Abstract
Carbapenem-resistant Gram-negative bacterial infections are a major public health threat due to the limited therapeutic options available. The introduction of the new β-lactam/β-lactamase inhibitors (BL/BLIs) has, however, altered the treatment options for such pathogens. Thus, four new BL/BLI combinations—namely, ceftazidime/avibactam, meropenem/vaborbactam, imipenem/relebactam, and ceftolozane/tazobactam—have been approved for infections attributed to carbapenem-resistant Enterobacterales species and Pseudomonas aeruginosa. Nevertheless, although these antimicrobials are increasingly being used in place of other drugs such as polymyxins, their optimal clinical use is still challenging. Furthermore, there is evidence that resistance to these agents might be increasing, so urgent measures should be taken to ensure their continued effectiveness. Therefore, clinical laboratories play an important role in the judicious use of these new antimicrobial combinations by detecting and characterizing carbapenem resistance, resolving the presence and type of carbapenemase production, and accurately determining the minimum inhibitor concentrations (MICs) for BL/BLIs. These three targets must be met to ensure optimal BL/BLIs use and prevent unnecessary exposure that could lead to the development of resistance. At the same time, laboratories must ensure that results are interpreted in a timely manner to avoid delays in appropriate treatment that might be detrimental to patient safety. Thus, we herein present an overview of the indications and current applications of the new antimicrobial combinations and explore the diagnostic limitations regarding both carbapenem resistance detection and the interpretation of MIC results. Moreover, we suggest the use of alternative narrower-spectrum antibiotics based on susceptibility testing and present data regarding the effect of synergies between BL/BLIs and other antimicrobials. Finally, in order to address the absence of a standardized approach to using the novel BL/BLIs, we propose a diagnostic and therapeutic algorithm, which can be modified based on local epidemiological criteria. This framework could also be expanded to incorporate other new antimicrobials, such as cefiderocol, or currently unavailable BL/BLIs such as aztreonam/avibactam and cefepime/taniborbactam.
1. Introduction
Antimicrobial resistance represents a major threat to public health and healthcare systems worldwide. Approximately 4.9 million deaths were attributed to antimicrobial resistance in 2019 [1], while its economic cost might reach one trillion USD by 2050 [2]. Among antimicrobial-resistant pathogens, carbapenem-resistant Gram-negative bacteria (CR-GNB) pose a significant threat to public health due to their resistance to most β-lactams in addition to other antimicrobial agents [3].
However, four novel β-lactam/β-lactamase inhibitors (BL/BLIs)—namely, ceftazidime/avibactam (CZA), meropenem/vaborbactam (MEV), imipenem/relebactam (IMR), and ceftolozane/tazobactam (C/T)—have been approved for the treatment of various CR-GNB infections, enhancing our antibiotic arsenal. Although these antimicrobial combinations have been in clinical use for approximately eight years, there is evidence that bacterial resistance to these agents might be increasing [4,5], thus demonstrating the importance of constant antimicrobial and diagnostic stewardship. Furthermore, the inherent heterogeneity and variable sensitivity and specificity of the available diagnostic tests used to characterize carbapenem resistance complicates the use of these antibiotics.
Herein, we present an overview of carbapenemases and novel BL/BLIs and subsequently discuss the role of BL/BLIs alone or in combination with other antimicrobials in the treatment of CR-GNB infections. We also discuss methods to approach discordant minimum inhibitory concentrations (MICs) and phenotypic results during susceptibility testing, and, finally, we propose a diagnostic and therapeutic algorithm based on regional epidemiological data, which will allow for the rapid and streamlined identification and subsequent treatment of CR-GNB isolates.
2. Overview of Resistance Mechanisms to Carbapenems
Carbapenems are a family of β-lactam antibiotics that includes meropenem, imipenem, doripenem, and ertapenem. They have a broad spectrum of activity against numerous antimicrobial-resistant Gram-positive and Gram-negative pathogens. Carbapenem resistance was first identified in 1997 [6] and has spread globally ever since [7]. The primary resistance mechanism is the production of carbapenemases, which are β-lactamase enzymes capable of hydrolyzing most β-lactam antibiotics, including carbapenems [8]. Other resistance mechanisms include multidrug efflux pumps and a reduced expression of porins. Although infections by carbapenemase-producing isolates are typically hospital-acquired, the spread of carbapenemase genes to zoonotic pathogens such as Salmonella enterica raises concerns for the permeation of carbapenem resistance across the food chain, affecting individuals without prior exposure to broad-spectrum antibiotics [9].
Carbapenemases belong to Ambler classes A, B, and D and are capable of hydrolyzing aminopenicillins, ureidopenicillins, narrow- and broad-spectrum cephalosporins, cephamycins, and carbapenems [10]. The most important and common class A carbapenemase is the Klebsiella pneumoniae carbapenemase (KPC), which has spread amongst numerous Enterobacterales species. Examples of class D carbapenemases are the OXA-derived carbapenemases, commonly found in Acinetobacter baumannii. Certain OXA-carbapenemases, such as the OXA-48-like carbapenemases, have spread amongst Enterobacterales and have become an important mechanism of carbapenem resistance among such isolates in several countries, including Turkey [11,12]. Metallo-β-lactamases (MBLs) are class B carbapenemases and include (i) the New Delhi MBLs (NDMs), which are common in India and Pakistan but have also spread globally [13,14], (ii) the Verona integron-encoded MBL (VIM) types, and (iii) various imipenemase (IMP) types. Initially, MBL production was commonly found in Pseudomonas aeruginosa but has also spread to several Enterobacterales species [15]. MBL producers are particularly difficult to treat due to their frequent co-expression of other resistance genes against numerous antimicrobial classes [16,17]. It is noteworthy that aztreonam (AZT) is a β-lactamic antibiotic that is resistant to the enzymatic activity of MBLs but not to that of class A and D carbapenemases [13,16]. Most carbapenemases are plasmid-mediated, which allows for the rapid dissemination of resistance among different bacterial strains [12,18].
It is also important to note that not all carbapenemases have the same affinity for carbapenems. For example, OXA-48-like carbapenemases have a low affinity for carbapenems but are usually co-expressed with numerous other mechanisms, such as efflux pumps and porin deletions which synergistically increase carbapenem resistance [18]. Also, not all carbapenemases of the same group have the same hydrolyzing capability for all carbapenems. For example, clinical isolates of K. pneumoniae that produce mutated IMP enzymes have been identified, which confer resistance to meropenem but not imipenem [19].
3. The New β-Lactam and β-Lactamase Inhibitor Combinations: Indications and Resistance Mechanisms
The spread of carbapenem-resistant Enterobacterales and P. aeruginosa is a global concern due to the few treatment options available. Four novel BL/BLIs have been approved for the treatment of infections caused by these strains, and an overview of all these combinations is available in Table 1. Current guidelines do not propose the use of these combinations in the treatment of carbapenem-resistant A. baumannii [20,21].

Table 1.
Novel BL/BLI combinations and their susceptibility based on carbapenemase type.
3.1. Meropenem/Vaborbactam
The combination of MEV was approved in 2017 by the FDA to treat complicated urinary tract infections (cUTIs) only; however, it has been used off-label for other infections caused by non-CR-GNB [22]. The EMA approved the combination to treat cUTIs, complicated intra-abdominal infections (cIAIs), as well as hospital-acquired pneumonia (HAP) cases [23]. Vaborbactam is a broad-spectrum β-lactamase inhibitor that inhibits serine carbapenemases and Ambler class A extended-spectrum β-lactamase (ESBL) enzymes. It also inhibits AmpC enzymes, but it does not inhibit MBLs or class D ESBLs and carbapenemases such as OXA-derived ESBLs and OXA-derived carbapenemases [24]. MEV has been proven effective in treating infections caused by KPC-producing Enterobacterales [25]. However, it is not considered effective against carbapenem-resistant P. aeruginosa [26].
OmpK35 and OmpK36 porins are important for vaborbactam function, and, frequently, the downregulation or mutation of these porins is associated with the development of resistance. OmpK36 mutations appear to have a greater effect on vaborbactam efficacy, as demonstrated by Lomovskaya et al., with gene deletions and amino acid duplications resulting in reduced vaborbactam efficacy [24]. A loss of transcriptional factors associated with porin gene expression has also been shown to reduce vaborbactam efficacy [27]. It is of note that isolates with porin mutations frequently co-express other resistance mechanisms including the increased expression of KPCs [28,29]. Multidrug efflux pumps may also contribute to MEV resistance when co-expressed with other resistance mechanisms [24].
3.2. Ceftazidime/Avibactam
CZA was approved by the FDA in 2015 for the treatment of cIAI and cUTI and, in 2018, for the treatment of HAP and ventilator-associated pneumonia (VAP) [30]. The EMA also approved the combination for the same indications in 2016. Avibactam has an identical spectrum to vaborbactam, in addition to having the ability of inhibiting certain D class carbapenemases, such as OXA-48-like enzymes [31], and, thus, can be used to treat infections caused by KPC- and OXA-48-producing Enterobacterales [21]. CZA can be considered a treatment option against carbapenem-resistant P. aeruginosa, but, in general, it should not be used without documented MIC susceptibility testing due to its unpredictable efficacy [32,33].
No benefit was noted when CZA was used in combination therapies [34], and guidelines do not recommend combination therapies in infections caused by CR-GNB who are susceptible to CZA or MEV. However, CZA can be used in combination therapies with AZT to treat infections caused by MBL-producing isolates. AZT is inherently resistant to MBLs, and avibactam readily inhibits the action of AmpC, ESBL, and KPC β-lactamases that could hydrolyze AZT [35]. CZA + AZT could be used for the treatment of MBL-producing P. aeruginosa [36]. Interestingly, although avibactam does not inhibit MBL action, in vitro studies demonstrate that the exposure of MBL-producing bacteria to avibactam increases bacterial clearance by increasing the susceptibility of isolates to complement neutrophil and cathelicidin function [37].
CZA resistance is primarily associated with KPC mutations or the increased expression of KPC enzymes [38,39]. CZA appears to be more susceptible to specific KPC variants, such as KPC-3, with KPC-3 having an increased affinity to ceftazidime [40,41]. Moreover, KPC-3 mutants that confer resistance to CZA are, surprisingly, frequently susceptible to meropenem [42,43]. These mutations, which primarily affect the Ω-loop, increase the enzyme’s affinity to ceftazidime while simultaneously reducing its affinity to avibactam [43]. Such mutations, however, confer an ESBL-like phenotype to the enzyme, thus restoring carbapenem efficacy. These mutants are frequently associated with false-negative phenotypic test results [44]. Isolated clinical reports have demonstrated the efficacy of meropenem combinations in treating CZA-resistant isolates due to these KPC mutations [45]. However, Shields et al. demonstrated that serial passages of such isolates through media containing sublethal meropenem concentrations resulted in the selection of carbapenem-resistant isolates that retain their CZA resistance profile [42]. Vaborbactam appears to be effective against certain KPC mutants that result in CZA resistance [46]. Finally, porin mutations responsible for MEV resistance are frequently associated with CZA resistance [47,48].
3.3. Imipenem/Relebactam
IMR was approved by the FDA in 2019 for the treatment of cIAI and cUTIs and, in 2020, for HAP and VAP [49]. The EMA approved the combination for HAP, VAP, and bacteremia caused by susceptible strains [50]. Moreover, the EMA approved the use of this combination in any infection caused by aerobic Gram-negative bacteria that are susceptible and for which no other treatment option is available. Relebactam readily inhibits class A carbapenemases; however, it exhibits variable activity against class D carbapenemases [51]. It has no effect on MBLs. IMR is the only combination that has demonstrated efficacy against P. aeruginosa infections [52], possibly by enhancing the stability and activity of imipenem [53].
Resistance to IMR remains the least studied of all these combinations. Epidemiological data indicate that resistance is primarily associated with the production of carbapenemases not inhibited by relebactam [54], with certain studies demonstrating a role for ompK35 and ompK36 mutations [55].
3.4. Ceftolozane/Tazobactam
Ceftolozane is a novel fifth generation cephalosporin, which was approved by the FDA in 2014 for the treatment of cIAIs and UTIs and, in 2019, for the treatment of HAP and VAP [56]. It has also been approved by the EMA with the same indications [57]. Ceftolozane’s action is compromised when exposed to ESBLs and carbapenemases, and the subsequent addition of tazobactam restores ceftolozane’s action against certain ESBL-producing bacteria [58]. The addition of tazobactam does not increase the antipseudomonal activity of ceftolozane [59]. Ceftolozane is, nevertheless, resistant to the action of AmpC β-lactamases and not affected by multidrug resistance efflux pump systems, such as the MexAB-OprM system, or by the deletion of porins, which are commonly observed mechanism of carbapenem resistance in P. aeruginosa [60]. The combination is, therefore, used primarily in the treatment of multidrug-resistant P. aeruginosa infections and for the treatment of non-carbapenemase-producing CR-GNB. It has already been demonstrated to be non-inferior to carbapenems in the setting of HAP and VAP caused by Gram-negative organisms [61], and one retrospective study has demonstrated the efficacy of C/T over polymyxin- or aminoglycoside-based combinations in P. aeruginosa infections [62]. C/T resistance is less frequent than IMR resistance in P. aeruginosa [63,64].
The most well-characterized mechanism is the mutation of an AmpC-type cephalosporinase, PDC. The overexpression or mutation of this enzyme, both of which would increase the rate of ceftolozane hydrolysis, results in resistance amongst P. aeruginosa isolates [65]. In addition, mutated Ambler C class β-lactamases and the inactivation of AmpC-negative regulators have also been implicated [66]. Moreover, C/T can be ineffective against isolates that produce carbapenemases or ESBLs that are not inhibited by tazobactam [67].
4. Future Directions in Antimicrobial and Diagnostic Stewardship of Novel β-Lactam/β-Lactamase Inhibitors
Clinical laboratories play an important role in the judicious use of novel BL/BLIs by detecting and characterizing carbapenem resistance, resolving the presence and type of carbapenemase production, and accurately determining the MICs for BL/BLIs. These three targets must be met to ensure optimal BL/BLIs use and prevent unnecessary exposure that can lead to the development of resistance.
Phenotypic tests are the methods primarily deployed by diagnostic laboratories to detect and characterize carbapenemase production. Examples of such tests include combination disc testing (CDT), colorimetric tests such as the Carba-NP, lateral flow assays such as the NG-Test Carba-5, and the carbapenem inactivation method (CIM). There are no recommendations or guidelines on the optimal way of using these detection methods, and each laboratory uses different methods based on their ease of use, cost, and availability. A review of the advantages and limitations of the most common phenotypic detection tests is provided in Table 2. Genotypic testing methods are more sensitive and can detect the presence of efflux pumps or porin deletions that can contribute to carbapenem resistance [68]. However, they are associated with increased costs and require specialized equipment. EUCAST does not recommend the direct detection of carbapenemase genes among carbapenemase-producing Enterobacterales [69,70].

Table 2.
Advantages and limitations of major phenotypic carbapenemase detection methods.
Current laboratory practices leave much to be desired both in terms of the efficacy and accuracy of current methods. Moreover, the rapid emergence of resistance to the novel BL/BLIs dictates that antimicrobial use must be targeted, efficient, and rational. To address these issues, we hereby discuss the role of antimicrobial synergy for the treatment of CR-GNB, identify methods to accurately combine MIC and phenotypic test results, and, finally, propose a diagnostic and therapeutic framework based on objective epidemiological data to streamline CR-GNB identification and treatment.
5. Antibiotic Synergy and Treatment of Carbapenem-Resistant Enterobacterales
CZA has been proven to be an important addition to our therapeutic armamentarium for treating various infections, such as those caused by KPC- or OXA-48-like-producing CR-GNB. However, resistance and treatment failures appear when CZA is used as a single antimicrobial agent [39,44]. Nevertheless, CZA has been found to act synergistically with carbapenems against carbapenem-resistant KPC producers. In that respect, a recent study has demonstrated excellent synergistic effects with a fractional inhibitory concentration index (FICI) < 0.5 when CZA was combined with meropenem or AZT even in isolates that were resistant to CZA, meropenem, and AZT [93]. Similarly, Gaibani et al. showed that the combination of CZA with imipenem or meropenem resulted in a synergistic effect (FICI < 0.5) in all K. pneumoniae isolates, including two which exhibited resistance to CZA due to mutations in the blaKPC-3 gene [94]. In both studies, the combination resulted in the restoration of carbapenem efficacy. Another study demonstrated a synergistic effect of MEV with CZA and imipenem even in initially CZA- and MEV-resistant strains [95]. Zhang et al. demonstrated the efficacy of CZA in combination with imipenem in the treatment of XDR-P. aeruginosa isolates that do not produce MBL but possess porin and AmpC mutations and multidrug efflux pumps and overexpress AmpC enzymes. Although counterintuitive, the efficacy of this combination may be explained by the reduction in the AmpC levels by ceftazidime, which acts as a suicide molecule, and by the inhibition of the AmpC enzymes by avibactam, in addition to the known reduced affinity of imipenem to efflux pumps [96].
Finally, the use of novel BL/BLIs synergies has been studied in the treatment of double carbapenemase producers. Double carbapenemase producers are frequently associated with high-carbapenem MICs and an extensive resistance profile, reducing the available antimicrobial agents [97]. The treatment of such isolates is further complicated by the inability of many phenotypic testing methods to accurately detect and characterize double carbapenemase production (Table 1). In theory, the combination of CZA + AZT is a rational choice for strains producing multiple carbapenemases, due to the proven efficacy of the combination against MBL producers and the known efficacy of CZA against OXA enzymes. Romina et al. have demonstrated the synergistic effect of CZA + AZT in carbapenemase-producing isolates, including those with a double production of KPC plus NDM [98], and Jayol et al. have demonstrated the efficacy of this combination against strains that co-produce NDM and OXA enzymes [99]. Additionally, it has been demonstrated that both CZA + AZT and MEV + AZT combinations can be used for the treatment of double carbapenemase producers, with MEV + AZT possibly being more effective [100].
Synergies have also been reported with other antibiotics such as fosfomycin, polymyxins, and aminoglycosides [101]. It has been shown that CZA + colistin is effective in producing more than two-log reductions in all CZA-resistant P. aeruginosa isolates [102]. A case report has also demonstrated the synergistic effect of MEV with fosfomycin in the treatment of a patient with a severe K. pneumoniae infection. The patient did not respond to CZA therapy and subsequently relapsed after MEV was administered. The relapsing strain was resistant to MEV, but the patient was successfully treated with the addition of fosfomycin [103].
Therefore, numerous in vitro data suggest that antimicrobial synergies could expand the spectrum of novel BL/BLIs to initially resistant isolates, thus providing new treatment options for MDR and XDR pathogens. Moreover, these synergies could reduce the emergence of resistance amongst susceptible isolates [93].
6. Interpreting Carbapenem and Novel BL/BLI MIC Results in CR-GNB
EUCAST recommends further screening for carbapenemase production in all Enterobacterales isolates with a meropenem MIC > 0.125 mg/L, which corresponds to a disk-diffusion zone diameter of <28 mm [69,70]. These screening MIC and zone diameter cutoffs proposed by EUCAST are different from the clinical breakpoints, which characterize an isolate as either susceptible or resistant. This reflects the fact that certain carbapenemase producers can be classified as being susceptible based on clinical cutoff criteria due to the low affinity of the produced carbapenemase for the selected carbapenem in susceptibility testing or even due to the low carbapenemase production rates [69,104]. In fact, a recent study has demonstrated that approximately 50% of NDM producers were susceptible to meropenem, and approximately 60% of NDM producers were susceptible to imipenem [105]. However, pursuing further testing in many isolates that are within the clinical susceptibility range but harbor carbapenem resistance genes can result in the overuse of last-line antibiotics including the novel BL/BLIs [106].
These conflicting results present a therapeutic dilemma. Should MIC be the sole criterion for carbapenem use or should phenotypic and/or genotypic detection of a carbapenemase, regardless of the MIC values, automatically preclude the use of a carbapenem? A 2015 study demonstrated that the meropenem that is used in combination therapies can be used in the treatment of infections caused by Gram-negative bacteria with reduced susceptibility to meropenem (i.e., MIC ≤ 8 mg/L) in high-risk patients [107]. This is reflected in the 2022 ESCMID guidelines, according to which high-dose extended-infusion meropenem therapy in combination with other in vitro susceptible antibiotics is proposed as an alternative, albeit with a low certainty of evidence, to novel BL/BLI combinations, provided that the MIC remains ≤8 mg/L [21]. This dilemma is not novel and is frequently encountered in infections caused by ESBL bacteria. Ever since the MERINO trial, carbapenems have been the mainstay for the treatment of ESBL infections [108]. However, the use of piperacillin/tazobactam for the treatment of UTIs and biliary tract infections caused by susceptible ESBL Escherichia coli and K. pneumoniae strains has been shown to be safe and effective [109], with one retrospective study concluding that amoxicillin/clavulanic acid and piperacillin/tazobactam were non-inferior to carbapenem when used in bacteremia caused by susceptible strains of E. coli [110]. Therefore, one could conclude that carbapenem combination therapies could be used provided that meropenem’s MIC is ≤8 mg/L. Colistin could further augment carbapenem action, possibly reducing treatment failures and the emergence of resistance and minimizing novel BL/BLI use [111].
Another important parameter that can affect the efficacy of carbapenems in infections caused by carbapenemase-producing bacteria is the inoculum effect, which refers to the increase in MIC as the bacterial inoculum increases. In vitro studies have shown that the inoculum effect can have a profound effect on the efficacy of carbapenems in carbapenemase-producing Enterobacterales, which depends on the underlying carbapenem resistance mechanism and the type of carbapenemase produced. Adler et al. have shown that non-carbapenemase resistance mechanisms (e.g., porin mutations) are rarely associated with an inoculum effect [112], whereas Golikova et al. have demonstrated that OXA-48 producers exhibit a less-pronounced inoculum effect compared to KPC and NDM producers [113]. Therefore, infections caused by OXA producers or non-carbapenemase producers, initially susceptible to meropenem, are less likely to be associated with clinical failures, since an initial MIC result within the susceptible range will not be severely affected by a higher inoculum. Conversely, high-inoculum infections should not be treated with carbapenems regardless of the initial MIC.
Similar to carbapenems, discordant MICs and phenotypic results have been described for the novel BL/BLI combinations. A recent study demonstrated that 57% of MBL producers tested susceptible to MEV [114]. These results could be caused by laboratory misidentification, a phenomenon more common with commercially available identification methods such as automated systems or Etest strips. CZA Etests have been shown to result in very major errors (i.e., false susceptibility) in up to 6% of isolates and, thus, might not fulfill the ISO performance standards [115]; thus, the results obtained from CZA Etest strips should be cautiously interpreted [116]. Clinicians and clinical microbiologists need to be aware of the limitations of current commercially available MIC determination techniques for novel BL/BLIs when interpreting the MICs for these agents, especially when discordant results are present. These results need to be verified via reference standard methods (broth microdilution or disc diffusion) and additional phenotypic or genotypic testing. A true discordant result can be attributed either to a low production of the detected carbapenemase or a low affinity of the carbapenemase for the tested combination. Regardless, few data are available addressing this phenomenon, in a similar fashion to the use of carbapenems for carbapenemase producers mentioned previously. Therefore, we suggest that phenotypic results be the deciding factor for treatment.
7. Carbapenemase Detection—Rational Testing and Treatment Options Based on Epidemiological Data
The absence of a systematic approach to the detection and characterization of carbapenem resistance might be attributed to the numerous pitfalls surrounding the available diagnostic tests [117,118] (see Table 2). Diagnostic uncertainties remain, especially in relation to the detection of OXA-48-like carbapenemase producers, the identification of carbapenemase production in P. aeruginosa isolates, and the proper characterization of bacteria with complex carbapenem resistance mechanisms (e.g., production of numerous carbapenemases of different types and/or co-expression of non-carbapenemase-related resistance mechanisms). These uncertainties translate to ambiguous treatment decisions.
Another important factor that needs to be accounted for is time. The early initiation of an appropriate antimicrobial therapy is associated with improved survival in patients with bloodstream infections, and the accurate characterization of the underlying resistance mechanisms in carbapenem-resistant organisms is time-consuming, considering the extra time needed for some phenotypic tests such as CDT. The entire process, starting from the moment in which a blood culture is drawn to final carbapenemase detection and MIC reporting, for novel BL/BLI combinations could extend over more than 72 h [119] (Figure 1). Although this time gap can be bridged using molecular methods, their cost and need for expensive equipment preclude their widespread use.
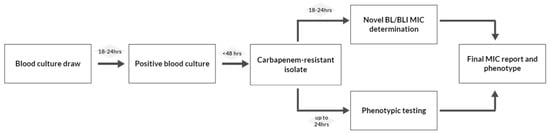
Figure 1.
Current laboratory process in detecting and characterizing carbapenemase producers. BL/BLI: β-lactam/β-lactamase inhibitor.
Therefore, there is a need for a clear diagnostic framework that could accurately detect most carbapenemase producers whilst simultaneously providing clear guidelines on which antibiotic regimen should be prescribed pending confirmatory testing and MIC results. In addition, streamlined processes that allow for the rapid identification of resistant isolates would allow for the notification of clinicians and administrators in order to deploy infection control measures such as nurse and patient cohorting. These measures are necessary in order to curb the horizontal transmission of plasmid-mediated carbapenemase genes [120]. Here, we propose a diagnostic and treatment algorithm based on key epidemiological data that could be altered based on local and national epidemiology and provide the example of Greece, a country with a high burden of CR-GNB infections. The algorithm is centered around an initial rapid diagnostic test that will allow for the initiation of treatment based on local epidemiology on the day when carbapenem resistance is detected. Subsequent confirmatory testing can be pursued as dictated by the epidemiology via CDT or CIM or even genotypic testing in selected cases.
Epidemiological data such as what are the most common mechanisms of carbapenem resistance among Enterobacterales and P. aeruginosa strains should be addressed to pursue epidemiologically appropriate diagnoses and treatments. For example, in Greece, the most common carbapenem-resistant microorganism is K. pneumoniae, with most strains being KPC producers. However, non-carbapenemase mechanisms are also common, and certain studies have demonstrated that double carbapenemase production (e.g., KPC + VIM) can be encountered in up to 25% of isolates [121,122,123]. OXA-type carbapenemase production is uncommon in non-Acinetobacter cases, with rare isolations of K. pneumoniae strains co-expressing OXA-48-like enzymes in addition to other carbapenemases [122]. Epidemiological data regarding the resistance phenotypes of other carbapenem-resistant Gram-negative bacteria such as E. coli are missing, but no double carbapenemase production has been reported. Epi-net data indicate that carbapenem-resistant E. coli strains are presumed to be carbapenemase producers [124]. Studies have shown that 35% of P. aeruginosa isolates are carbapenem-resistant, with most of them expressing numerous resistance mechanisms. As far as carbapenemases are concerned, most carbapenemase-producing P. aeruginosa strains produce MBLs [125]. Based on these epidemiological data, diagnostic and therapeutic algorithms are proposed in Figure 2 and Figure 3. These algorithms are proposed for three different microorganisms: K. pneumoniae, non-K. pneumoniae Enterobacterales (most commonly E. coli) species, and P. aeruginosa.
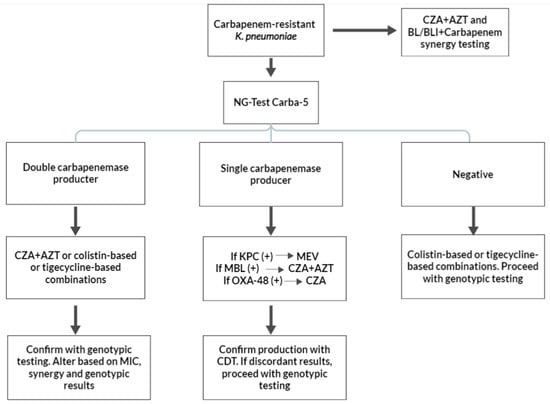
Figure 2.
Suggested diagnostic and treatment algorithms of carbapenem-resistant Klebsiella pneumoniae based on carbapenemase production. KPC: Klebsiella pneumoniae carbapenemase; MBL: metallo-β-lactamase; MEV: meropenem/vaborbactam; CZA: ceftazidime/avibactam; AZT: aztreonam; CDT: combination disc testing; and OXA-48: OXA-48 oxacillinase.
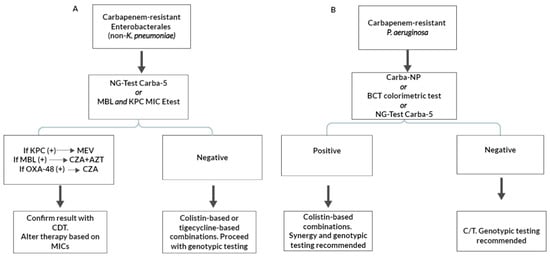
Figure 3.
Suggested diagnostic and treatment algorithms for carbapenem-resistant Enterobacterales (non-Klebsiella pneumoniae) (A) and Pseudomonas aeruginosa (B) based on carbapenemase production. KPC: Klebsiella pneumoniae carbapenemase; MBL: metallo-β-lactamase; MEV: meropenem/vaborbactam; CZA: ceftazidime/avibactam; AZT: aztreonam; CDT: combination disc testing; BCT: Blue Carba Test; and C/T: ceftolozane/tazobactam.
K. pneumoniae isolates can produce KPC, MBL, or both and might even possess other non-carbapenemase-related resistance mechanisms. A colorimetric test such as the Blue Carba Test (BCT) or the Carba NP cannot distinguish carbapenemase types and, therefore, has limited use for the initial screening of carbapenem-resistant K. pneumoniae. NG-Test Carba-5 testing is a reasonable choice due to its ability to detect most carbapenemases as well as double carbapenemase producers. If the rapid test is positive for a double carbapenemase (such as KPC + VIM or KPC + NDM), the treatment options include CZA + AZT or tigecycline- or polymyxin-based therapies. A subsequent CDT, primed for detecting double carbapenemase producers, is a suitable confirmatory test [71,72]. Genotypic testing is also not unreasonable, considering the relatively unknown sensitivity of most phenotypic testing in accurately detecting double producers. If the initial rapid test is positive for one carbapenemase type, the diagnostic algorithm continues with CDT, with the aim of confirming the absence of double carbapenemase producers. The initial treatment should be tailored to the initial rapid results: if the result is KPC-positive, treatment is recommended with MEV; if it is MBL-positive, CZA + AZT should be administered; and, if the result is OXA-48-positive, CZA should be used. If CDT confirms the initial rapid testing result, no further diagnostic action is needed. If the CDT and NG-Test Carba-5 results are discordant, treatment should be changed to CZA + AZT, and genotypic testing is considered mandatory in this case.
A negative NG-Test Carba-5 result has two possible interpretations: either the isolate is carbapenem-resistant due to non-carbapenemase-related mechanisms or the production of a rare carbapenemase type (e.g., GES carbapenemase), or, alternatively, the result is a false negative. In all cases, genotypic testing is recommended to determine the presence of non-carbapenemase-mediated resistance. Although CZA + AZT could be considered for treatment, polymyxin- or tigecycline-based therapies are recommended pending confirmatory genotypic and MIC results. In all isolated K. pneumoniae isolates, synergy testing with CZA + AZT is recommended due to the high rate of MBL production. Laboratories should consider adding additional antimicrobial synergy testing based on the novel BL/BLI combinations and colistin with carbapenems, aminoglycosides, and fosfomycin for all double carbapenemase producers, as indicated by NG-Test Carba 5, and for all patients with severe diseases. This is based on studies that, although demonstrating no differences in terms of a reduction in mortality between combination and monotherapies, have cited possible indication biases as a cause for the lack of benefit seen from combination therapies in addition to the delays associated with initiating CZA therapies [126].
Determining carbapenem resistance in non-K. pneumoniae Enterobacterales, such as carbapenem-resistant E. coli, is less-challenging considering the more predictable phenotype (Figure 3). A simple KPC or MBL Etest strip [127] could suffice as an initial and cheap screening tool, while NG-Test Carba 5 is a suitable alternative. If the initial screening test is positive for KPC production, MEV should be used. If an MBL is detected, then CZA + AZT should be provided. In the latter case, CZA + AZT synergy should be tested in vitro. A confirmatory CDT could also be suggested, since the detection of MBL by means of MBL Etests, especially when EDTA is used as the MBL inhibitor, has been associated with false-positive results [128]. If however, initial testing with either Etest strips or NG-Test Carba-5 are negative, genotypic testing is highly recommend, and treatment with polymyxin- or tigecycline-based combinations are needed.
Finally, P. aeruginosa isolates should initially be approached with a simple colorimetric test (Figure 3). The high specificity of the test means that a negative result excludes carbapenemase production, and, thus, C/T can be administered pending MIC results. Genotypic results are also recommended, but not expected, to provide valuable data that would alter treatment decisions. An NG-Test Carba-5 might also be a suitable alternative.If the test is positive and a carbapenemase is detected (most likely an MBL), the initial treatment should be polymyxin-based, which, while more toxic, might be safer, considering the unpredictable MICs for CZA, IMR, and CZA + AZT [17]. The determination of CZA and IMR MICs and CZA + AZT synergy is considered necessary for carbapenemase-producing P. aeruginosa prior to their use. Moreover, a further characterization of the underlying mechanisms of resistance using molecular methods is highly recommended.
It is of note that different countries can develop various diagnostic approaches based on these epidemiological data. For example, in the USA, KPC production predominates, with double carbapenemase producers being a rare exception [121,129]. Therefore, treatment can be initiated with a positive NG-Test Carba-5, a KPC and MBL Etest strip, or a modified colorimetric test. Subsequent confirmatory testing, if deemed necessary, should be conducted with EDTA-based CIM (eCIM) and modified-CIM (mCIM), since there is no need for double carbapenemase testing.
The diagnostic and therapeutic frameworks proposed can easily be altered and augmented to add new antimicrobial combinations, especially against MBL-producing isolates. The combination of AZT/avibactam has already been demonstrated to be non-inferior to meropenem, without or with colistin, in the treatment of severe Gram-negative infections. Data related to the efficacy of this combination against MBL producers are awaited [130]. In addition, the combination of cefepime with a novel β-lactamase inhibitor, taniborbactam, the first inhibitor which is active against all β-lactamase classes, has been shown to be superior to meropenem in a recent clinical trial featuring patients with cUTIs [131]. More trials are awaited in order to compare the efficacy of these two combinations against CZA + AZT or colistin-based regimens in the treatment of infections caused by MBL-producing Enterobacterales and P. aeruginosa. In addition, cefiderocol, a siderophore cephalosporin resistant to most β-lactamases, including carbapenemases, is increasingly being used for the treatment of multidrug-resistant Gram-negative infections, including carbapenem-resistant Enterobacterales and P. aeruginosa [132]. Although clinical experience with this agent is limited, it has shown promise as a last-line therapy against numerous carbapenem-resistant isolates, including carbapenem-resistant P. aerugionsa and A. baumanni [17,133]. The versatility of streamlined algorithmic processes allows for treatment flexibility based on newer guidelines.
There are, however, certain limitations with our proposition, and the drafting of the algorithm proposed in this paper is based on certain assumptions: (1) rare carbapenemase types such as GES-type carbapenemases are indeed rare among Enterobacterales and P. aeruginosa strains, and, as such, the false-negative rate of the initial NG-Test Carba-5 screening is low; (2) the resistance phenotype of other non-K. pneumoniae carbapenem-resistant Enterobacterales (mainly E. coli) is associated primarily with class A carbapenemases, with no double carbapenemase production; and (3) the patient at hand has not been recently exposed to novel BL/BLIs. In such cases, the probability of mutations either in the carbapenemase itself, in the produced porins, or in efflux pumps is relatively high, and, thus, phenotypic diagnostic tests may have significant disadvantages.
8. Conclusions
Novel BL/BLI combinations have provided new possibilities for the treatment of infections caused by certain CR-GNB. However, given the rising resistance to these agents and the difficulties surrounding current carbapenemase identification methods, adjustments in our diagnostic and therapeutic approaches are needed. Here, we presented in vitro evidence for the use of the novel BL/BLIs as single agents as well as in synergies with other antimicrobials for treating carbapenem-resistant isolates, a practice which might increase BL/BLIs efficacy and reduce resistance rates. Moreover, we discussed the importance of properly interpreting MIC and phenotypic testing results, especially in isolates with conflicting results, and proposed means to identify isolates that might benefit from narrower-spectrum therapies, such as carbapenems, thus sparing the use of the novel BL/BLIs. Finally, to address the absence of a standardized approach to detect carbapenemase production, we proposed a diagnostic algorithm, based on epidemiological data, that will streamline diagnostic pathways and ensure sensible broad antimicrobial coverage, with proper de-escalation when necessary.
Author Contributions
Conceptualization, G.V. and A.T.; methodology, S.F. and C.A.; investigation and formal analysis, S.F., C.A., V.P. and G.V.; writing—original draft preparation, S.F. and C.A.; editing of the draft, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All the data of this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AZT | aztreonam |
| BL/BLI | β-lactam/β-lactamase inhibitor |
| BCT | Blue Carba Test |
| CDT | combination disc testing |
| cIAI | complicated intra-abdominal infection |
| CIM | carbapenem inactivation method |
| CR-GNB | carbapenem-resistant Gram-negative bacteria |
| C/T | ceftolozane/tazobactam |
| cUTI | complicated urinary tract infection |
| CZA | ceftazidime/avibactam |
| eCIM | EDTA-based CIM |
| ESBL | extended-spectrum-β-lactamase |
| FICI | fractional inhibitory concentration index |
| HAP | hospital-acquired pneumonia |
| IMR | imipenem/relebactam |
| IMP | imipenemase |
| KPC | Klebsiella pneumoniae carbapenemase |
| MBL | metallo-β-lactamase |
| mCIM | modified CIM test |
| MEV | meropenem/vaborbactam |
| MIC | minimum inhibitory concentration |
| NDM | New Delhi metallo-β-lactamase |
| OXA | oxacillinase |
| VAP | ventilator-associated pneumonia |
| VIM | Verona integron-mediated metallo-β-lactamase |
| XDR | extensive drug-resistant |
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in Lancet 2022, 400, 1102. [Google Scholar] [CrossRef]
- World Bank Group. Drug-Resistant Infections a Threat to Our Economic Future. 2017. Available online: www.worldbank.org (accessed on 12 December 2023).
- Ruppé, E.; Woerther, P.-L.; Barbier, F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann. Intensive Care 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Shields, R.K.; Chen, L.; Cheng, S.; Chavda, K.D.; Press, E.G.; Snyder, A.; Pandey, R.; Doi, Y.; Kreiswirth, B.N.; Nguyen, M.H.; et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob. Agents Chemother. 2017, 61, e02097-16. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Huang, L.; Zhang, Y.; Sun, Q.; Lu, J.; Zeng, Y.; Dong, N.; Cai, C.; Shen, Z.; et al. The Rapid Emergence of Ceftazidime-Avibactam Resistance Mediated by KPC Variants in Carbapenem-Resistant Klebsiella pneumoniae in Zhejiang Province, China. Antibiotics 2022, 11, 731. [Google Scholar] [CrossRef]
- A Bradford, P.; Urban, C.; Mariano, N.; Projan, S.J.; Rahal, J.J.; Bush, K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC beta-lactamase, and the foss of an outer membrane protein. Antimicrob. Agents Chemother. 1997, 41, 563–569. [Google Scholar] [CrossRef]
- Jean, S.-S.; Harnod, D.; Hsueh, P.-R. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef]
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Guerra, B.; Rodicio, M.R. Resistance to carbapenems in non-typhoidal Salmonella enterica serovars from humans, animals and food. Veter Sci. 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Evans, B.A.; Amyes, S.G.B. OXA β-Lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef]
- Uddin, F.; Imam, S.H.; Khan, S.; Khan, T.A.; Ahmed, Z.; Sohail, M.; Elnaggar, A.Y.; Fallatah, A.M.; El-Bahy, Z.M. NDM Production as a Dominant Feature in Carbapenem-Resistant Enterobacteriaceae Isolates from a Tertiary Care Hospital. Antibiotics 2021, 11, 48. [Google Scholar] [CrossRef]
- Poirel, L.; Hombrouck-Alet, C.; Freneaux, C.; Bernabeu, S.; Nordmann, P. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect. Dis. 2010, 10, 832. [Google Scholar] [CrossRef]
- Deshmukh, D.G.; Damle, A.S.; Bajaj, J.K.; Bhakre, J.B. Metallo-β-lactamase-producing clinical isolates from patients of a tertiary care hospital. J. Lab. Physicians 2011, 3, 93–97. [Google Scholar] [CrossRef]
- Masoud, S.M.; El-Baky, R.M.A.; Aly, S.A.; Ibrahem, R.A. Co-Existence of Certain ESBLs, MBLs and Plasmid Mediated Quinolone Resistance Genes among MDR E. coli Isolated from Different Clinical Specimens in Egypt. Antibiotics 2021, 10, 835. [Google Scholar] [CrossRef]
- Zakhour, J.; El Ayoubi, L.W.; Kanj, S.S. Metallo-beta-lactamases: Mechanisms, treatment challenges, and future prospects. Expert Rev. Anti-Infective Ther. 2024, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Walther-Rasmussen, J.; Høiby, N. OXA-type carbapenemases. J. Antimicrob. Chemother. 2006, 57, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Suzuki, Y.; Okuno, R.; Uchitani, Y.; Ariyoshi, T.; Takemura, N.; Mihara, F.; Mezaki, K.; Ohmagari, N.; Matsui, M.; et al. IMP-68, a Novel IMP-Type Metallo-β-Lactamase in Imipenem-Susceptible Klebsiella pneumoniae. mSphere 2019, 4, e00736-19. [Google Scholar] [CrossRef] [PubMed]
- IDSA Guidelines. Available online: https://www.idsociety.org/practice-guideline/amr-guidance/#Carbapenem-ResistantAcinetobacterbaumannii%C2%A0 (accessed on 10 January 2024).
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, B.R.; Casapao, A.M.; Venugopalan, V. An Update on Existing and Emerging Data for Meropenem-Vaborbactam. Clin. Ther. 2020, 42, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Topola, E. Meropenem/Vaborbactam: β-Lactam/β-Lactamase Inhibitor Combination, the Future in Eradicating Multidrug Resistance. Antibiotics 2023, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of β-lactamase inhibition and impact of resistance mechanisms on activity in Enter-obacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e01443-17. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Tsergouli, K.; Lowrie, K. Efficacy and safety of ceftazidime-avibactam compared to other antimicrobials for the treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae strains, a systematic review and meta-analysis. Microb. Pathog. 2023, 179, 106090. [Google Scholar] [CrossRef]
- Pogue, J.M.; A Bonomo, R.; Kaye, K.S. Ceftazidime/Avibactam, Meropenem/Vaborbactam, or Both? Clinical and Formulary Considerations. Clin. Infect. Dis. 2018, 68, 519–524. [Google Scholar] [CrossRef]
- Dulyayangkul, P.; Ismah, W.A.K.W.N.; Douglas, E.J.A.; Avison, M.B.; Dulyayangkul, P.; Ismah, W.A.K.W.N.; Douglas, E.J.A.; Avison, M.B. Mutation of kvrA Causes OmpK35 and OmpK36 Porin Downregulation and Reduced Meropenem-Vaborbactam Susceptibility in KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2020, 64, e02208-19. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.; Poirel, L.; Nordmann, P. In vitro-obtained meropenem-vaborbactam resistance mechanisms among clinical Klebsiella pneumoniae carbapenemase-producing K. pneumoniae isolates. J. Glob. Antimicrob. Resist. 2023, 32, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Dudley, M.N.; Lomovskaya, O. Meropenem-Vaborbactam Resistance Selection, Resistance Prevention, and Molecular Mechanisms in Mutants of KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2017, 61, e01694-17. [Google Scholar] [CrossRef] [PubMed]
- Di Pietrantonio, M.; Brescini, L.; Candi, J.; Gianluca, M.; Pallotta, F.; Mazzanti, S.; Mantini, P.; Candelaresi, B.; Olivieri, S.; Ginevri, F.; et al. Ceftazidime–Avibactam for the Treatment of Multidrug-Resistant Pathogens: A Retrospective, Single Center Study. Antibiotics 2022, 11, 321. [Google Scholar] [CrossRef]
- Hachem, R.; Reitzel, R.; Rolston, K.; Chaftari, A.-M.; Raad, I. Antimicrobial Activities of Ceftazidime-Avibactam and Comparator Agents against Clinical Bacteria Isolated from Patients with Cancer. Antimicrob. Agents Chemother. 2017, 61, e02106-16. [Google Scholar] [CrossRef]
- Humphries, R.M.; Hindler, J.A.; Wong-Beringer, A.; Miller, S.A. Activity of Ceftolozane-Tazobactam and Ceftazidime-Avibactam against Beta-Lactam-Resistant Pseudomonas aeruginosa Isolates. Antimicrob. Agents Chemother. 2017, 61, e01858-17. [Google Scholar] [CrossRef]
- Alatoom, A.; Elsayed, H.; Lawlor, K.; AbdelWareth, L.; El-Lababidi, R.; Cardona, L.; Mooty, M.; Bonilla, M.-F.; Nusair, A.; Mirza, I. Comparison of antimicrobial activity between ceftolozane–tazobactam and ceftazidime–avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int. J. Infect. Dis. 2017, 62, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-Avibactam Use for Klebsiella pneumoniae Carbapenemase–Producing K. pneumoniae Infections: A Retrospective Observational Multicenter Study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients with Bloodstream Infections Caused by Metallo-β-lactamase–Producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Abbey, T.; Biagi, M.; Wenzler, E. Activity of aztreonam in combination with ceftazidime–avibactam against serine- and metallo-β-lactamase–producing Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2021, 99, 115227. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, E.R.; Dillon, N.; Tsunemoto, H.; Pogliano, J.; Sakoulas, G.; Nizet, V. Avibactam Sensitizes Carbapenem-Resistant NDM-1–Producing Klebsiella pneumoniae to Innate Immune Clearance. J. Infect. Dis. 2019, 220, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Yang, C.; Li, J.; Wang, J.; Huang, W.; Zeng, L.; Liang, X.; Long, W.; Zhang, X. Increased Expression and Amplification of bla KPC-2 Contributes to Resistance to Ceftazidime/Avibactam in a Sequence Type 11 Carbapenem-Resistant Klebsiella pneumoniae Strain. Microbiol. Spectr. 2022, 10, e0095522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Wang, R.; Cai, Y. Resistance to ceftazidime–avibactam and underlying mechanisms. J. Glob. Antimicrob. Resist. 2020, 22, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Alba, J.; Ishii, Y.; Thomson, K.; Moland, E.S.; Yamaguchi, K. Kinetics study of KPC-3, a plasmid-encoded class A carbapenem-hydrolyzing beta-lactamase. Antimicrob. Agents Chemother. 2005, 49, 4760–4762. [Google Scholar] [CrossRef]
- Göttig, S.; Frank, D.; Mungo, E.; Nolte, A.; Hogardt, M.; Besier, S.; Wichelhaus, T.A. Emergence of ceftazidime/avibactam resistance in KPC-3-producing Klebsiella pneumoniae in vivo. J. Antimicrob. Chemother. 2019, 74, 3211–3216. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Chen, L.; Kreiswirth, B.N.; Clancy, C.J. In Vitro Selection of Meropenem Resistance among Ceftazidime-Avibactam-Resistant, Meropenem-Susceptible Klebsiella pneumoniae Isolates with Variant KPC-3 Carbapenemases. Antimicrob. Agents Chemother. 2017, 61, e00079-17. [Google Scholar] [CrossRef]
- Haidar, G.; Clancy, C.J.; Shields, R.K.; Hao, B.; Cheng, S.; Nguyen, M.H. Mutations in bla KPC-3 That Confer Ceftazidime-Avibactam Resistance Encode Novel KPC-3 Variants That Function as Extended-Spectrum β-Lactamases. Antimicrob. Agents Chemother. 2017, 61, e02534-16. [Google Scholar] [CrossRef]
- Gaibani, P.; Lombardo, D.; Foschi, C.; Re, M.C.; Ambretti, S. Evaluation of five carbapenemase detection assays for Enterobacteriaceae harbouring blaKPC variants associated with ceftazidime/avibactam resistance. J. Antimicrob. Chemother. 2020, 75, 2010–2013. [Google Scholar] [CrossRef]
- Cano, Á.; Guzmán-Puche, J.; García-Gutiérrez, M.; Castón, J.J.; Gracia-Ahufinger, I.; Pérez-Nadales, E.; Recio, M.; Natera, A.M.; Marfil-Pérez, E.; Martínez-Martínez, L.; et al. Use of carbapenems in the combined treatment of emerging ceftazidime/avibactam-resistant and carbapenem-susceptible KPC-producing Klebsiella pneumoniae infections: Report of a case and review of the literature. J. Glob. Antimicrob. Resist. 2020, 22, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Kline, E.G.; Jones, C.E.; Morder, K.T.; Mettus, R.T.; Doi, Y.; Nguyen, M.H.; Clancy, C.J.; Shields, R.K. Effects of KPC Variant and Porin Genotype on the In Vitro Activity of Meropenem-Vaborbactam against Carbapenem-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e02048-18. [Google Scholar] [CrossRef]
- Gaibani, P.; Lombardo, D.; Bussini, L.; Bovo, F.; Munari, B.; Giannella, M.; Bartoletti, M.; Viale, P.; Lazzarotto, T.; Ambretti, S. Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella pneumoniae Causing Bloodstream Infections in Northern Italy, 2018. Antibiotics 2021, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Giani, T.; Bovo, F.; Lombardo, D.; Amadesi, S.; Lazzarotto, T.; Coppi, M.; Rossolini, G.M.; Ambretti, S. Resistance to Ceftazidime/Avibactam, Meropenem/Vaborbactam and Imipenem/Relebactam in Gram-Negative MDR Bacilli: Molecular Mechanisms and Susceptibility Testing. Antibiotics 2022, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- FDA. Imipenem/Cilastatin-Relebactam Indications. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-antibiotic-treat-hospital-acquired-bacterial-pneumonia-and-ventilator-associated (accessed on 15 December 2023).
- EMA. Imipenem/Cilastatin-Relebactam Approval. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/recarbrio (accessed on 15 December 2023).
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Zhanel, M.; Lagacé-Wiens, P.R.S.; Walkty, A.; Denisuik, A.; Golden, A.; et al. Imipenem–Relebactam and Meropenem–Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations. Drugs 2017, 78, 65–98. [Google Scholar] [CrossRef]
- Motsch, J.; Murta de Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients with Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020, 70, 1799–1808. [Google Scholar] [CrossRef]
- Livermore, D.M.; Warner, M.; Mushtaq, S. Activity of MK-7655 combined with imipenem against Enterobacteri-aceae and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2013, 68, 2286–2290. [Google Scholar]
- Lob, S.H.; Karlowsky, J.A.; Young, K.; Motyl, M.R.; Hawser, S.; Kothari, N.D.; Sahm, D.F. In vitro activity of imipenem-relebactam against resistant phenotypes of Enterobacteriaceae and Pseudomonas aeruginosa isolated from intraabdominal and urinary tract infection samples–SMART Surveillance Europe 2015–2017. J. Med Microbiol. 2020, 69, 207–217. [Google Scholar] [CrossRef]
- Galani, I.; Souli, M.; Nafplioti, K.; Adamou, P.; Karaiskos, I.; Giamarellou, H.; Antoniadou, A.; Study Collaborators. In vitro activity of imipenem-relebactam against non-MBL carbapenemase-producing Klebsiella pneumoniae isolated in Greek hospitals in 2015–2016. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1143–1150. [Google Scholar] [CrossRef]
- Haidar, G.; Philips, N.J.; Shields, R.K.; Snyder, D.; Cheng, S.; A Potoski, B.; Doi, Y.; Hao, B.; Press, E.G.; Cooper, V.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin. Infect. Dis. 2017, 65, 110–120. [Google Scholar] [CrossRef]
- EMA. Ceftolozane/Tazobactam Approval. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zerbaxa (accessed on 16 December 2023).
- Zhanel, G.G.; Chung, P.; Adam, H.; Zelenitsky, S.; Denisuik, A.; Schweizer, F.; Lagacé-Wiens, P.; Rubinstein, E.; Gin, A.S.; Walkty, A.; et al. Ceftolozane/tazobactam: A novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 2014, 74, 31–51. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Satlin, M.J.; Elabor, A.; Saraiya, N.; McCreary, E.K.; Molnar, E.; El-Beyrouty, C.; Jones, B.M.; Dixit, D.; Heil, E.L.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: A Multicenter Study. Open Forum Infect. Dis. 2018, 5, ofy280. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Siddiqui, F.; Akrich, B.; DeRyke, C.A.; Young, K.; Motyl, M.R.; Hawser, S.P.; Sahm, D.F. In vitro activity of ceftolozane/tazobactam against multidrug-resistant Pseudomonas aeruginosa from patients in Western Europe: SMART 2017–2020. Int. J. Antimicrob. Agents 2023, 61, 106772. [Google Scholar] [CrossRef]
- Kollef, M.H.; Nováček, M.; Kivistik, Ü.; Réa-Neto, Á.; Shime, N.; Martin-Loeches, I.; Timsit, J.-F.; Wunderink, R.G.; Bruno, C.J.; Huntington, J.A.; et al. Ceftolozane–tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): A randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2019, 19, 1299–1311. [Google Scholar] [CrossRef]
- Pogue, J.M.; Kaye, K.S.; Veve, M.P.; Patel, T.S.; Gerlach, A.T.; Davis, S.L.; A Puzniak, L.; File, T.M.; Olson, S.; Dhar, S.; et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-based Regimens for the Treatment of Drug-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2020, 71, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Lob, S.H.; DePestel, D.D.; DeRyke, C.A.; Kazmierczak, K.M.; Young, K.; Motyl, M.R.; Sahm, D.F. Ceftolozane/Tazobactam and Imipenem/Relebactam Cross-Susceptibility Among Clinical Isolates of Pseudomonas aeruginosa from Patients with Respiratory Tract Infections in ICU and Non-ICU Wards-SMART Unit-ed States 2017–2019. Open Forum Infect. Dis. 2021, 8, ofab320. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Lob, S.H.; DeRyke, C.A.; Hilbert, D.W.; Wong, M.T.; Young, K.; Siddiqui, F.; Motyl, M.R.; Sahm, D.F. In Vitro Activity of Ceftolozane-Tazobactam, Imipenem-Relebactam, Ceftazidime-Avibactam, and Comparators against Pseudomonas aeruginosa Isolates Collected in United States Hospitals According to Results from the SMART Surveillance Program, 2018 to 2020. Antimicrob. Agents Chemother. 2022, 66, e0018922. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; Carrière, R.; Bour, M.; Grisot, E.; Triponney, P.; Muller, C.; Lemoine, J.; Jeannot, K.; Plésiat, P.; the GERPA Study Group. Mechanisms of Resistance to Ceftolozane/Tazobactam in Pseudomonas aeruginosa: Results of the GERPA Multicenter Study. Antimicrob. Agents Chemother. 2021, 65, e01117-20. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.F.; De La Cadena, E.; Ríos, R.; García-Betancur, J.C.; Díaz, L.; Reyes, J.; Hernández-Gómez, C.; Radice, M.; Gales, A.C.; Méndez, P.C.; et al. Molecular mechanisms leading to ceftolozane/tazobactam resistance in clinical isolates of Pseudomonas aeruginosa from five Latin American countries. Front. Microbiol. 2022, 13, 1035609. [Google Scholar] [CrossRef]
- Lizza, B.D.; Betthauser, K.D.; Ritchie, D.J.; Micek, S.T.; Kollef, M.H. New Perspectives on Antimicrobial Agents: Ceftolozane-Tazobactam. Antimicrob. Agents Chemother. 2021, 65, e0231820. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Q.; Ponnampalavanar, S.S.L.S.; Chong, C.W.; Karunakaran, R.; Vellasamy, K.M.; Jabar, K.A.; Kong, Z.X.; Lau, M.Y.; Teh, C.S.J. Characterisation of Non-Carbapenemase-Producing Carbapenem-Resistant Klebsiella pneumoniae Based on Their Clinical and Molecular Profile in Malaysia. Antibiotics 2022, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Breakpoint Table. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.1_Breakpoint_Tables.pdf (accessed on 20 December 2023).
- EUCAST. Guidelines for Detection of Carbapenem Resistance. 2017. Available online: https://www.eucast.org/resistance_mechanisms (accessed on 20 December 2023).
- Miriagou, V.; Tzelepi, E.; Kotsakis, S.D.; Daikos, G.L.; Casals, J.B.; Tzouvelekis, L.S. Combined disc methods for the detection of KPC- and/or VIM-positive Klebsiella pneumoniae: Improving reliability for the double carbapenemase producers. Clin. Microbiol. Infect. 2013, 19, E412–E415. [Google Scholar] [CrossRef] [PubMed]
- Tsakris, A.; Poulou, A.; Pournaras, S.; Voulgari, E.; Vrioni, G.; Themeli-Digalaki, K.; Petropoulou, D.; Sofianou, D. A simple phenotypic method for the differentiation of metallo-β-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J. Antimicrob. Chemother. 2010, 65, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, K.; Voets, G.M.; Scharringa, J.; Voskuil, S.; Fluit, A.C.; Rottier, W.C.; Hall, M.A.L.; Stuart, J.W.T.C. A disc diffusion assay for detection of class A, B and OXA-48 carbapenemases in Enterobacteriaceae using phenyl boronic acid, dipicolinic acid and temocillin. Clin. Microbiol. Infect. 2014, 20, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Tsakris, A.; Poulou, A.; Bogaerts, P.; Dimitroulia, E.; Pournaras, S.; Glupczynski, Y. Evaluation of a new phenotypic OXA-48 disk test for differentiation of OXA-48 carbapenemase-producing Enterobacteriaceae clinical isolates. J. Clin. Microbiol. 2015, 53, 1245–1251. [Google Scholar] [CrossRef]
- Sattler, J.; Brunke, A.; Hamprecht, A. Systematic Comparison of Three Commercially Available Combination Disc Tests and the Zinc-Supplemented Carbapenem Inactivation Method (zCIM) for Carbapenemase Detection in Enterobacterales Isolates. J. Clin. Microbiol. 2021, 59, e0314020. [Google Scholar] [CrossRef]
- Pires, J.; Novais, A.; Peixe, L. Blue-carba, an easy biochemical test for detection of diverse carbapenemase producers directly from bacterial cultures. J. Clin. Microbiol. 2013, 51, 4281–4283. [Google Scholar] [CrossRef]
- Tijet, N.; Boyd, D.; Patel, S.N.; Mulvey, M.R.; Melano, R.G. Evaluation of the Carba NP test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4578–4580. [Google Scholar] [CrossRef]
- Pancotto, L.R.; Nodari, C.S.; Rozales, F.P.; Soldi, T.; Siqueira, C.G.; Freitas, A.L.; Barth, A.L. Performance of rapid tests for carbapenemase detection among Brazilian Enterobacteriaceae isolates. Braz. J. Microbiol. 2018, 49, 914–918. [Google Scholar] [CrossRef]
- Noël, A.; Huang, T.-D.; Berhin, C.; Hoebeke, M.; Bouchahrouf, W.; Yunus, S.; Bogaerts, P.; Glupczynski, Y. Comparative Evaluation of Four Phenotypic Tests for Detection of Carbapenemase-Producing Gram-Negative Bacteria. J. Clin. Microbiol. 2017, 55, 510–518. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, V.A.; Beniwal, V.; Pottathil, S. Modified Carba NP Test: Simple and rapid method to differentiate KPC- and MBL-producing Klebsiella species. J. Clin. Lab. Anal. 2018, 32, e22448. [Google Scholar] [CrossRef]
- Nordmann, P.; Kerbol, A.; Bouvier, M.; Sadek, M.; Poirel, L.; Raro, O.H.F. Rapid meropenem/vaborbactam NP test for detecting susceptibility/resistance in Enterobacterales. J. Antimicrob. Chemother. 2023, 78, 2428–2434. [Google Scholar] [CrossRef]
- Kon, H.; Abramov, S.; Frenk, S.; Schwartz, D.; Shalom, O.; Adler, A.; Carmeli, Y.; Lellouche, J. Multiplex lateral flow immunochromatographic assay is an effective method to detect carbapenemases without risk of OXA-48-like cross reactivity. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 61. [Google Scholar] [CrossRef]
- Tarlton, N.J.; Wallace, M.A.; Potter, R.F.; Zhang, K.; Dantas, G.; Dubberke, E.R.; Burnham, C.-A.D.; Yarbrough, M.L. Evaluation of the NG-Test CARBA 5 Lateral Flow Assay with an IMP-27-Producing Morganella morganii and Other Morganellaceae. Microbiol. Spectr. 2023, 11, e0079323. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Sotelo, B.J.; López-Jácome, L.E.; Colín-Castro, C.A.; Hernández-Durán, M.; Martínez-Zavaleta, M.G.; Rivera-Buendía, F.; Velázquez-Acosta, C.; Rodríguez-Zulueta, A.P.; Morfín-Otero, M.d.R.; Franco-Cendejas, R. Comparison of Lateral Flow Immunochromatography and Phenotypic Assays to PCR for the Detection of Carbapenemase-Producing Gram-Negative Bacteria, a Multicenter Experience in Mexico. Antibiotics 2023, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Gelmez, G.A.; Can, B.; Hasdemir, U.; Soyletir, G. Evaluation of phenotypic tests for detection of carbapenemases: New modifications with new interpretation. J. Infect. Chemother. 2021, 27, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jia, P.; Li, X.; Wang, T.; Zhang, J.; Zhang, G.; Duan, S.; Kang, W.; Xu, Y.; Yang, Q. Carbapenemase detection by NG-Test CARBA 5—A rapid immunochromatographic assay in carbapenem-resistant Enterobacterales diagnosis. Ann. Transl. Med. 2021, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M. CIM City: The Game Continues for a Better Carbapenemase Test. J. Clin. Microbiol. 2019, 57, e00353-19. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, M.M.; Hayden, J.A.; Fauntleroy, K.A.; Mazur, C.; Johnson, J.K.; Simner, P.J.; Das, S.; Satlin, M.J.; Jenkins, S.G.; Westblade, L.F. EDTA-modified carbapenem inactivation method: A phenotypic method for detecting metal-lo-β-lactamase-producing Enterobacteriaceae. J. Clin. Microbiol. 2019, 57, e01757-18. [Google Scholar] [CrossRef] [PubMed]
- Haung, S.; Qin, C.; Pu, B.; Zhou, C.; Ma, Y.; Wang, B.; Pan, B.; Hu, B.; Guo, W. 199. Fast Detection of ceftazidime-avibactam-resistant Enterobacterales with VITEK-MSTM incorporating a direct-on-target micro-droplet growth assay. Open Forum. Infect. Dis. 2023, 10, ofad500.272. [Google Scholar] [CrossRef]
- Ghebremedhin, B.; Halstenbach, A.; Smiljanić, M.; Kaase, M.; Ahmad-Nejad, P. MALDI-TOF MS based carbapenemase detection from culture isolates and from positive blood culture vials. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Papagiannitsis, C.C.; Študentová, V.; Izdebski, R.; Oikonomou, O.; Pfeifer, Y.; Petinaki, E.; Hrabák, J. Matrix-assisted laser desorption ionization-time of flight mass spectrometry meropenem hydrolysis assay with NH4HCO3, a reliable tool for direct detection of carbapenemase activity. J. Clin. Microbiol. 2015, 53, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Y.; Cai, J.-C.; Zhou, H.-W.; Zhang, R.; Chen, G.-X. Rapid detection of porins by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Front. Microbiol. 2015, 6, 784. [Google Scholar] [CrossRef] [PubMed]
- Kuai, J.; Zhang, Y.; Lu, B.; Chen, H.; Zhang, Y.; Li, H.; Wang, Y.; Wang, Q.; Wang, H.; Wang, X. In vitro synergistic activity of ceftazidime-avibactam in combination with aztreonam or meropenem against clinical Enterobacterales producing blaKPC or blaNDM. Infect. Drug Resist. 2023, 16, 3171–3182. [Google Scholar] [CrossRef]
- Gaibani, P.; Lewis, R.E.; Volpe, S.L.; Giannella, M.; Campoli, C.; Landini, M.P.; Viale, P.; Re, M.C.; Ambretti, S. In vitro interaction of ceftazidime–avibactam in combination with different antimicrobials against KPC-producing Klebsiella pneumoniae clinical isolates. Int. J. Infect. Dis. 2017, 65, 1–3. [Google Scholar] [CrossRef]
- Gaibani, P.; Ambretti, S.; Viale, P.; Re, M.C. In vitro synergistic activity of meropenem/vaborbactam in combination with ceftazidime/avibactam against KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2019, 74, 1457–1459. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Han, J.; Fan, Y.; Xiong, Z.; Zou, X.; Li, B.; Liu, X.; Li, Z.; Lu, B.; et al. Synergistic activity of imipenem in combination with ceftazidime/avibactam or avibactam against non-MBL-producing extensively drug-resistant Pseudomonas aeruginosa. Microbiol. Spectr. 2022, 10, e0274021. [Google Scholar] [CrossRef]
- Bedenić, B.; Luxner, J.; Car, H.; Sardelić, S.; Bogdan, M.; Varda-Brkić, D.; Šuto, S.; Grisold, A.; Beader, N.; Zarfel, G. Emergence and Spread of Enterobacterales with Multiple Carbapenemases after COVID-19 Pandemic. Pathogens 2023, 12, 677. [Google Scholar] [CrossRef]
- Romina, P.-E.; Lucía, A.; Leticia, C.; Federica, F.; Pablo, Á.; Verónica, S.; Antonio, G.; Inés, B.; Rafael, V. In vitro effectiveness of ceftazidime-avibactam in combination with aztreonam on carbapenemase-producing Enterobacterales. J. Glob. Antimicrob. Resist. 2023, 35, 62–66, Erratum in J. Glob. Antimicrob. Resist. 2023, 35, 355. [Google Scholar] [CrossRef] [PubMed]
- Jayol, A.; Nordmann, P.; Poirel, L.; Dubois, V. Ceftazidime/avibactam alone or in combination with aztreonam against colistin-resistant and carbapenemase-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2017, 73, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Boattini, M.; Comini, S.; Casale, R.; Iannaccone, M.; Cavallo, R.; Costa, C. Occurrence of multi-carbapenemases producers among carbapenemase-producing Enterobacterales and in vitro activity of combinations including cefiderocol, ceftazidime-avibactam, meropenem-vaborbactam, and aztreonam in the COVID-19 era. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, S.; Singh, N.B.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Castanheira, M.; Rybak, M.J. Evaluation of the Synergy of Ceftazidime-Avibactam in Combination with Meropenem, Amikacin, Aztreonam, Colistin, or Fosfomycin against Well-Characterized Multidrug-Resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e00779-19. [Google Scholar] [CrossRef]
- Montero, M.M.; Ochoa, S.D.; López-Causapé, C.; Luque, S.; Sorlí, L.; Campillo, N.; Montesinos, I.L.; Padilla, E.; Prim, N.; Angulo-Brunet, A.; et al. Time-Kill Evaluation of antibiotic combinations containing ceftazidime-avibactam against extensively drug-resistant Pseudomonas aeruginosa and their potential role against ceftazidime-avibactam-resistant isolates. Microbiol. Spectr. 2021, 9, e0058521. [Google Scholar] [CrossRef]
- Oliva, A.; Curtolo, A.; Volpicelli, L.; Dezza, F.C.; De Angelis, M.; Cairoli, S.; Dell’utri, D.; Goffredo, B.M.; Raponi, G.; Venditti, M. Synergistic Meropenem/Vaborbactam Plus Fosfomycin Treatment of KPC producing K. pneumoniae septic thrombosis unresponsive to ceftazidime/avibactam: From the bench to the bedside. Antibiotics 2021, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Miriagou, V.; Cornaglia, G.; Edelstein, M.; Galani, I.; Giske, C.; Gniadkowski, M.; Malamou-Lada, E.; Martinez-Martinez, L.; Navarro, F.; Nordmann, P.; et al. Acquired carbapenemases in Gram-negative bacterial pathogens: Detection and surveillance issues. Clin. Microbiol. Infect. 2010, 16, 112–122. [Google Scholar] [CrossRef]
- Charan, J.; Mulla, S.; Rajdev, S. Antibiotic sensitivity pattern in blaNDM-1-positive and carbapenemase-producing Enterobacteriaceae. Int. J. Appl. Basic Med. Res. 2016, 6, 14–17. [Google Scholar] [CrossRef]
- Haldorsen, B.; Giske, C.G.; Hansen, D.S.; Helgason, K.O.; Kahlmeter, G.; Löhr, I.H.; Matuschek, E.; Österblad, M.; Rantakokko-Jalava, K.; Wang, M.; et al. Performance of the EUCAST disc diffusion method and two MIC methods in detection of Enterobacteriaceae with reduced susceptibility to meropenem: The NordicAST CPE study. J. Antimicrob. Chemother. 2018, 73, 2738–2747, Erratum in J. Antimicrob. Chemother. 2018, 73, 2905. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; De Rosa, F.G.; Giannella, M.; Giacobbe, D.R.; Bassetti, M.; Losito, A.R.; Bartoletti, M.; Del Bono, V.; Corcione, S.; et al. Infections caused by KPC-producing Klebsiella pneumoniae: Differences in therapy and mortality in a multicentre study. J. Antimicrob. Chemother. 2015, 70, 2133–2143. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients with E coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994, Erratum in JAMA 2019, 321, 2370. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Rodriguez-Baňo, J. The Use of Noncarbapenem β-Lactams for the Treatment of Extended-Spectrum β-Lactamase Infections. Clin. Infect. Dis. 2017, 64, 972–980. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Navarro, M.D.; Retamar, P.; Picón, E.; Pascual, Á. Extended-Spectrum Beta-Lactamases–Red Es-pañola de Investigación en Patología Infecciosa/Grupo de Estudio de Infección Hospitalaria Group. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: A post hoc analysis of prospective cohorts. Clin Infect Dis. 2012, 54, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ontong, J.C.; Ozioma, N.F.; Voravuthikunchai, S.P.; Chusri, S. Synergistic antibacterial effects of colistin in combination with aminoglycoside, carbapenems, cephalosporins, fluoroquinolones, tetracyclines, fosfomycin, and piperacillin on multidrug resistant Klebsiella pneumoniae isolates. PLoS ONE 2021, 16, e0244673, Erratum in PLoS ONE 2021, 16, e0251994. [Google Scholar] [CrossRef]
- Adler, A.; Ben-Dalak, M.; Chmelnitsky, I.; Carmeli, Y. Effect of Resistance Mechanisms on the Inoculum Effect of Carbapenem in Klebsiella pneumoniae Isolates with Borderline Carbapenem Resistance. Antimicrob. Agents Chemother. 2015, 59, 5014–5017. [Google Scholar] [CrossRef] [PubMed]
- Golikova, M.V.; Strukova, E.N.; Alieva, K.N.; Ageevets, V.A.; Avdeeva, A.A.; Sulian, O.S.; Zinner, S.H. Meropenem MICs at Standard and High Inocula and Mutant Prevention Concentration Inter-Relations: Comparative Study with Non-Carbapenemase-Producing and OXA-48-, KPC- and NDM-Producing Klebsiella pneumoniae. Antibiotics 2023, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Doyle, T.B.; Kantro, V.; Mendes, R.E.; Shortridge, D. Meropenem-Vaborbactam Activity against Carbapenem-Resistant Enterobacterales Isolates Collected in U.S. Hospitals during 2016 to 2018. Antimicrob. Agents Chemother. 2020, 64, e01951-19. [Google Scholar] [CrossRef]
- Papadomanolaki, A.; Siopi, M.; Karakosta, P.; Vourli, S.; Pournaras, S. Comparative Evaluation of Vitek 2 and Etest versus Broth microdilution for ceftazidime/avibactam and ceftolozane/tazobactam susceptibility testing of Enterobacterales and Pseudomonas aeruginosa. Antibiotics 2022, 11, 865. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Kuo, Y.-W.; Teng, L.-J.; Liao, C.-H.; Hsueh, P.-R. Comparison of Etest and broth microdilution for evaluating the susceptibility of Staphylococcus aureus and Streptococcus pneumoniae to ceftaroline and of carbapenem-resistant Enterobacterales and Pseudomonas aeruginosa to ceftazidime/avibactam. J. Glob. Antimicrob. Resist. 2021, 26, 301–307. [Google Scholar] [CrossRef]
- Tamma, P.D.; Simner, P.J. Phenotypic Detection of Carbapenemase-Producing Organisms from Clinical Isolates. J. Clin. Microbiol. 2018, 56, e01140-18. [Google Scholar] [CrossRef]
- Teo, J.Q.M.; Cai, Y.; Lim, T.-P.; Tan, T.T.; Kwa, A.L.-H. Carbapenem resistance in gram-negative bacteria: The not-so-little problem in the little red dot. Microorgan-isms. Microorganisms 2016, 4, 13. [Google Scholar] [CrossRef]
- Hamprecht, A.; Vehreschild, J.J.; Seifert, H.; Saleh, A. Rapid detection of NDM, KPC and OXA-48 carbapenemases directly from positive blood cultures using a new multiplex immunochromatographic assay. PLoS ONE 2018, 13, e0204157. [Google Scholar] [CrossRef]
- Poulou, A.; Voulgari, E.; Vrioni, G.; Xidopoulos, G.; Pliagkos, A.; Chatzipantazi, V.; Markou, F.; Tsakris, A. Imported Klebsiella pneumoniae carbapenemase-producing K. pneumoniae clones in a Greek hospital: Impact of infection control measures for restraining their dissemination. J. Clin. Microbiol. 2012, 50, 2618–2623. [Google Scholar] [CrossRef]
- Ma, J.; Song, X.; Li, M.; Yu, Z.; Cheng, W.; Yu, Z.; Zhang, W.; Zhang, Y.; Shen, A.; Sun, H.; et al. Global spread of carbapenem-resistant Enterobacteriaceae: Epidemiological features, resistance mechanisms, detection and therapy. Microbiol. Res. 2023, 266, 127249. [Google Scholar] [CrossRef]
- Protonotariou, E.; Meletis, G.; Pilalas, D.; Mantzana, P.; Tychala, A.; Kotzamanidis, C.; Papadopoulou, D.; Papadopoulos, T.; Polemis, M.; Metallidis, S.; et al. Polyclonal endemicity of carbapenemase-producing Klebsiella pneumoniae in ICUs of a Greek tertiary care hos-pital. Antibiotics 2022, 11, 149. [Google Scholar] [CrossRef]
- Tsilipounidaki, K.; Athanasakopoulou, Z.; Müller, E.; Burgold-Voigt, S.; Florou, Z.; Braun, S.D.; Monecke, S.; Gatselis, N.K.; Zachou, K.; Stefos, A.; et al. Plethora of resistance genes in carbapenem-resistant gram-negative bacteria in Greece: No end to a continuous genetic evolution. Microorganisms 2022, 10, 159. [Google Scholar] [CrossRef]
- Epinet Website. Available online: https://epi-net.eu/records/13553/13553/ (accessed on 18 January 2024).
- Tsilipounidaki, K.; Gkountinoudis, C.-G.; Florou, Z.; Fthenakis, G.C.; Miriagou, V.; Petinaki, E. First Detection and Molecular Characterization of Pseudomonas aeruginosa blaNDM-1 ST308 in Greece. Microorganisms 2023, 11, 2159. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Halimi, D.; Zambardi, G.; Nordmann, P. Evaluation of Etest strips for detection of KPC and metallo-carbapenemases in Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2013, 77, 200–201. [Google Scholar] [CrossRef]
- Ratkai, C.; Quinteira, S.; Grosso, F.; Monteiro, N.; Nagy, E.; Peixe, L. Controlling for false positives: Interpreting MBL Etest and MBL combined disc test for the detection of metallo-beta-lactamases. J. Antimicrob. Chemother. 2009, 64, 657–658. [Google Scholar] [CrossRef] [PubMed]
- CDC. Healthcare Associated Infections Report. Available online: https://www.cdc.gov/hai/organisms/cre/trackingcre.html (accessed on 9 January 2024).
- Carmeli, Y.; Cisneros, J.-M.; Paul, M.; Daikos, G.L.; Wang, M.; Cisneros, J.T.; Singer, G.; Titov, I.; Gumenchuk, I.; Zhao, Y.; et al. 2893 A. Efficacy and Safety of Aztreonam-Avibactam for the Treatment of Serious Infections Due to Gram-Negative Bacteria, Including Metallo-β-Lactamase-Producing Pathogens: Phase 3 REVISIT Study. Open Forum Infect. Dis. 2023, 10, ofad500.2476. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Gasink, L.B.; McGovern, P.C.; Moeck, G.; McLeroth, P.; Dorr, M.; Dane, A.; Henkel, T.; CERTAIN-1 Study Team. Cefepime–Taniborbactam in Complicated Urinary Tract Infection. N. Engl. J. Med. 2024, 390, 611–622. [Google Scholar] [CrossRef]
- Wu, J.Y.; Srinivas, P.; Pogue, J.M. Cefiderocol: A Novel Agent for the Management of Multidrug-Resistant Gram-Negative Organisms. Infect. Dis. Ther. 2020, 9, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Fendian, Á.M.; Albanell-Fernández, M.; Tuset, M.; Pitart, C.; Castro, P.; Soy, D.; Bodro, M.; Soriano, A.; Del Río, A.; Martínez, J.A. Real-Life Data on the Effectiveness and Safety of Cefiderocol in Severely Infected Patients: A Case Series. Infect. Dis. Ther. 2023, 12, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).