Insights into the Evolution of IncR Plasmids Found in the Southern European Clone of the Monophasic Variant of Salmonella enterica Serovar Typhimurium
Abstract
1. Introduction
2. Results
2.1. Origin and Typing of the Isolates Belonging to the Southern European Clone
2.2. Patterns of Antibiotic Resistance and Identification of the Responsible Genes
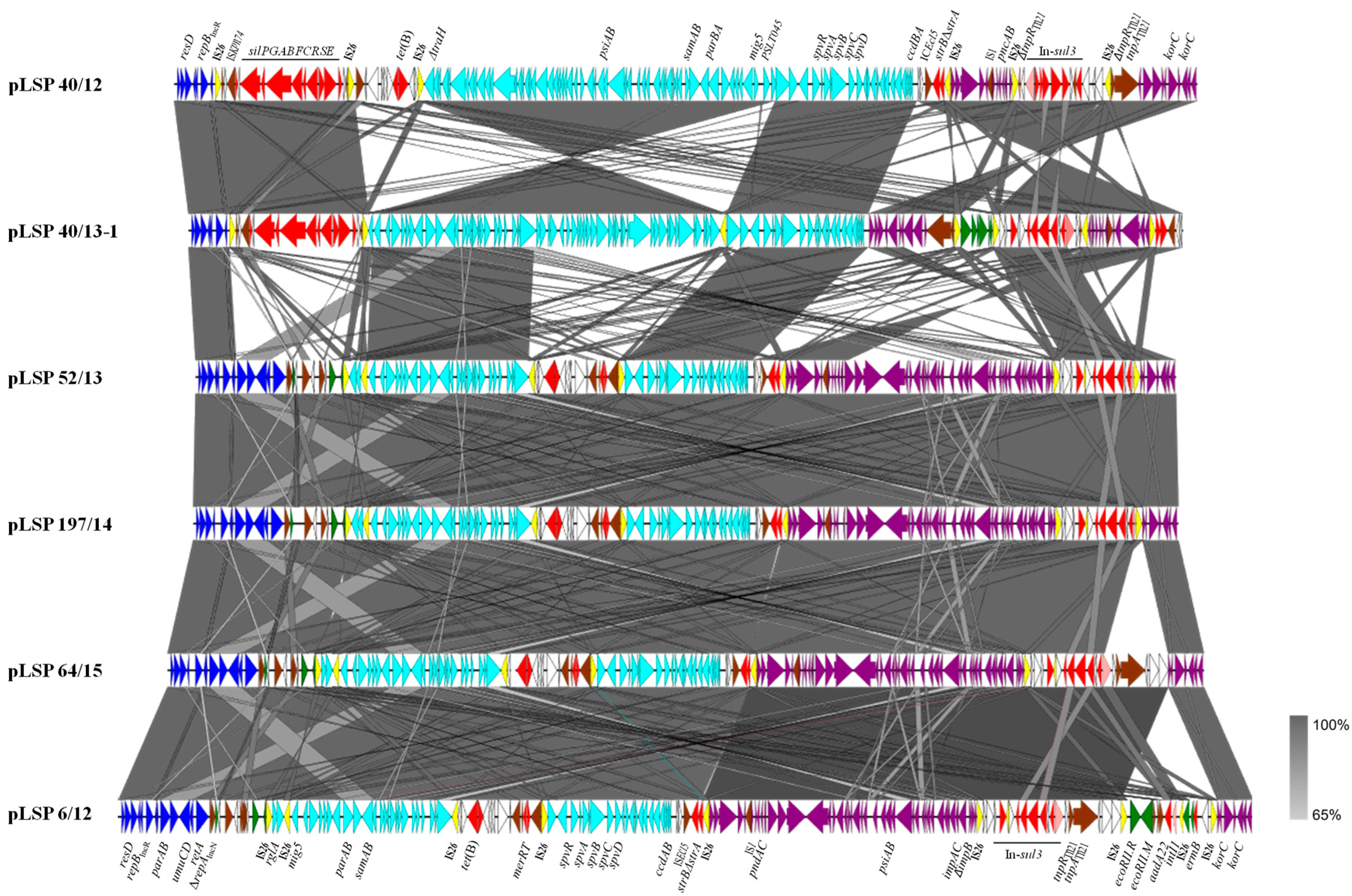
2.3. Plasmid Analysis
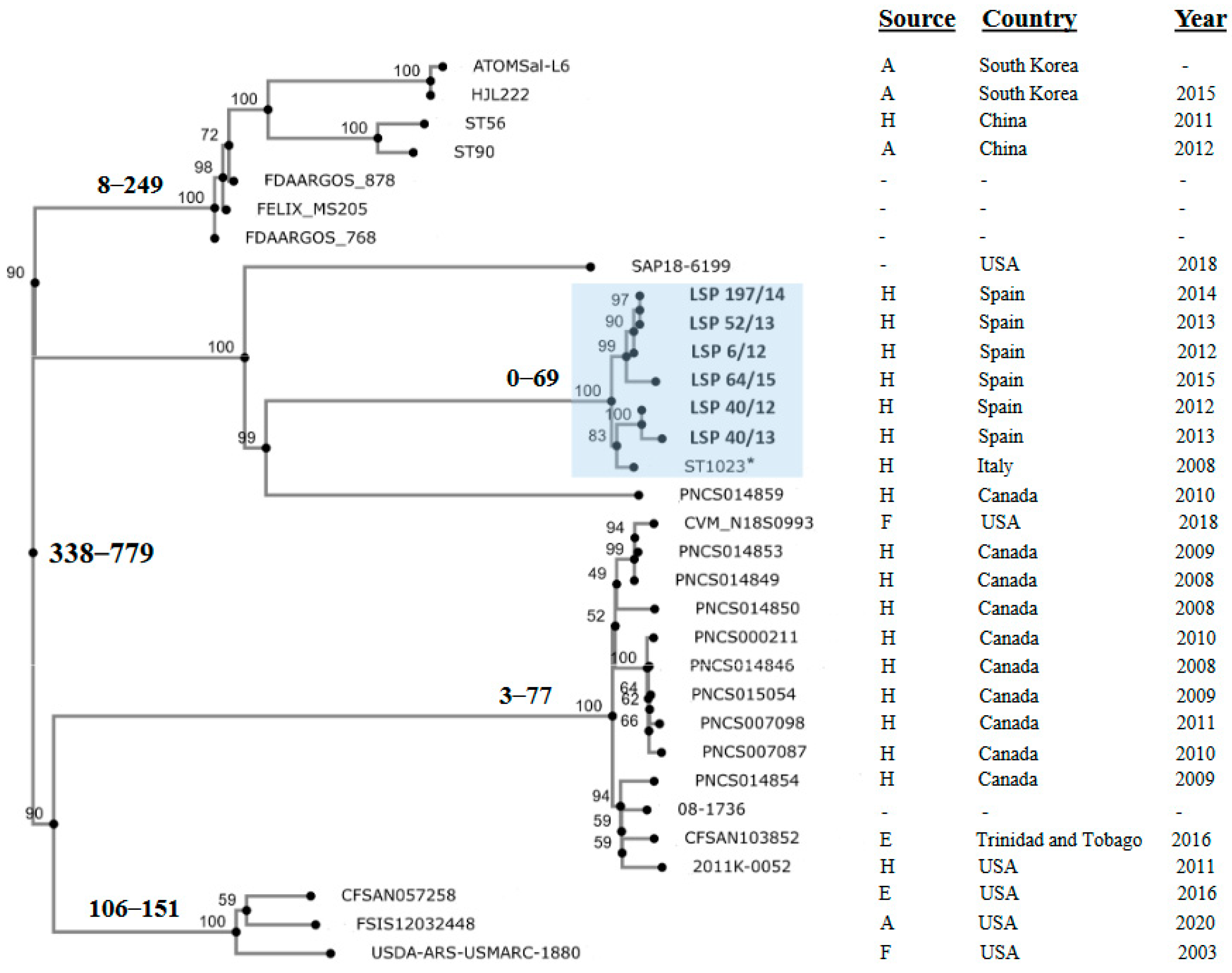
2.3.1. Comparative Analysis of the IncR Plasmids and Phylogenetic Relationships
2.3.2. The Second Resistance Plasmid of LSP 40/13
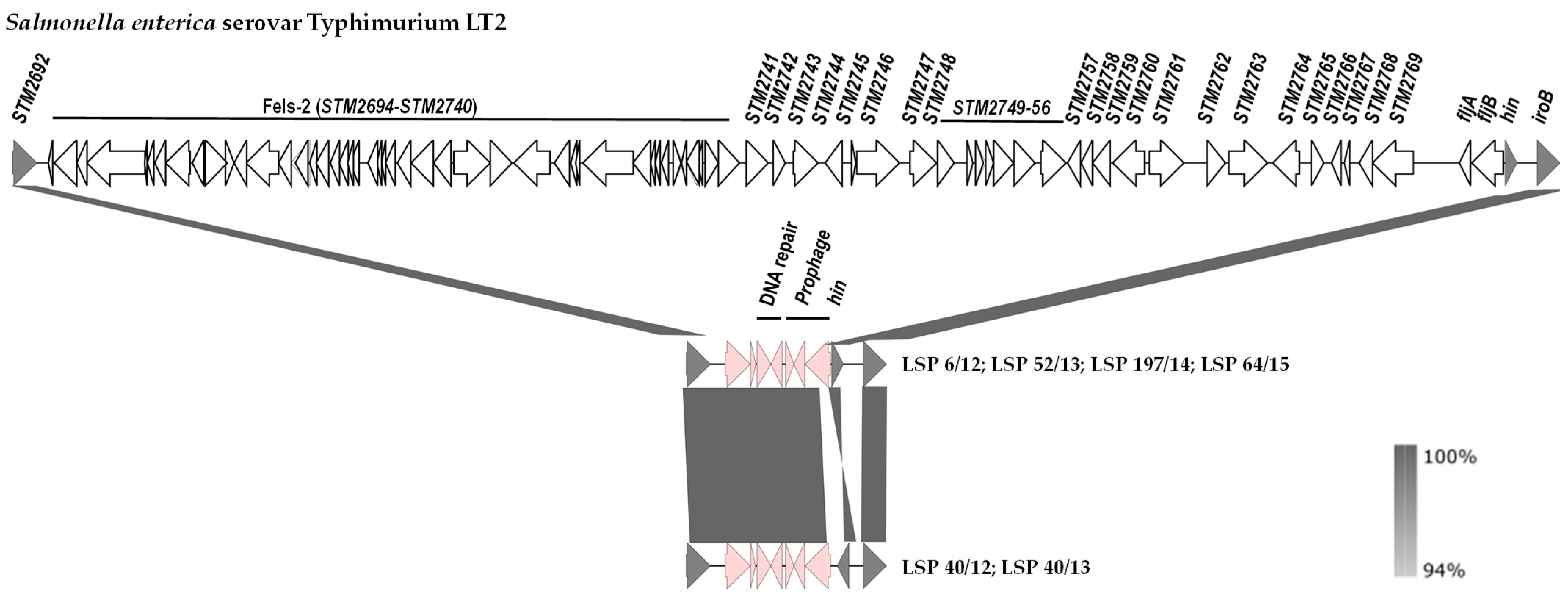
2.4. Genetic Basis of the Monophasic Phenotype and Phylogenetic Analysis
3. Materials and Methods
3.1. Isolate Selection
3.2. Whole Genome Sequencing, Assembly, Annotation and Bioinformatics Analysis
3.3. Phylogenetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease “Burden of Illness” Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Issenhuth-Jeanjean, S.; Roggentin, P.; Mikoleit, M.; Guibourdenche, M.; de Pinna, E.; Nair, S.; Fields, P.I.; Weill, F.X. Supplement 2008–2010 (no. 48) to the White-Kauffmann-Le Minor scheme. Res. Microbiol. 2014, 165, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.; Simon, M. Phase variation: Genetic analysis of switching mutants. Cell 1980, 19, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, A.M.; Graziani, C.; Lucarelli, C.; Filetici, E.; Villa, L.; Owczarek, S.; Caprioli, A.; Luzzi, I. Molecular characterization of multidrug-resistant strains of Salmonella enterica serotype Typhimurium and Monophasic variant (S. 4,[5],12:i:-) isolated from human infections in Italy. Foodborne Pathog. Dis. 2009, 6, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Echeita, M.A.; Herrera, S.; Usera, M.A. Atypical, fljB-negative Salmonella enterica subsp. enterica strain of serovar 4,5,12:i:- appears to be a monophasic variant of serovar Typhimurium. J. Clin. Microbiol. 2001, 39, 2981–2983. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, L.; Mather, A.E.; AbuOun, M.; Branchu, P.; Harris, S.R.; Connor, T.; Hopkins, K.L.; Underwood, A.; Lettini, A.A.; Page, A.; et al. Microevolution of monophasic Salmonella Typhimurium during epidemic, United Kingdom, 2005–2010. Emerg. Infect. Dis. 2016, 22, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wan, Y.; Du, P.; Bai, L. The epidemiology of monophasic Salmonella Typhimurium. Foodborne Pathog. Dis. 2020, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Switt, A.I.; Soyer, Y.; Warnick, L.D.; Wiedmann, M. Emergence, distribution, and molecular and phenotypic characteristics of Salmonella enterica serotype 4,5,12:i. Foodborne Pathog. Dis. 2009, 6, 407–415. [Google Scholar] [CrossRef]
- Zamperini, K.; Soni, V.; Waltman, D.; Sanchez, S.; Theriault, E.C.; Bray, J.; Maurer, J.J. Molecular characterization reveals Salmonella enterica serovar 4,[5],12:i:- from poultry is a variant Typhimurium serovar. Avian Dis. 2007, 51, 958–964. [Google Scholar] [CrossRef]
- Clark, C.G.; Landgraff, C.; Robertson, J.; Pollari, F.; Parker, S.; Nadon, C.; Gannon, V.P.J.; Johnson, R.; Nash, J. Distribution of heavy metal resistance elements in Canadian Salmonella 4,[5],12:i:- populations and association with the monophasic genotypes and phenotype. PLoS ONE 2020, 15, e0236436. [Google Scholar] [CrossRef] [PubMed]
- Echeita, M.A.; Aladuena, A.; Cruchaga, S.; Usera, M.A. Emergence and spread of an atypical Salmonella enterica subsp. enterica serotype 4,5,12:i:- strain in Spain. J. Clin. Microbiol. 1999, 37, 3425. [Google Scholar] [CrossRef] [PubMed]
- EFSA; EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on monitoring and assessment of the public health risk of “Salmonella Typhimurium-like” strains. EFSA J. 2010, 8, 1826. [Google Scholar] [CrossRef]
- Elnekave, E.; Hong, S.; Mather, A.E.; Boxrud, D.; Taylor, A.J.; Lappi, V.; Johnson, T.J.; Vannucci, F.; Davies, P.; Hedberg, C.; et al. Salmonella enterica serotype 4,[5],12:i:- in swine in the United States Midwest: An emerging multidrug-resistant clade. Clin. Infect. Dis. 2018, 66, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.L.; Kirchner, M.; Guerra, B.; Granier, S.A.; Lucarelli, C.; Porrero, M.C.; Jakubczak, A.; Threlfall, E.J.; Mevius, D.J. Multiresistant Salmonella enterica serovar 4,[5],12:i:- in Europe: A new pandemic strain? Euro Surveill. 2010, 15, 19580. [Google Scholar] [CrossRef]
- Soyer, Y.; Moreno Switt, A.; Davis, M.A.; Maurer, J.; McDonough, P.L.; Schoonmaker-Bopp, D.J.; Dumas, N.B.; Root, T.; Warnick, L.D.; Grohn, Y.T.; et al. Salmonella enterica serotype 4,5,12:i:-, an emerging Salmonella serotype that represents multiple distinct clones. J. Clin. Microbiol. 2009, 47, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Sekizuka, T.; Tamamura, Y.; Tanaka, K.; Barco, L.; Izumiya, H.; Kusumoto, M.; Hinenoya, A.; Yamasaki, S.; Iwata, T.; et al. Phylogenetic characterization of Salmonella enterica serovar Typhimurium and its monophasic variant isolated from food animals in Japan revealed replacement of major epidemic clones in the last 4 decades. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Agasan, A.; Kornblum, J.; Williams, G.; Pratt, C.C.; Fleckenstein, P.; Wong, M.; Ramon, A. Profile of Salmonella enterica subsp. enterica (subspecies I) serotype 4,5,12:i:- strains causing food-borne infections in New York City. J. Clin. Microbiol. 2002, 40, 1924–1929. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ambrose, S.J.; Harmer, C.J.; Hall, R.M. Compatibility and entry exclusion of IncA and IncC plasmids revisited: IncA and IncC plasmids are compatible. Plasmid 2018, 96–97, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Gisasola, A.; Atxaerandio-Landa, A.; Garrido, V.; Grillo, M.J.; Martinez-Ballesteros, I.; Laorden, L.; Garaizar, J.; Bikandi, J. Genotyping study of Salmonella 4,[5],12:i:- monophasic variant of serovar Typhimurium and characterization of the second-phase flagellar deletion by whole genome sequencing. Microorganisms 2020, 8, 2049. [Google Scholar] [CrossRef] [PubMed]
- Calia, C.; Oliva, M.; Ferrara, M.; Minervini, C.F.; Scrascia, M.; Monno, R.; Mule, G.; Cumbo, C.; Marzella, A.; Pazzani, C. Identification and characterisation of pST1023 a mosaic, multidrug-resistant and mobilisable IncR plasmid. Microorganisms 2022, 10, 1592. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Guerra, B.; Bances, M.; Mendoza, M.C.; Rodicio, M.R. IncA/C plasmids mediate antimicrobial resistance linked to virulence genes in the Spanish clone of the emerging Salmonella enterica serotype 4,[5],12:i. J. Antimicrob. Chemother. 2011, 66, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Hopkins, K.L.; Garcia, V.; Beutlich, J.; Mendoza, M.C.; Threlfall, J.; Mevius, D.; Helmuth, R.; Rodicio, M.R.; Guerra, B.; et al. Diversity of plasmids encoding virulence and resistance functions in Salmonella enterica subsp. enterica serovar Typhimurium monophasic variant 4,[5],12:i:- strains circulating in Europe. PLoS ONE 2014, 9, e89635. [Google Scholar] [CrossRef]
- Guerra, B.; Soto, S.M.; Arguelles, J.M.; Mendoza, M.C. Multidrug resistance is mediated by large plasmids carrying a class 1 integron in the emergent Salmonella enterica serotype [4,5,12:i:-]. Antimicrob. Agents Chemother. 2001, 45, 1305–1308. [Google Scholar] [CrossRef]
- Mourao, J.; Machado, J.; Novais, C.; Antunes, P.; Peixe, L. Characterization of the emerging clinically-relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- (monophasic variant of S. Typhimurium) clones. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, X.; Garcia, P.; Garcia, V.; de Toro, M.; Ladero, V.; Heinisch, J.J.; Fernandez, J.; Rodicio, R.; Rodicio, M.R. Genomic analysis and phylogenetic position of the complex IncC plasmid found in the Spanish monophasic clone of Salmonella enterica serovar Typhimurium. Sci. Rep. 2021, 11, 11482. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; Pong, C.H.; Hall, R.M. Structures bounded by directly-oriented members of the IS26 family are pseudo-compound transposons. Plasmid 2020, 111, 102530. [Google Scholar] [CrossRef] [PubMed]
- Nohmi, T.; Hakura, A.; Nakai, Y.; Watanabe, M.; Murayama, S.Y.; Sofuni, T. Salmonella typhimurium has two homologous but different umuDC operons: Cloning of a new umuDC-like operon (samAB) present in a 60-megadalton cryptic plasmid of S. typhimurium. J. Bacteriol. 1991, 173, 1051–1063. [Google Scholar] [CrossRef]
- Valdivia, R.H.; Falkow, S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 1997, 277, 2007–2011. [Google Scholar] [CrossRef]
- Antunes, P.; Machado, J.; Peixe, L. Dissemination of sul3-containing elements linked to class 1 integrons with an unusual 3′ conserved sequence region among Salmonella isolates. Antimicrob. Agents Chemother. 2007, 51, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, F.; Di Lecce, C.; Galli, E.; Barbieri, P. Activation and inactivation of Pseudomonas stutzeri methylbenzene catabolism pathways mediated by a transposable element. Appl. Environ. Microbiol. 1999, 65, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, A.; Fortini, D.; Veldman, K.; Mevius, D.; Carattoli, A. Characterization of plasmids harbouring qnrS1, qnrB2 and qnrB19 genes in Salmonella. J. Antimicrob. Chemother. 2009, 63, 274–281. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI supplement M100; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- Vazquez, X.; Garcia-Fierro, R.; Fernandez, J.; Bances, M.; Herrero-Fresno, A.; Olsen, J.E.; Rodicio, R.; Ladero, V.; Garcia, V.; Rodicio, M.R. Incidence and Genomic Background of Antibiotic Resistance in Food-Borne and Clinical Isolates of Salmonella enterica Serovar Derby from Spain. Antibiotics 2023, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Vielva, L.; de Toro, M.; Lanza, V.F.; de la Cruz, F. PLACNETw: A web-based tool for plasmid reconstruction from bacterial genomes. Bioinformatics 2017, 33, 3796–3798. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- McClelland, M.; Sanderson, K.E.; Spieth, J.; Clifton, S.W.; Latreille, P.; Courtney, L.; Porwollik, S.; Ali, J.; Dante, M.; Du, F.; et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 2001, 413, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Rodicio, M.R.; Herrero, A.; Rodríguez, I.; García, P.; Montero, I.; Beutlich, J.; Rodicio, R.; Guerra, B.; Mendoza, M.C. Acquisition of antimicrobial resistance determinants by virulence plasmids specific for nontyphoid serovars of Salmonella enterica. Rev. Med. Microbiol. 2011, 22, 55–65. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Wang, X.M.; Li, H.; Shang, Y.H.; Pan, Y.S.; Wu, C.M.; Wang, Y.; Du, X.D.; Shen, J.Z. Novel lnu(G) gene conferring resistance to lincomycin by nucleotidylation, located on Tn6260 from Enterococcus faecalis E531. J. Antimicrob. Chemother. 2017, 72, 993–997. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, Y.; She, J.; Wang, X.; Dai, X.; Zhang, L. Genomic characterization of a Proteus sp. strain of animal origin co-carrying blaNDM-1 and lnu(G). Antibiotics 2021, 10, 1411. [Google Scholar] [CrossRef]
- Gomes, C.; Martinez-Puchol, S.; Palma, N.; Horna, G.; Ruiz-Roldan, L.; Pons, M.J.; Ruiz, J. Macrolide resistance mechanisms in Enterobacteriaceae: Focus on azithromycin. Crit. Rev. Microbiol. 2017, 43, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Spizek, J.; Rezanka, T. Lincosamides: Chemical structure, biosynthesis, mechanism of action, resistance, and applications. Biochem. Pharmacol. 2017, 133, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Stock, I.; Wiedemann, B. Natural antibiotic susceptibility of Salmonella enterica strains. Int. J. Antimicrob. Agents 2000, 16, 211–217. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, e07209. [Google Scholar] [CrossRef]
- Chiou, C.S.; Hong, Y.P.; Wang, Y.W.; Chen, B.H.; Teng, R.H.; Song, H.Y.; Liao, Y.S. Antimicrobial resistance and mechanisms of azithromycin resistance in nontyphoidal Salmonella isolates in Taiwan, 2017 to 2018. Microbiol. Spectr. 2023, 11, e0336422. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.P.; Chen, Y.T.; Wang, Y.W.; Chen, B.H.; Teng, R.H.; Chen, Y.S.; Chiou, C.S. Integrative and conjugative element-mediated azithromycin resistance in multidrug-resistant Salmonella enterica serovar Albany. Antimicrob. Agents Chemother. 2023, 65. [Google Scholar] [CrossRef] [PubMed]
- Hooda, Y.; Sajib, M.S.I.; Rahman, H.; Luby, S.P.; Bondy-Denomy, J.; Santosham, M.; Andrews, J.R.; Saha, S.K.; Saha, S. Molecular mechanism of azithromycin resistance among typhoidal Salmonella strains in Bangladesh identified through passive pediatric surveillance. PLoS Negl. Trop. Dis. 2019, 13, e0007868. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.L.; Panzenhagen, P.; Ferrari, R.G.; Dos Santos, A.; Paschoalin, V.M.F.; Conte-Junior, C.A. Frequency of antimicrobial resistance genes in Salmonella from Brazil by in silico whole-genome sequencing analysis: An overview of the last four decades. Front. Microbiol. 2020, 11, 1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Xu, X.; Liang, B.; Wu, F.; Yang, X.; Ma, Q.; Yang, C.; Hu, X.; Liu, H.; et al. Antimicrobial resistance of Salmonella enterica serovar Typhimurium in Shanghai, China. Front. Microbiol. 2017, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tran, J.H.; Jacoby, G.A.; Zhang, Y.; Wang, F.; Hooper, D.C. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob. Agents Chemother. 2003, 47, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, X.; Shi, C. Global spread of MCR-producing Salmonella enterica isolates. Antibiotics 2022, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Fierer, J. Extra-intestinal Salmonella infections: The significance of spv genes. Clin. Infect. Dis. 2001, 32, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Fierer, J.; Krause, M.; Tauxe, R.; Guiney, D. Salmonella typhimurium bacteremia: Association with the virulence plasmid. J. Infect. Dis. 1992, 166, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Guiney, D.G.; Fierer, J. The role of the spv genes in Salmonella pathogenesis. Front. Microbiol. 2011, 2, 129. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Hickman, A.B.; Varani, A.M.; Siguier, P.; Chandler, M.; Dekker, J.P.; Dyda, F. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio 2015, 6, e00762. [Google Scholar] [CrossRef]
- Leeper, M.M.; Tolar, B.M.; Griswold, T.; Vidyaprakash, E.; Hise, K.B.; Williams, G.M.; Im, S.B.; Chen, J.C.; Pouseele, H.; Carleton, H.A. Evaluation of whole and core genome multilocus sequence typing allele schemes for Salmonella enterica outbreak detection in a national surveillance network, PulseNet USA. Front. Microbiol. 2023, 14, 1254777. [Google Scholar] [CrossRef]
- Ji, H.J.; Jang, A.Y.; Song, J.Y.; Ahn, K.B.; Han, S.H.; Bang, S.J.; Jung, H.K.; Hur, J.; Seo, H.S. Development of live attenuated Salmonella Typhimurium vaccine strain using radiation mutation enhancement technology (R-MET). Front. Immunol. 2022, 13, 931052. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.; Khan, A.S.; Adesiyun, A.A.; Georges, K.; Gonzalez-Escalona, N. Genomic comparison of eight closed genomes of multidrug-resistant Salmonella enterica strains isolated from broiler farms and processing plants in Trinidad and Tobago. Front. Microbiol. 2022, 13, 863104. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tyson, G.H.; Hsu, C.H.; Harrison, L.; Strain, E.; Tran, T.T.; Tillman, G.E.; Dessai, U.; McDermott, P.F.; Zhao, S. Long-Read Sequencing Reveals Evolution and Acquisition of Antimicrobial Resistance and Virulence Genes in Salmonella enterica. Front. Microbiol. 2021, 12, 777817. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Mukherjee, S.; Li, C.; Harrison, L.B.; Hsu, C.H.; Tran, T.T.; Whichard, J.M.; Dessai, U.; Singh, R.; Gilbert, J.M.; et al. Genomic analysis of azithromycin-resistant Salmonella from food animals at slaughter and processing, and retail meats, 2011–2021, United States. Microbiol. Spectr. 2024, 12, e0348523. [Google Scholar] [CrossRef]
- Timme, R.E.; Lafon, P.C.; Balkey, M.; Adams, J.K.; Wagner, D.; Carleton, H.; Strain, E.; Hoffmann, M.; Sabol, A.; Rand, H.; et al. Gen-FS coordinated proficiency test data for genomic foodborne pathogen surveillance, 2017 and 2018 exercises. Sci. Data 2020, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kuang, D.; Xu, X.; Gonzalez-Escalona, N.; Erickson, D.L.; Brown, E.; Meng, J. Genomic analyses of multidrug-resistant Salmonella Indiana, Typhimurium, and Enteritidis isolates using MinION and MiSeq sequencing technologies. PLoS ONE 2020, 15, e0235641. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.V.; Harhay, D.M.; Bono, J.L.; Smith, T.P.; Fields, P.I.; Dinsmore, B.A.; Santovenia, M.; Kelley, C.M.; Wang, R.; Bosilevac, J.M.; et al. Complete and Closed Genome Sequences of 10 Salmonella enterica subsp. enterica Serovar Anatum Isolates from Human and Bovine Sources. Genome Announc. 2016, 4, e00447-16. [Google Scholar] [CrossRef] [PubMed]

| Isolate a | Patient: Sex b/Age (Sample) c Hospital d | Antigenic Formula | Phage Type e | R-Profile | AMR-Phenotype/Genotype f | R-Plasmid (Inc; Size in bp) g Other Plasmids (Size in bp) |
|---|---|---|---|---|---|---|
| LSP 64/15 | F/56 (Fc) HFJ | 4,5,12:i:- | RDNC | R1 | CHL-STR-SUL-TET-TMP/ cmlA1, [aadA1, aadA2, strA-Δ1, strB], sul3, tet(B), dfrA12 qacH | pLSP 64/15 (IncR; 126,138) |
| LSP 197/14 | F/1 (Fc) HUC | 4,5,12:i:- | DT18 | R1 | CHL-STR-SUL-TET-TMP/ cmlA1, [aadA1, aadA2, strA-Δ2, strB], sul3, tet(B), dfrA12 qacH | pLSP 197/14 (IncR; 119,881) |
| LSP 52/13 | M/81 (Fc) HFJ | 4,5,12:i:- | DT18 | R1 | CHL, STR, SUL, TET, TMP/ cmlA1, [aadA1, aadA2, strA-Δ2, strB], sul3, tet(B), dfrA12 qacH | pLSP52/13 (IncR; 113,363) |
| LSP 40/13 | F/33 (Fc) HUCA | 4,12:i:- | DT120 | R2 | AMP, CHL, STR, SUL, TMP/ cmlA1, [aadA1, aadA2, strA-Δ2, strB], sul3, dfrA12 qacH, silPGABFCRSE | pLSP 40/13-1 (IncR; 121,068) |
| blaTEM-1B, lnu(G) | pLSP 40/13-2 (ni; 33,942) | |||||
| - | ni (4593); oriColE (3830); ni (3374) | |||||
| LSP 40/12 | F/53 (Fc) HUC | 4,12:i:- | DT104 | R3 | CHL, STR, SUL, TET, TMP/ cmlA1, [aadA1, aadA2, strA-Δ2, strB], sul3, tet(B), dfrA12 qacH, silPGABFCRSE | pLSP 40/12 (IncR; 124,546) |
| - | ni (4593); ni (4,066); ni (3374) | |||||
| LSP 6/12 | M/89 (B) HUCA | 4,5,12:i:- | RDNC | R4 | CHL, STR, SUL, TET, TMP/ cmlA1, [aadA1, aadA2, aadA22, strA-Δ2, strB], sul3, tet(B), dfrA12, erm(B) qacH | pLSP 6/12 (IncR; 138,093) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, X.; Fernández, J.; Heinisch, J.J.; Rodicio, R.; Rodicio, M.R. Insights into the Evolution of IncR Plasmids Found in the Southern European Clone of the Monophasic Variant of Salmonella enterica Serovar Typhimurium. Antibiotics 2024, 13, 314. https://doi.org/10.3390/antibiotics13040314
Vázquez X, Fernández J, Heinisch JJ, Rodicio R, Rodicio MR. Insights into the Evolution of IncR Plasmids Found in the Southern European Clone of the Monophasic Variant of Salmonella enterica Serovar Typhimurium. Antibiotics. 2024; 13(4):314. https://doi.org/10.3390/antibiotics13040314
Chicago/Turabian StyleVázquez, Xenia, Javier Fernández, Jürgen J. Heinisch, Rosaura Rodicio, and M. Rosario Rodicio. 2024. "Insights into the Evolution of IncR Plasmids Found in the Southern European Clone of the Monophasic Variant of Salmonella enterica Serovar Typhimurium" Antibiotics 13, no. 4: 314. https://doi.org/10.3390/antibiotics13040314
APA StyleVázquez, X., Fernández, J., Heinisch, J. J., Rodicio, R., & Rodicio, M. R. (2024). Insights into the Evolution of IncR Plasmids Found in the Southern European Clone of the Monophasic Variant of Salmonella enterica Serovar Typhimurium. Antibiotics, 13(4), 314. https://doi.org/10.3390/antibiotics13040314









