Dalbavancin for Acute Bacterial Skin and Skin Structure Infections in Pediatrics: Insights from Continuation Therapy Experience
Abstract
1. Introduction
2. Cases Presentation
2.1. Patient #1
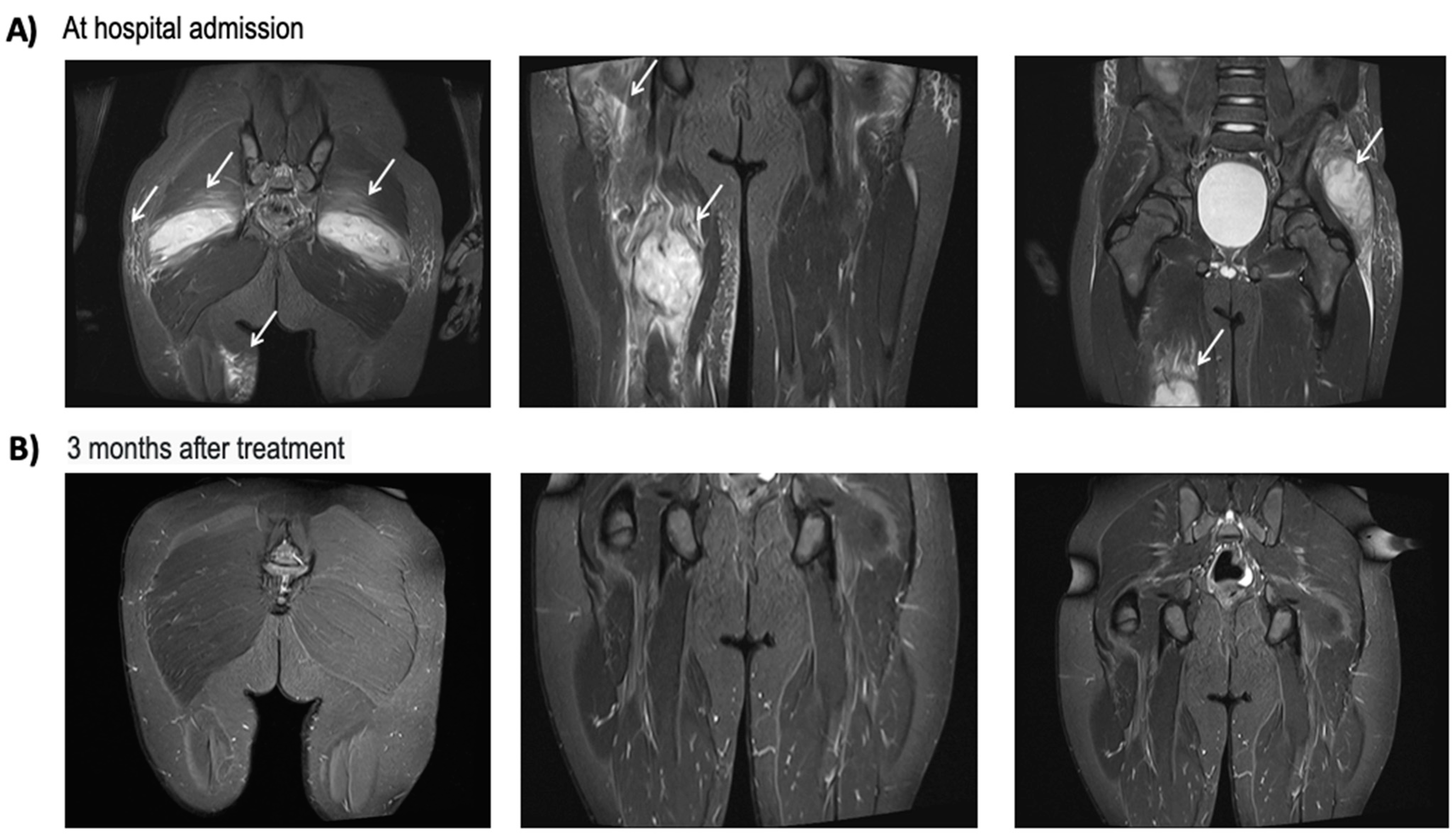
2.2. Patient #2
2.3. Patient #3
2.4. Patient #4
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, S.; Bassetti, M.; Concia, E.; De Simone, G.; De Rosa, F.G.; Grossi, P.; Novelli, A.; Menichetti, F.; Petrosillo, N.; Tinelli, M.; et al. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: An update. J. Chemother. 2017, 29, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Daum, R.S.; Creech, C.B.; Young, D.; Downing, M.D.; Eells, S.J.; Pettibone, S.; Hoagland, R.J.; Chambers, H.F.; DMID 07-0051 Team. Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N. Engl. J. Med. 2015, 372, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.D. Skin and soft tissue infections. Pediatr. Clin. N. Am. 2013, 60, 1063–1082. [Google Scholar] [CrossRef] [PubMed]
- Dukic, V.M.; Lauderdale, D.S.; Wilder, J.; Daum, R.S.; David, M.Z. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: A meta-analysis. PLoS ONE 2013, 8, e52722. [Google Scholar] [CrossRef] [PubMed]
- Bamford, A.; Whittaker, E. Resurgence of group A streptococcal disease in children. BMJ 2023, 380, 43. [Google Scholar] [CrossRef] [PubMed]
- Eurpean Medical Agency. Xydalba (dalbavancin). Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/xydalba-epar-product-information_en.pdf (accessed on 26 March 2024).
- Pfaller, M.A.; Mendes, R.E.; Duncan, L.R.; Flamm, R.K.; Sader, H.S. Activity of dalbavancin and comparator agents against Gram-positive cocci from clinical infections in the USA and Europe 2015–16. J. Antimicrob. Chemother. 2018, 73, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Sivori, F.; Cavallo, I.; Kovacs, D.; Guembe, M.; Sperduti, I.; Truglio, M.; Pasqua, M.; Prignano, G.; Mastrofrancesco, A.; Toma, L.; et al. Role of Extracellular DNA in Dalbavancin Activity against Methicillin-Resistant Staphylococcus aureus (MRSA) Biofilms in Patients with Skin and Soft Tissue Infections. Microbiol. Spectr. 2022, 10, e0035122. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Stefani, S.; Venditti, M.; Di Domenico, E.G. Biofilm-Related Infections in Gram-Positive Bacteria and the Potential Role of the Long-Acting Agent Dalbavancin. Front. Microbiol. 2021, 12, 749685. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Bradley, J.S.; Blumer, J.; Yogev, R.; Watt, K.M.; James, L.P.; Palazzi, D.L.; Bhatt-Mehta, V.; Sullivan, J.E.; Zhang, L.; et al. Dalbavancin Pharmacokinetics and Safety in Children 3 Months to 11 Years of Age. Pediatr. Infect. Dis. J. 2017, 36, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Giorgobiani, M.; Burroughs, M.H.; Antadze, T.; Carrothers, T.J.; Riccobene, T.A.; Patel, R.; Lin, T.; Stefanova, P. The Safety and Efficacy of Dalbavancin and Active Comparator in Pediatric Patients with Acute Bacterial Skin and Skin Structure Infections. Pediatr. Infect. Dis. J. 2023, 42, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Teran, C.G.; Sura, S.; Mohamed, T.; Lin, T.; Meadows, M.; Cynthia, D.; Wong, S.H. Current role of community-acquired methicillin-resistant Staphylococcus aureus among children with skin and soft tissue infections. Pediatr. Rep. 2012, 4, e5. [Google Scholar] [CrossRef] [PubMed]
- Regional Committee for Antimicrobial Stewardship and Antibiotic Resistances. Regional Surveillance Report for Antibiotic Resistances Rapporto 2019 sull’antibiotico resistenza e sull’uso di antibiotici rilevati nelle strutture pubbliche del Sistema Sanitario della Campania. Assessorato alla sanità della Regione Campania. Direzione Generale per la Tutela della Salute ed il Coordinamento del Sistema Sanitario Regionale. Available at the official website of Regione Campania–Italy. Available online: https://www.regione.campania.it/assets/documents/report-2019-sull-antibotico-resistenza-8rjc6720un7pwyo7.pdf (accessed on 26 March 2024).
- Zhang, C.; Guo, Y.; Chu, X. In Vitro generation of Panton-Valentine leukocidin (PVL) in clinical Methicillin-Resistant Staphylococcus aureus (MRSA) and its correlation with PVL variant, clonal complex, infection type /692/308/174/692/308/409 article. Sci. Rep. 2018, 8, 7696. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Gould, I.; Esposito, S.; Ahmad-Saeed, N.; Ahmed, S.S.; Alp, E.; Bal, A.M.; Bassetti, M.; Bonnet, E.; Chan, M.; et al. Panton-Valentine leukocidin-positive Staphylococcus aureus: A position statement from the International Society of Chemotherapy. Int. J. Antimicrob. Agents 2018, 51, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wu, P.H.; Lu, M.C.; Ho, M.W.; Hsueh, P.R.; SMART Program Study Group. National surveillance of antimicrobial susceptibilities to ceftaroline, dalbavancin, telavancin, tedizolid, eravacycline, omadacycline, and other comparator antibiotics, and genetic characteristics of bacteremic Staphylococcus aureus isolates in adults: Results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) program in 2020. Int. J. Antimicrob. Agents 2023, 61, 106745. [Google Scholar] [PubMed]
- Gatti, M.; Andreoni, M.; Pea, F.; Viale, P. Real-World Use of Dalbavancin in the Era of Empowerment of Outpatient Antimicrobial Treatment: A Careful Appraisal beyond Approved Indications Focusing on Unmet Clinical Needs. Drug Des. Devel. Ther. 2021, 15, 3349–3378. [Google Scholar] [CrossRef] [PubMed]
- Caselli, D.; Mariani, M.; Colomba, C.; Ferrecchi, C.; Cafagno, C.; Trotta, D.; Carloni, I.; Dibello, D.; Castagnola, E.; Aricò, M. Real-World Use of Dalbavancin for Treatment of Soft Tissue and Bone Infection in Children: Safe, Effective and Hospital-Time Sparing. Children 2024, 11, 78. [Google Scholar] [CrossRef] [PubMed]
| Patient #1 | Patient #2 | Patient #3 | Patient #4 | |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age (years) | 4 | 9 | 2 | 2 |
| Underlying conditions | Autism spectrum disorder | Congenital factor VII deficiency | None | Atopic dermatitis |
| WBC at admission (103/mm3) | 12.95 | 24.58 | 12.72 | 31.66 |
| CRP at admission (mg/L) | 71.0 | 221.7 | 72.3 | 77.2 |
| Indication for antibiotic treatment | Cellulitis | Muscle abscesses | Cellulitis | Cellulitis |
| Disease localization | Right calf | Thigh and gluteal region | Chest | Right lower limb |
| Microbiological evaluation | ||||
| Blood culture or NAAT * | Negative | Negative | Negative | Negative |
| Nasal swab | Negative | NP | Negative | NP |
| Lesion sample (aspiration, evacuation) | NP | Positive | Positive | NP |
| Bacterial isolation | NP | PVL-MRSA | PVL-MRSA | NP |
| Primary antibiotic treatment | Clindamycin | Linezolid+ rifampicin | Clindamycin | Clindamycin |
| Dalbavancin administration | ||||
| Dosage (mg/kg) | 22.5 | 18 | 22.5 | 22.5 |
| Number of doses | 1 | 1 | 1 | 1 |
| Time from hospitalization (weeks) | 1 | 3 | 1 | 1 |
| Reason for dalbavancin administration | - Poor acceptance of hospital stay - Poor acceptance of venous access - Poor adherence to oral drugs | - Oral drugs available only as off-label therapy | - Poor adherence to oral drugs - Pediatric formulation not available | - Poor adherence to oral drugs - Pediatric formulation not available |
| WBC at dalbavancin infusion | 9.71 | 6.57 | 8.99 | 10.89 |
| CRP at dalbavancin infusion | 5.95 | 1.26 | 0.6 | 4.86 |
| Associated antibiotic treatment | None | Rifampin | Rifampin | None |
| Adverse events at administration | None | None | None | None |
| Adverse events after 2 months of follow-up | None | None | None | None |
| Clinical outcomes | Healed | Healed | Healed | Healed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, S.M.; Bruzzese, E.; Poeta, M.; Del Bene, M.; Guarino, A.; Lo Vecchio, A. Dalbavancin for Acute Bacterial Skin and Skin Structure Infections in Pediatrics: Insights from Continuation Therapy Experience. Antibiotics 2024, 13, 327. https://doi.org/10.3390/antibiotics13040327
Scarano SM, Bruzzese E, Poeta M, Del Bene M, Guarino A, Lo Vecchio A. Dalbavancin for Acute Bacterial Skin and Skin Structure Infections in Pediatrics: Insights from Continuation Therapy Experience. Antibiotics. 2024; 13(4):327. https://doi.org/10.3390/antibiotics13040327
Chicago/Turabian StyleScarano, Sara Maria, Eugenia Bruzzese, Marco Poeta, Margherita Del Bene, Alfredo Guarino, and Andrea Lo Vecchio. 2024. "Dalbavancin for Acute Bacterial Skin and Skin Structure Infections in Pediatrics: Insights from Continuation Therapy Experience" Antibiotics 13, no. 4: 327. https://doi.org/10.3390/antibiotics13040327
APA StyleScarano, S. M., Bruzzese, E., Poeta, M., Del Bene, M., Guarino, A., & Lo Vecchio, A. (2024). Dalbavancin for Acute Bacterial Skin and Skin Structure Infections in Pediatrics: Insights from Continuation Therapy Experience. Antibiotics, 13(4), 327. https://doi.org/10.3390/antibiotics13040327






