). Z. Fuer Naturforschung Sect. C J. Biosci. 1973, 28, 354–355. [Google Scholar]
- Gill, M.; Smrdel, A.F.; Strauch, R.J.; Begley, M.J. Pigments of Fungi. Part 12. Structure and Absolute Stereochemistry of Antibiotic Tetrahydroanthraquinones from the Fungus Dermocybe Splendida Horak. X-ray Structure Determination of Austrocortirubin Phenylboronate and Austrocortilutein Acetonide. J. Chem. Soc. Perkin Trans. 1 Org. Bio-Org. Chem. 1990, 6, 1583–1592. [Google Scholar] [CrossRef]
- Wahab, N.S.A.; Chua, L.S. Partitioning Phytochemicals in Orthosiphon Aristatus Extract with Antioxidant and Antibacterial Properties. Biointerface Res. Appl. Chem. 2023, 13, 28. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of Lovastatin, γ-Aminobutyric Acid and Ergothioneine in Mushroom Fruiting Bodies and Mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse Role of γ-Aminobutyric Acid in Dynamic Plant Cell Responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. γ-Aminobutyric Acid and Related Amino Acids in Plant Immune Responses: Emerging Mechanisms of Action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Steglich, W.; Steffan, B.; Stroech, K.; Wolf, M. Pistillarin a Characteristic Metabolite of Clavariadelphus Pistillaris and Several Ramaria Spp. (Basidiomycetes). Z. Fuer Naturforschung Sect. C J. Biosci. 1984, 39, 10–12. [Google Scholar] [CrossRef]
- Choomuenwai, V.; Schwartz, B.D.; Beattie, K.D.; Andrews, K.T.; Khokhar, S.; Davis, R.A. The Discovery, Synthesis and Antimalarial Evaluation of Natural Product-Based Polyamine Alkaloids. Tetrahedron Lett. 2013, 54, 5188–5191. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Hang, D.T.T.; Van Minh, C.; Dat, N.T. Anti-Inflammatory Effects of Fatty Acids Isolated from Chromolaena Odorata. Asian Pac. J. Trop. Med. 2011, 4, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Mundt, S.; Kreitlow, S.; Jansen, R. Fatty Acids with Antibacterial Activity from the Cyanobacterium Oscillatoria Redekei HUB 051. J. Appl. Phycol. 2003, 15, 263–267. [Google Scholar] [CrossRef]
- Ko, Y.-C.; Choi, H.S.; Kim, J.-H.; Kim, S.-L.; Yun, B.-S.; Lee, D.-S. Coriolic Acid (13-(S)-Hydroxy-9Z, 11E-Octadecadienoic Acid) from Glasswort (Salicornia herbacea L.) Suppresses Breast Cancer Stem Cell through the Regulation of c-Myc. Molecules 2020, 25, 4950. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, S.; Weber, H.; Stolz, S.; Chételat, A.; Farmer, E.E. Fatty Acid Ketodienes and Fatty Acid Ketotrienes: Michael Addition Acceptors That Accumulate in Wounded and Diseased Arabidopsis Leaves. Plant J. 2000, 24, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Jennemann, R.; Geyer, R.; Sandhoff, R.; Gschwind, R.M.; Levery, S.B.; Gröne, H.; Wiegandt, H. Glycoinositolphosphosphingolipids (Basidiolipids) of Higher Mushrooms. Eur. J. Biochem. 2001, 268, 1190–1205. [Google Scholar] [CrossRef] [PubMed]
- Niedens, B.R.; Parker, S.R.; Stierle, D.B.; Stierle, A.A. First Fungal Aromatic L-Amino Acid Decarboxylase from a Paclitaxel-Producing Penicillium Raistrickii. Mycologia 1999, 91, 619–626. [Google Scholar] [CrossRef]
- Heim, R. Les Champignons Toxiques et Hallucinogènes; Boubée: Paris, France, 1963. [Google Scholar]
- Stadelmann, R.J.; Eugster, C.H.; Müller, E. Über die Verbreitung der stereomeren Muscarine innerhalb der Ordnung der Agaricales. Helv. Chim. Acta 1976, 59, 2432–2436. [Google Scholar] [CrossRef]
- Aronsen, A.; Læssøe, T. The Genus Mycena s.l. In The Fungi of Northern Europe; Danish Mycological Society: Copenhagen, Denmark, 2016; Volume 5. [Google Scholar]
- Peters, S.; Spiteller, P. Sanguinones A and B, Blue Pyrroloquinoline Alkaloids from the Fruiting Bodies of the Mushroom Mycena sanguinolenta. J. Nat. Prod. 2007, 70, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Jaeger, R.J.R.; Spiteller, P. Red Pyrroloquinoline Alkaloids from the Mushroom Mycena haematopus. Eur. J. Org. Chem. 2008, 2008, 319–323. [Google Scholar] [CrossRef]
- Pulte, A.; Wagner, S.; Kogler, H.; Spiteller, P. Pelianthinarubins A and B, Red Pyrroloquinoline Alkaloids from the Fruiting Bodies of the Mushroom Mycena pelianthina. J. Nat. Prod. 2016, 79, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, J.S.; Wagner, S.; von Nussbaum, M.; Pulte, A.; Steglich, W.; Spiteller, P. Mycenaflavin A, B, C, and D: Pyrroloquinoline Alkaloids from the Fruiting Bodies of the Mushroom Mycena haematopus. Chem. Eur. J. 2018, 24, 8609–8614. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, R.J.R.; Spiteller, P. Mycenaaurin A, an Antibacterial Polyene Pigment from the Fruiting Bodies of Mycena Aurantiomarginata. J. Nat. Prod. 2010, 73, 1350–1354. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality Control for Plant Metabolomics: Reporting MSI-compliant Studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J. Universal Chemical Assay for the Detection and Determination of Siderophore. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- R Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
| Ion Formula | Exact m/z | Accurate m/z | Delta ppm | RDB |
|---|---|---|---|---|
| C33H29O10 | +585.1760 | +585.1764 | 1.27 | 19.5 |
| RT | Putative Compound | Ion Formula | Accurate (+/−Polarity) m/z | Exact m/z | Delta ppm | Fragments |
|---|---|---|---|---|---|---|
| 27.84 | LPC 18:2 isomer 1 | C26H51NO7P | (+) 520.3410 | 520.3421 | −2.1 | 502.3308 (C26H49NO6P) 184.0724 (C5H15NO4P) 443.2571 (C23H40O6P) |
| 28.61 | LPC 18:2 isomer 2 | C26H51NO7P | (+) 520.3416 | 520.3421 | −1.0 | 502.3308 (C26H49NO6P) 184.0724 (C5H15NO4P) 443.2571 (C23H40O6P) |
| 31.99 | LPC 18:2 isomer 3 | C26H51NO7P | (+) 520.3416 | 520.3421 | −1.0 | 502.3308 (C26H49NO6P) 184.0724 (C5H15NO4P) 443.2571 (C23H40O6P) |
| 33.11 | 13-Oxooctadecadienoic acid Isomer 1 | C18H31O3 | (+) 295.2269 | 295.2269 | −0.01 | 277.2165 (C18H29O2) 221.1534 (C14H21O2) |
| 34.0 | 13-Oxo-octadecadienoic acid Isomer 2 | C18H30O3 | (+) 295.2270 | 295.2269 | −1.4 | 277.2165 (C18H29O2) 221.1534 (C14H21O2) |
| Proposed Name | Ion Formula | Exact m/z | Accurate m/z | Delta ppm | RDB |
|---|---|---|---|---|---|
| Pistillarin | +C21H28O6N3 −C21H26O6N3 | +418.1945 −416.1789 | +418.1966 −416.1809 | +1.61 −2.26 | 10.5 |
| RT | Putative Compound | Ion Formula | Accurate (+/−Polarity) m/z | Exact m/z | Delta ppm | Fragments |
|---|---|---|---|---|---|---|
| 23.3 | Oxo-octadecadienoic acid Isomer 1 | C18H31O3 | (+)295.2267 | 295.2269 | 0.43 | 277.2165 (C18H29O2) 221.1534 (C14H21O2) |
| 24.5 | Oxo-octadecadienoic acid Isomer 2 | C18H31O3 | (+) 295.2267 | 295.2269 | 0.43 | 277.2165 (C18H29O2) 221.1534 (C14H21O2) |
| 25.9 | Oxo-octadecadienoic acid Isomer 3 | C18H31O3 | (+) 295.2267 | 295.2269 | 0.43 | 277.2165 (C18H29O2) 221.1534 (C14H21O2) |
| 27.8 | LPC 18:2 isomer 1 | C26H51NO7P | (+) 520.3397 | 520.3406 | 2.0 | 502.3308 (C26H49NO6P) 184.0724 (C5H15NO4P) 443.2571 (C23H40O6P) |
| 28.3 | LPC 18:2 isomer 2 | C26H51NO7P | (+) 520.3397 | 520.3406 | 2.0 | 502.3308 (C26H49NO6P) 184.0724 (C5H15NO4P) 443.2571 (C23H40O6P) |
| 32.0 | Linolenic Acid | C18H31O2 | (−) 279.2318 | 279.2320 | 0.37 | 261.2207 (C18H29O) 243.2110 (C18H2) |
| 35.7 | Coriolic acid | +C18H33O3 −C18H31O3 | (+) 297.2424 (−) 295.2267 | 297.2425 | 0.23 | 279.2314 (C18H31O2) 183.1373 (C11H19O2) |
| RT | Putative Compound | Ion Formula | Accurate (+/−Polarity) m/z | Exact m/z | Delta ppm | Fragments |
|---|---|---|---|---|---|---|
| 21.67 | Phytosphingosine | C18H40NO3 | (+) 318.3005 | 318.3003 | 0.721 | 300.2896 (C18H40NO2) 270.2790 (C17H36NO) 265.2525 (C18H33O) |
| 23.18 | Saginfol | C18H40NO2 | (+) 302.3051 | 302.3054 | −0.847 | 284.2944 (C18H38NO) 266.2843 (C18H36N) 240.2677 (C16H34N) |
| RT | Putative Compound | Ion Formula | Accurate (+/−Polarity) m/z | Exact m/z | Delta ppm | Fragments |
|---|---|---|---|---|---|---|
| 8.01 | Phenethyl amine | C8H12N | (+) 122.0956 | 122.0964 | 6.7 | 105.0690 (C8H8) 79.0352 (C6H6) |
| 10.36 | Propanamide, N, N-dibutyl- | C11H24NO | (+) 186.1848 | 186.1852 | 2.3 | n.d. |
| 12.29 | 4-[[(1S)-1-phenylethyl-amino hexan-2-one | C14H22NO | (+) 220.1692 | 220.1696 | −1.7 | 162.1271 (C11H15N) 105.0691 (C8H8) |
| 16.58 | Heptanamide | C7H16NO | (+) 130.1218 | 130.1226 | −6.5 | 60.0434 (C7H15NO) |
| 17.51 | N-(2-Phenylethyl) acetamide | C10H14NO | (+) 164.1064 | 164.1070 | −3.6 | 105.0690 (C8H8) |
| 25.1 | N-Pentacosa-10,12-diynoylglycine | C27H46NO3 | (+) 432.3479 | 432.3472 | 1.6 | 290.2121 (C18H27O2) 246.2216 (C17H27N) 232.2053 (C16H25N) |
| RT | Putative Compound | Formula | Accurate (+/−Polarity) m/z | Exact m/z | Delta ppm | Fragments |
|---|---|---|---|---|---|---|
| 16.7 | (3R)-3-[(2R)-piperidin-2-yl] pentanoic acid | C10H20NO2 | (+) 186.1484 | 186.1489 | −2.4 | 168.1379 (C10H18NO) 71.0846 (C5H11) |
| 18.6 | 4-(1-Phenylprop-2-enylamino) butanoic acid | C13H18NO2 | (+) 220.1328 | 220.1332 | −1.8 | 122.0957 (C8H12N) 105.0689 (C8H10) |
| 19.62 | Monomethyl suberate | C9H17O4 | (+) 189.1116 | 189.1121 | −2.8 | 171.1006 (C9H15O3) 139.0746 (C8H11O2) |
| 21.35 | 2-(2-Oxo-1-propan-2-yl piperidin-3-yl) propanenitrile | C11H19N2O | (+) 195.1488 | 195.1492 | −2.0 | 125.1063 (C7H13N2) 180.1247 (C10H16N2O) 167.1538 (C10H19N2) |
| 22.22 | Monomethyl azelate | C10H17O3 | (+) 185.1167 | 203.1278 | 0.01 | 153.0903 (C9H13O2) 125.0952 (C8H13O) 135.0797 (C9H11O) 107.0846 (C8H11) |
| RT | Putative Compound | Formula | Accurate (+/−Polarity) m/z | Exact m/z | Delta ppm | Fragments |
|---|---|---|---|---|---|---|
| 23.5 | Methyl 2,6-bis(octanoylamino)hexanoate | C23H45N2O4 | (+) 413.3377 | 413.3373 | −0.2 | 310.2739 (C19H36NO2) 176.0915 (C7H14NO4) |
| 24.04 | 2-[[(Z)-13-[heptanoyl(propan-2-yl) amino] tridec-8-enoyl] amino]acetic acid | C25H47N2O4 | (+) 439.3537 | 439.3530 | 1.5 | 379.3330 (C23H43N2O2) 338.3060 (C21H34NO2) 336.2890 (C21H38NO2) |
| 27.84 | LPC 18:2 isomer 1 | C26H51NO7P | (+) 520.3410 | 520.3421 | −2.1 | 502.3308 (C26H49NO6P) 184.0724 (C5H15NO4P) 443.2571 (C23H40O6P) |
| 28.36 | LPC 18:2 isomer 2 | C26H51NO7P | (+) 520.3416 | 520.3421 | −1.0 | 502.3308 (C26H49NO6P) 184.0724 (C5H15NO4P) 443.2571 (C23H40O6P) |
| 34.3 | 2-(Linoleylamino)-1,3-propanediol | C21H40NO3 | (+) 354.3005 | 354.3002 | 0.64 | 336.2892 (C21H35O2) 175.1475 (C13H19) |
| Fraction Eluent | H2O | MeOH | Acetonitrile | Acetone |
|---|---|---|---|---|
| A | 75 | 25 | 0 | 0 |
| B | 45 | 55 | 0 | 0 |
| C | 30 | 70 | 0 | 0 |
| D | 15 | 85 | 0 | 0 |
| E | 0 | 0 | 100 | 0 |
| F | 0 | 0 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clericuzio, M.; Novello, G.; Bivona, M.; Gamalero, E.; Bona, E.; Caramaschi, A.; Massa, N.; Asteggiano, A.; Medana, C. A Study of Metabolites from Basidiomycota and Their Activities against Pseudomonas aeruginosa. Antibiotics 2024, 13, 326. https://doi.org/10.3390/antibiotics13040326
Clericuzio M, Novello G, Bivona M, Gamalero E, Bona E, Caramaschi A, Massa N, Asteggiano A, Medana C. A Study of Metabolites from Basidiomycota and Their Activities against Pseudomonas aeruginosa. Antibiotics. 2024; 13(4):326. https://doi.org/10.3390/antibiotics13040326
Chicago/Turabian StyleClericuzio, Marco, Giorgia Novello, Mattia Bivona, Elisa Gamalero, Elisa Bona, Alice Caramaschi, Nadia Massa, Alberto Asteggiano, and Claudio Medana. 2024. "A Study of Metabolites from Basidiomycota and Their Activities against Pseudomonas aeruginosa" Antibiotics 13, no. 4: 326. https://doi.org/10.3390/antibiotics13040326
APA StyleClericuzio, M., Novello, G., Bivona, M., Gamalero, E., Bona, E., Caramaschi, A., Massa, N., Asteggiano, A., & Medana, C. (2024). A Study of Metabolites from Basidiomycota and Their Activities against Pseudomonas aeruginosa. Antibiotics, 13(4), 326. https://doi.org/10.3390/antibiotics13040326











