Phylogeny of Transferable Oxazolidinone Resistance Genes and Homologs
Abstract
1. Introduction
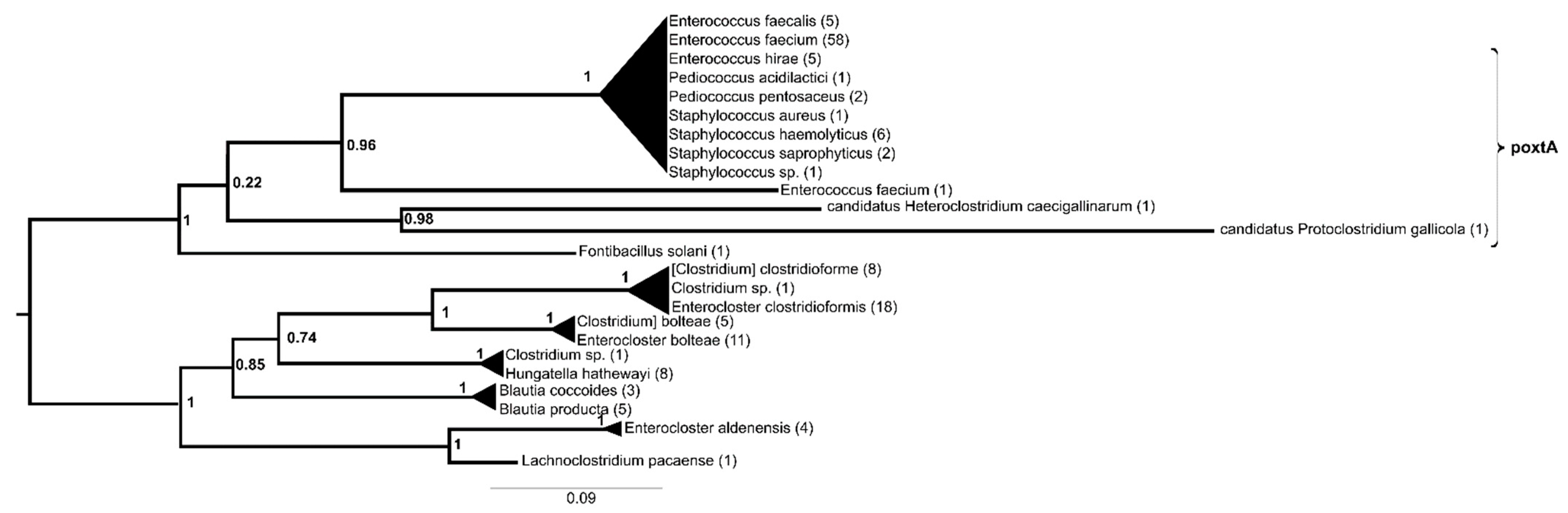
2. Results
3. Discussion
4. Materials and Methods
4.1. Assembly of the Dataset
4.2. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.G.; Yuan, X.L.; He, D.D.; Hu, G.Z.; Miao, M.S.; Xu, E.P. Research Progress on the Oxazolidinone Drug Linezolid Resistance. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9274–9281. [Google Scholar] [PubMed]
- Besier, S.; Ludwig, A.; Zander, J.; Brade, V.; Wichelhaus, T.A. Linezolid Resistance in Staphylococcus Aureus: Gene Dosage Effect, Stability, Fitness Costs, and Cross-Resistances. Antimicrob. Agents Chemother. 2008, 52, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Fu, Y.; Chen, Y.; Wang, Y.; Ye, D.; Wang, C.; Hu, X.; Zhou, L.; Du, J.; et al. Association of Florfenicol Residues with the Abundance of Oxazolidinone Resistance Genes in Livestock Manures. J. Hazard Mater. 2020, 399, 123059. [Google Scholar] [CrossRef] [PubMed]
- Bager, F.; Madsen, M.; Christensen, J.; Aarestrup, F.M. Avoparcin Used as a Growth Promoter is Associated with the Occurrence of Vancomycin-Resistant Enterococcus Faecium on Danish Poultry and Pig Farms. Prev. Vet. Med. 1997, 31, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in Agriculture and the Risk to Human Health: How Worried Should We Be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Silley, P. Antimicrobial Resistance in Zoonotic Bacteria: Lessons Learned from Host-Specific Pathogens. Anim. Health Res. Rev. 2008, 9, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Long, K.S.; Poehlsgaard, J.; Kehrenberg, C.; Schwarz, S.; Vester, B. The Cfr rRNA Methyltransferase Confers Resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A Antibiotics. Antimicrob. Agents Chemother. 2006, 50, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Sadowy, E. Linezolid Resistance Genes and Genetic Elements Enhancing their Dissemination in Enterococci and Streptococci. Plasmid 2018, 99, 89–98. [Google Scholar] [CrossRef]
- Cuong, N.V.; Padungtod, P.; Thwaites, G.; Carrique-Mas, J.J. Antimicrobial Usage in Animal Production: A Review of the Literature with a Focus on Low- and Middle-Income Countries. Antibiotics 2018, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Nüesch-Inderbinen, M.; Hausmann, A.; Treier, A.; Zurfluh, K.; Biggel, M.; Stephan, R. Fattening Pigs are a Reservoir of Florfenicol Resistant Enterococci Harboring Oxazolidinone Resistance Genes. J. Food Prot. 2022, 85, 740–746. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Wang, S.; Wang, Z.; Du, X.D.; Jiang, H.; Xia, X.; Shen, Z.; Ding, S.; Wu, C.; et al. Prevalence and Abundance of Florfenicol and Linezolid Resistance Genes in Soils Adjacent to Swine Feedlots. Sci. Rep. 2016, 6, 32192. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Werckenthin, C.; Kehrenberg, C. Identification of a Plasmid-Borne Chloramphenicol-Florfenicol Resistance Gene in Staphylococcus Sciuri. Antimicrob. Agents Chemother. 2000, 44, 2530–2533. [Google Scholar] [CrossRef] [PubMed]
- Stojković, V.; Ulate, M.F.; Hidalgo-Villeda, F.; Aguilar, E.; Monge-Cascante, C.; Pizarro-Guajardo, M.; Tsai, K.; Tzoc, E.; Camorlinga, M.; Paredes-Sabja, D.; et al. Cfr(B), Cfr(C), and a New Cfr-Like Gene, Cfr(E), in Clostridium Difficile Strains Recovered across Latin America. Antimicrob. Agents Chemother. 2019, 64, e01074-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A Novel Gene, OptrA, that Confers Transferable Resistance to Oxazolidinones and Phenicols and its Presence in Enterococcus Faecalis and Enterococcus Faecium of Human and Animal Origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; D’Andrea, M.M.; Brenciani, A.; Galeotti, C.L.; Morroni, G.; Pollini, S.; Varaldo, P.E.; Rossolini, G.M. Characterization of PoxtA, a Novel Phenicol-Oxazolidinone-Tetracycline Resistance Gene from an MRSA of Clinical Origin. J. Antimicrob. Chemother. 2018, 73, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.K.; Edwards, T.A.; O’Neill, A.J. ABC-F Proteins Mediate Antibiotic Resistance through Ribosomal Protection. mBio 2016, 7, e01975. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Y.; Schwarz, S. Presence and Dissemination of the Multiresistance Gene Cfr in Gram-Positive and Gram-Negative Bacteria. J. Antimicrob. Chemother. 2013, 68, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Lazaris, A.; Coleman, D.C.; Kearns, A.M.; Pichon, B.; Kinnevey, P.M.; Earls, M.R.; Boyle, B.; O’Connell, B.; Brennan, G.I.; Shore, A.C. Novel Multiresistance Cfr Plasmids in Linezolid-Resistant Methicillin-Resistant Staphylococcus Epidermidis and Vancomycin-Resistant Enterococcus Faecium (VRE) from a Hospital Outbreak: Co-Location of Cfr and OptrA in VRE. J. Antimicrob. Chemother. 2017, 72, 3252–3257. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Arthur, M.; Brisson-Noël, A.; Courvalin, P. Origin and Evolution of Genes Specifying Resistance to Macrolide, Lincosamide and Streptogramin Antibiotics: Data and Hypotheses. J. Antimicrob. Chemother. 1987, 20, 783–802. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of Polymyxin Resistance: Acquired and Intrinsic Resistance in Bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Trieu-Cuot, P.; Courvalin, P. Evolution and Transfer of Aminoglycoside Resistance Genes under Natural Conditions. J. Antimicrob. Chemother. 1986, 18 (Suppl. C), 93–102. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Wachino, J.; Doi, Y.; Kurokawa, H.; Arakawa, Y. Global Spread of Multiple Aminoglycoside Resistance Genes. Emerg. Infect. Dis. 2005, 11, 951–953. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Bush, K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Ishiguro, K.; Kimura, S.; Sakaguchi, Y.; Suzuki, T.; Suzuki, T. Single Methylation of 23S rRNA Triggers Late Steps of 50S Ribosomal Subunit Assembly. Proc. Natl. Acad. Sci. USA 2015, 112, E4707–E4716. [Google Scholar] [CrossRef] [PubMed]
- Schubert, H.L.; Blumenthal, R.M.; Cheng, X. Many Paths to Methyltransfer: A Chronicle of Convergence. Trends Biochem. Sci. 2003, 28, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Bhujbalrao, R.; Anand, R. Deciphering Determinants in Ribosomal Methyltransferases that Confer Antimicrobial Resistance. J. Am. Chem. Soc. 2019, 141, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.C.; Hansen, L.H.; Tenson, T.; Rasmussen, A.; Kirpekar, F.; Vester, B. Distinction between the Cfr Methyltransferase Conferring Antibiotic Resistance and the Housekeeping RlmN Methyltransferase. Antimicrob. Agents Chemother. 2013, 57, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, K.H.; Purta, E.; Hansen, L.H.; Bujnicki, J.M.; Vester, B.; Long, K.S. Insights into the Structure, Function and Evolution of the Radical-SAM 23S rRNA Methyltransferase Cfr that Confers Antibiotic Resistance in Bacteria. Nucleic Acids Res. 2010, 38, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.H.; Planellas, M.H.; Long, K.S.; Vester, B. The Order Bacillales Hosts Functional Homologs of the Worrisome Cfr Antibiotic Resistance Gene. Antimicrob. Agents Chemother. 2012, 56, 3563–3567. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.K.; Fleige, C.; Lange, D.; Klare, I.; Werner, G. Rapid Emergence of Highly Variable and Transferable Oxazolidinone and Phenicol Resistance Gene OptrA in German Enterococcus spp. Clinical Isolates. Int. J. Antimicrob. Agents 2018, 52, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Fioriti, S.; Morroni, G.; Coccitto, S.N.; Brenciani, A.; Antonelli, A.; Di Pilato, V.; Baccani, I.; Pollini, S.; Cucco, L.; Morelli, A.; et al. Detection of Oxazolidinone Resistance Genes and Characterization of Genetic Environments in Enterococci of Swine Origin, Italy. Microorganisms 2020, 8, 2021. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Tian, T.T.; Qiao, W.; Tian, Z.; Yang, M.; Zhang, Y.; Li, J.Y. Prevalence and Characterization of Oxazolidinone and Phenicol Cross-Resistance Gene OptrA in Enterococci Obtained from Anaerobic Digestion Systems Treating Swine Manure. Environ. Pollut. 2020, 267, 115540. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Bremer, N.; Knopp, M.; Martin, W.F.; Tria, F.D.K. Realistic Gene Transfer to Gene Duplication Ratios Identify Different Roots in the Bacterial Phylogeny Using a Tree Reconciliation Method. Life 2022, 12, 995. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Sledzieski, S.; Berger, B.; Wu, Y.-C.; Bansal, M.S. Phylogenetic Reconciliation Reveals Extensive Ancestral Recombination in Sarbecoviruses and the SARS-CoV-2 Lineage. bioRxiv 2021. [CrossRef]
- Tria, F.D.K.; Landan, G.; Dagan, T. Phylogenetic Rooting Using Minimal Ancestor Deviation. Nat. Ecol. Evol. 2017, 1, 193. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kardos, G.; Laczkó, L.; Kaszab, E.; Timmer, B.; Szarka, K.; Prépost, E.; Bányai, K. Phylogeny of Transferable Oxazolidinone Resistance Genes and Homologs. Antibiotics 2024, 13, 311. https://doi.org/10.3390/antibiotics13040311
Kardos G, Laczkó L, Kaszab E, Timmer B, Szarka K, Prépost E, Bányai K. Phylogeny of Transferable Oxazolidinone Resistance Genes and Homologs. Antibiotics. 2024; 13(4):311. https://doi.org/10.3390/antibiotics13040311
Chicago/Turabian StyleKardos, Gábor, Levente Laczkó, Eszter Kaszab, Bálint Timmer, Krisztina Szarka, Eszter Prépost, and Krisztián Bányai. 2024. "Phylogeny of Transferable Oxazolidinone Resistance Genes and Homologs" Antibiotics 13, no. 4: 311. https://doi.org/10.3390/antibiotics13040311
APA StyleKardos, G., Laczkó, L., Kaszab, E., Timmer, B., Szarka, K., Prépost, E., & Bányai, K. (2024). Phylogeny of Transferable Oxazolidinone Resistance Genes and Homologs. Antibiotics, 13(4), 311. https://doi.org/10.3390/antibiotics13040311






