The Clinical Efficacy of Adding Ceftazidime/Avibactam to Standard Therapy in Treating Infections Caused by Carbapenem-Resistant Klebsiella pneumonia with blaOXA-48-like Genes
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
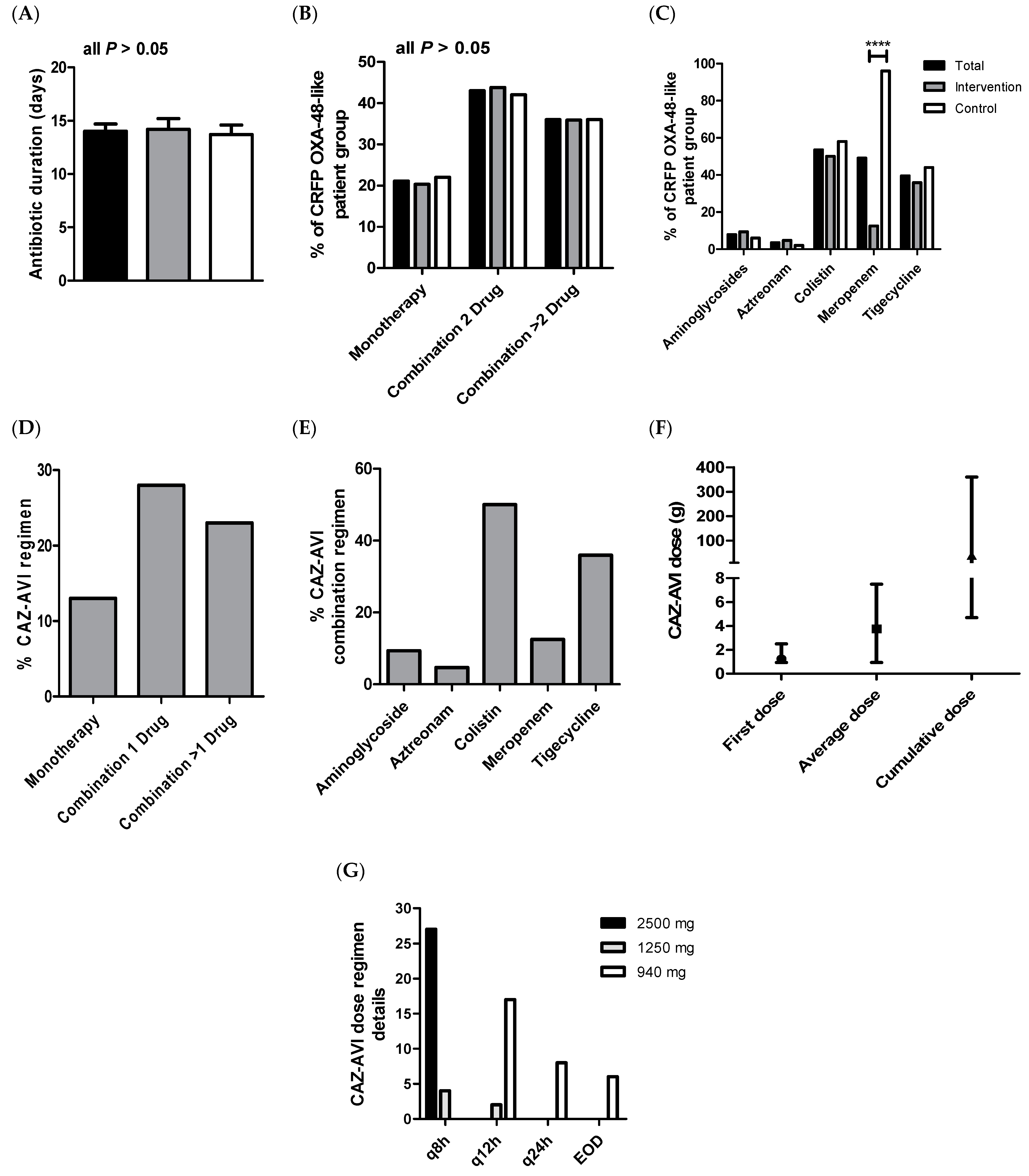
2.2. Antibiotic Medications
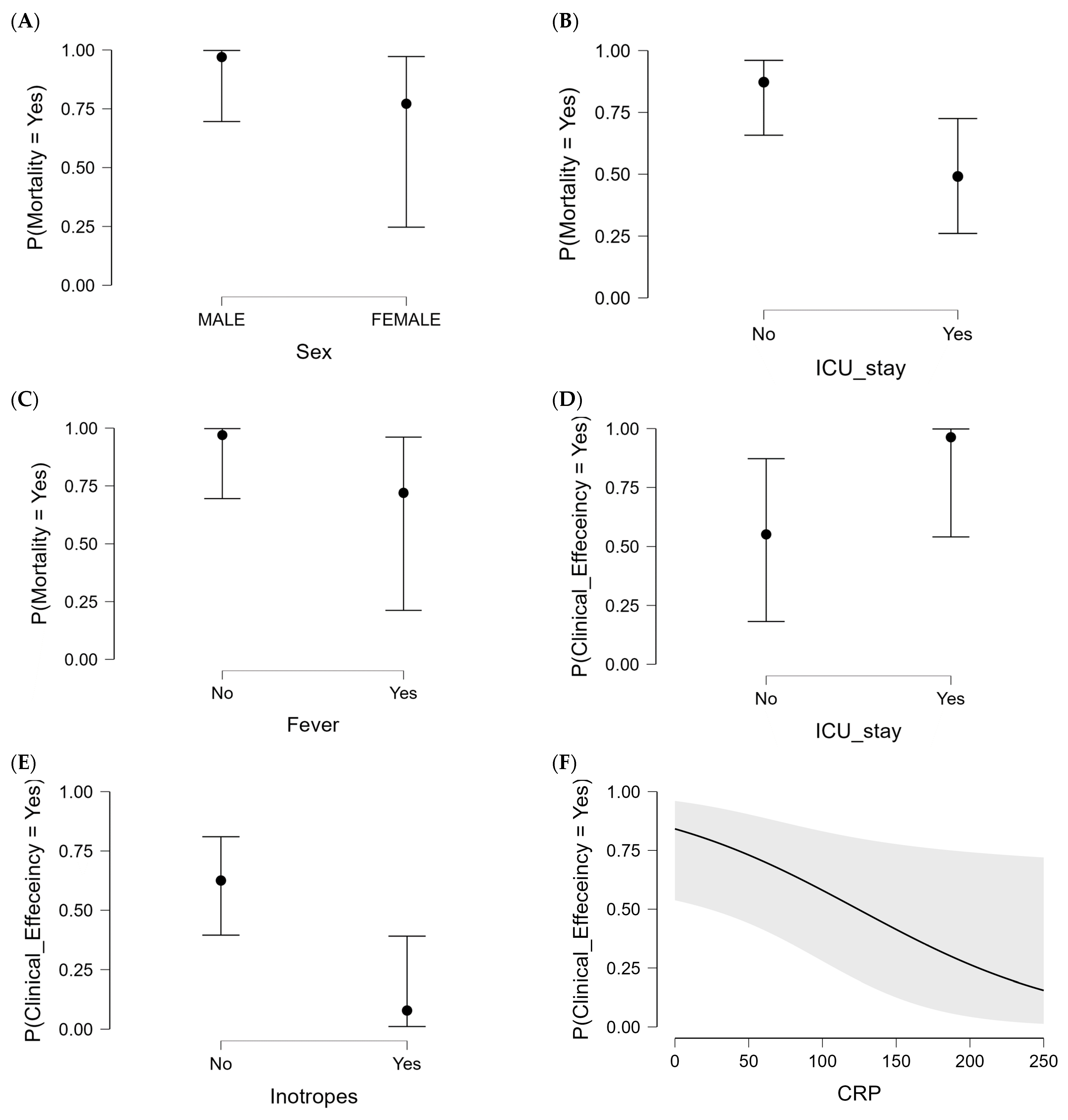
2.3. Parameters Associated with CAZ-AVI Clinical and Microbiological Outcomes
2.3.1. Clinical Efficiency in CAZ-AVI Patient Group
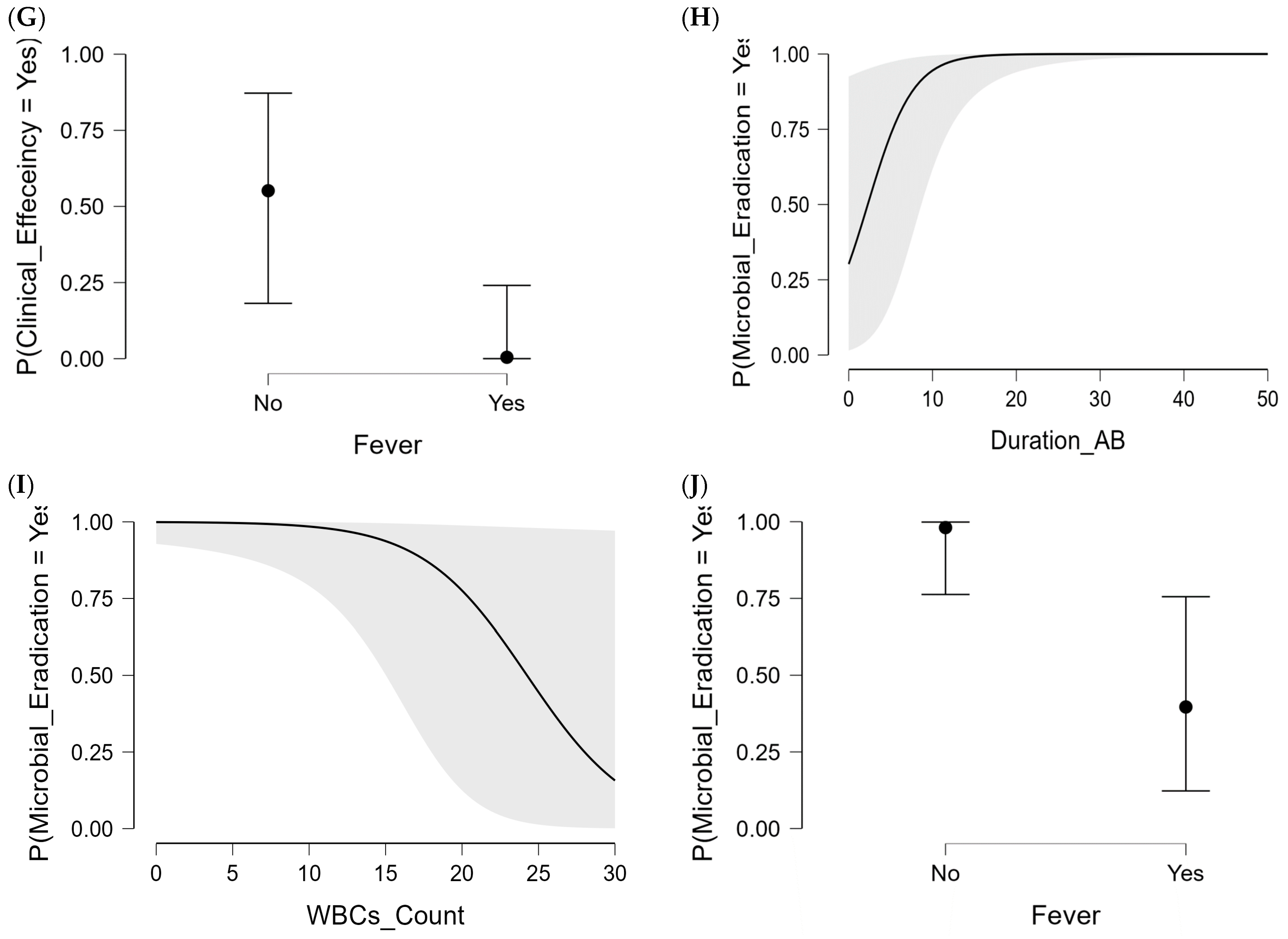
2.3.2. Microbiological Efficiency in CAZ-AVI Patient Group
3. Discussion
4. Materials and Methods
4.1. Ethical Statements and Study Design
4.2. Patients’ Inclusion and Exclusion Criteria
4.3. Data Collection
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surveillance of Antimicrobial Resistance in Europe 2018; Surveillance Report; European Centre for Disease Prevention and Control: Solna, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2018 (accessed on 15 December 2023).
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. MMBR 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, K.S.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: Epidemiology, genetic context, treatment options, and detection methods. Front. Microbiol. 2016, 7, 895. [Google Scholar] [CrossRef] [PubMed]
- Band, V.I.; Satola, S.W.; Burd, E.M.; Farley, M.M.; Jacob, J.T.; Weiss, D.S. Carbapenem-resistant Klebsiella pneumoniae exhibiting clinically undetected colistin heteroresistance leads to treatment failure in a murine model of infection. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Jean, S.S.; Lee, Y.L.; Lu, M.C.; Ko, W.C.; Liu, P.Y.; Hsueh, P.R. Carbapenem-resistant enterobacterales in long-term care facilities: A global and narrative review. Front. Cell Infect. Microbiol. 2021, 11, 601968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Yin, Y.; Chen, H.; Jin, L.; Gu, B.; Xie, L.; Yang, C.; Ma, X.; Li, H.; et al. Epidemiology of carbapenem-resistant enterobacteriaceae infections: Report from the China CRE network. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017; pp. 1–7. [Google Scholar]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the new Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Wu, Z.; Yin, W.; Schwarz, S.; Tyrrell, J.M.; Zheng, Y.; Wang, S.; Shen, Z.; et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2017, 2, 16260. [Google Scholar] [CrossRef]
- Albiger, B.; Glasner, C.; Struelens, M.J.; Grundmann, H.; Monnet, D.L. Carbapenemase-producing enterobacteriaceae in Europe: Assessment by national experts from 38 countries, May 2015. Euro Surveill. Bull. Eur. Mal. Transm. Eur. Commun. Dis. Bull. 2015, 20, 30062. [Google Scholar] [CrossRef]
- Kazi, M.; Drego, L.; Nikam, C.; Ajbani, K.; Soman, R.; Shetty, A.; Rodrigues, C. Molecular characterization of carbapenem-resistant enterobacteriaceae at a tertiary care laboratory in Mumbai. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 467–472. [Google Scholar] [CrossRef]
- Singh-Moodley, A.; Perovic, O. Antimicrobial susceptibility testing in predicting the presence of carbapenemase genes in enterobacteriaceae in South Africa. BMC Infect. Dis. 2016, 16, 536. [Google Scholar] [CrossRef] [PubMed]
- Akturk, H.; Sutcu, M.; Somer, A.; Aydın, D.; Cihan, R.; Ozdemir, A.; Coban, A.; Ince, Z.; Citak, A.; Salman, N. Carbapenem-resistant Klebsiella pneumoniae colonization in pediatric and neonatal intensive care units: Risk factors for progression to infection. Braz. J. Infect. Dis. 2016, 20, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, A.; Kärki, T.; Giambi, C.; Montano, C.; Sisto, A.; Bella, A.; D’Ancona, F. Attributable mortality of carbapenem-resistant Klebsiella pneumoniae infections in a prospective matched cohort study in Italy, 2012–2013. J. Hosp. Infect. 2016, 92, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Borer, A.; Saidel-Odes, L.; Riesenberg, K.; Eskira, S.; Peled, N.; Nativ, R.; Schlaeffer, F.; Sherf, M. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect. Control. Hosp. Epidemiol. 2009, 30, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Ullmann, U. Klebsiella spp. As nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, D.; Kordevani, R.; Keller, N.; Tal, I.; Marzel, A.; Gal-Mor, O.; Maor, Y.; Rahav, G. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin. Microbiol. Infect. 2012, 18, 54–60. [Google Scholar] [CrossRef]
- Kadri, S.S. Key takeaways from the U.S. Cdc’s 2019 antibiotic resistance threats report for frontline providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Hu, F.P.; Guo, Y.; Zhu, D.M. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin. Microbiol. Infect. 2016, 22 (Suppl. S1), S9–S14. [Google Scholar] [CrossRef]
- Rodríguez, O.L.; Sousa, A.; Pérez-Rodríguez, M.T.; Martínez-Lamas, L.; Suárez, R.L.; Martínez, C.T.; Pino, C.P.; Vidal, F.V.; Pérez-Landeiro, A.; Casal, M.C. Mortality-related factors in patients with OXA-48 carbapenemase-producing Klebsiella pneumoniae bacteremia. Medicine 2021, 100, e24880. [Google Scholar] [CrossRef]
- Wang, B.; Pan, F.; Wang, C.; Zhao, W.; Sun, Y.; Zhang, T.; Shi, Y.; Zhang, H. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China. Int. J. Infect. Dis. 2020, 93, 311–319. [Google Scholar] [CrossRef]
- Walsh, T.R. Clinically significant carbapenemases: An update. Curr. Opin. Infect. Dis. 2008, 21, 367–371. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; Nordmann, P.; Poirel, L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Neuner, E.A.; Yeh, J.Y.; Hall, G.S.; Sekeres, J.; Endimiani, A.; Bonomo, R.A.; Shrestha, N.K.; Fraser, T.G.; van Duin, D. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn. Microbiol. Infect. Dis. 2011, 69, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Potoski, B.A.; Marini, R.V.; Doi, Y.; Kreiswirth, B.N.; Clancy, C.J. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Krapp, F.; Grant, J.L.; Sutton, S.H.; Ozer, E.A.; Barr, V.O. Treating complicated carbapenem-resistant enterobacteriaceae infections with ceftazidime/avibactam: A retrospective study with molecular strain characterisation. Int. J. Antimicrob. Agents 2017, 49, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Trecarichi, E.M.; Corona, A.; De Rosa, F.G.; Bassetti, M.; Mussini, C.; Menichetti, F.; Viscoli, C.; Campoli, C.; Venditti, M.; et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin. Infect. Dis. 2019, 68, 355–364. [Google Scholar] [CrossRef]
- Castón, J.J.; Lacort-Peralta, I.; Martín-Dávila, P.; Loeches, B.; Tabares, S.; Temkin, L.; Torre-Cisneros, J.; Paño-Pardo, J.R. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing enterobacteriaceae in hematologic patients. Int. J. Infect. Dis. 2017, 59, 118–123. [Google Scholar] [CrossRef]
- van Duin, D.; Bonomo, R.A. Ceftazidime/avibactam and ceftolozane/tazobactam: Second-generation β-lactam/β-lactamase inhibitor combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xu, J.; Zuo, T.T.; Chen, Y.B. Ceftazidime-avibactam in the treatment of infections from carbapenem-resistant Klebsiella pneumoniae: Ceftazidime-avibactam against CR-KP infections. J. Glob. Antimicrob. Resist. 2021, 26, 20–25. [Google Scholar] [CrossRef]
- Soriano, A.; Carmeli, Y.; Omrani, A.S.; Moore, L.S.P.; Tawadrous, M.; Irani, P. Ceftazidime-avibactam for the treatment of serious gram-negative infections with limited treatment options: A systematic literature review. Infect. Dis. Ther. 2021, 10, 1989–2034. [Google Scholar] [CrossRef]
- Zhen, S.; Wang, H.; Feng, S. Update of clinical application in ceftazidime-avibactam for multidrug-resistant gram-negative bacteria infections. Infection 2022, 50, 1409–1423. [Google Scholar] [CrossRef]
- Mazuski, J.E.; Wagenlehner, F.; Torres, A.; Carmeli, Y.; Chow, J.W.; Wajsbrot, D.; Stone, G.G.; Irani, P.; Bharucha, D.; Cheng, K.; et al. Clinical and microbiological outcomes of ceftazidime-avibactam treatment in adults with gram-negative bacteremia: A subset analysis from the phase 3 clinical trial program. Infect. Dis. Ther. 2021, 10, 2399–2414. [Google Scholar] [CrossRef]
- Zheng, G.; Cai, J.; Zhang, L.; Chen, D.; Wang, L.; Qiu, Y.; Deng, H.; Bai, H.; Bian, X.; He, J. Ceftazidime/avibactam-based versus polymyxin b-based therapeutic regimens for the treatment of carbapenem-resistant Klebsiella pneumoniae infection in critically ill patients: A retrospective cohort study. Infect. Dis. Ther. 2022, 11, 1917–1934. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Tang, H.; Xia, Z.; Yang, W.; Xu, H.; Liu, Z.; Ni, S.; Wang, Z.; Shen, J. In vitro and in vivo antimicrobial activities of ceftazidime/avibactam alone or in combination with aztreonam against carbapenem-resistant enterobacterales. Infect. Drug Resist. 2022, 15, 7107–7116. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F. Carbapenem-resistant enterobacteriaceae: An update narrative review from Saudi Arabia. J. Infect. Public Health 2019, 12, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Hakeam, H.A.; Alsahli, H.; Albabtain, L.; Alassaf, S.; Al Duhailib, Z.; Althawadi, S. Effectiveness of ceftazidime-avibactam versus colistin in treating carbapenem-resistant enterobacteriaceae bacteremia. Int. J. Infect. Dis. 2021, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, A.R.; Korkmaz, L. How to define 30-day mortality? Anatol. J. Cardiol. 2021, 25, 368–369. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Heil, E.; Kuriakose, S.; Bias, T.; Huang, V.; El-Beyrouty, C.; McCoy, D.; Hiles, J.; Richards, L.; Gardner, J.; et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant enterobacteriaceae infections. Antimicrob. Agents Chemother. 2017, 61, e00449-17. [Google Scholar] [CrossRef]
- Shirley, M. Ceftazidime-avibactam: A review in the treatment of serious gram-negative bacterial infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious diseases society of America 2023 guidance on the treatment of antimicrobial resistant gram-negative infections. Clin. Infect. Dis. 2023, ciad428. [Google Scholar] [CrossRef]
- Marcoulides, K.M.; Raykov, T. Evaluation of variance inflation factors in regression models using latent variable modeling methods. Educ. Psychol. Meas. 2019, 79, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.; Mayberry, R.; Bae, S.; Singh, K.; Peter He, Q.; Lillard, J.W., Jr. A study of effects of multicollinearity in the multivariable analysis. Int. J. Appl. Sci. Technol. 2014, 4, 9–19. [Google Scholar]
- Goss-Sampson, M. JASP Documents: Statistical Analysis in JASP; Centre for Science and Medicine in Sport, University of Greenwich: London, UK, 2018. [Google Scholar]
- Lu, Q.; Zhu, H.H.; Li, G.H.; Qi, T.T.; Ye, L.J.; Teng, X.Q.; Qu, Q.; He, G.F.; Qu, J. A comparative study of the microbiological efficacy of polymyxin b on different carbapenem-resistant gram-negative bacteria infections. Front. Med. 2021, 8, 620885. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Harnod, D.; Hsueh, P.R. Global threat of carbapenem-resistant gram-negative bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, G.H.; Qu, Q.; Zhu, H.H.; Luo, Y.; Yan, H.; Yuan, H.Y.; Qu, J. Clinical efficacy of polymyxin b in patients infected with carbapenem-resistant organisms. Infect. Drug Resist. 2021, 14, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Doyle, T.B.; Collingsworth, T.D.; Sader, H.S.; Mendes, R.E. Increasing frequency of OXA-48-producing enterobacterales worldwide and activity of ceftazidime/avibactam, meropenem/vaborbactam and comparators against these isolates. J. Antimicrob. Chemother. 2021, 76, 3125–3134. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, B.L.; Karlowsky, J.A.; Kazmierczak, K.M.; Biedenbach, D.J.; Sahm, D.F.; Nichols, W.W. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible enterobacteriaceae isolates collected during the inform global surveillance study (2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 3163–3169. [Google Scholar] [CrossRef]
- Spiliopoulou, I.; Kazmierczak, K.; Stone, G.G. In vitro activity of ceftazidime/avibactam against isolates of carbapenem-non-susceptible enterobacteriaceae collected during the inform global surveillance programme (2015–17). J. Antimicrob. Chemother. 2020, 75, 384–391. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious diseases society of america guidance on the treatment of extended-spectrum β-lactamase producing enterobacterales (ESBL-E), carbapenem-resistant enterobacterales (CRE), and pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. Aeruginosa). Clin. Infect. Dis. 2020, 72, e169–e183. [Google Scholar]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Martin, K.; Arif, F.; Sultan-Ali, I.; Velamuri, S.R.; Hill, D.M. Analysis of ceftazidime/avibactam use for treating carbapenem-resistant infections in critically ill patients with thermal or inhalation injuries. J. Burn. Care Res. 2022, 43, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Di Pietrantonio, M.; Brescini, L.; Candi, J.; Gianluca, M.; Pallotta, F.; Mazzanti, S.; Mantini, P.; Candelaresi, B.; Olivieri, S.; Ginevri, F.; et al. Ceftazidime-avibactam for the treatment of multidrug-resistant pathogens: A retrospective, single center study. Antibiotics 2022, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Balandín, B.; Ballesteros, D.; Pintado, V.; Soriano-Cuesta, C.; Cid-Tovar, I.; Sancho-González, M.; Pérez-Pedrero, M.J.; Chicot, M.; Asensio-Martín, M.J.; Silva, J.A.; et al. Multicentre study of ceftazidime/avibactam for gram-negative bacteria infections in critically ill patients. Int. J. Antimicrob. Agents 2022, 59, 106536. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, P.; Zhou, Y. Analysis of the clinical application of ceftazidime-avibactam in China. J. Infect. Public Health 2022, 15, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, J.; Ding, H.; Liao, G. Efficacy of ceftazidime-avibactam in the treatment of carbapenem-resistant Klebsiella pneumoniae infection after kidney transplantation. Infect. Drug Resist. 2021, 14, 5165–5174. [Google Scholar] [CrossRef] [PubMed]
- Castón, J.J.; Gallo, M.; García, M.; Cano, A.; Escribano, A.; Machuca, I.; Gracia-Aufinger, I.; Guzman-Puche, J.; Pérez-Nadales, E.; Recio, M.; et al. Ceftazidime-avibactam in the treatment of infections caused by kpc-producing Klebsiella pneumoniae: Factors associated with clinical efficacy in a single-center cohort. Int. J. Antimicrob. Agents 2020, 56, 106075. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, H.; Alghamdi, A.; Alobaidallah, N.; Alfayez, A.; Almousa, R.; Albagli, R.; Shamas, N.; Farahat, F.; Mahmoud, E.; Bosaeed, M.; et al. Evaluation of ceftazidime/avibactam for treatment of carbapenemase-producing carbapenem-resistant enterobacterales with OXA-48 and/or NDM genes with or without combination therapy. JAC-Antimicrob. Resist. 2022, 4, dlac104. [Google Scholar] [CrossRef]
- Alraddadi, B.M.; Saeedi, M.; Qutub, M.; Alshukairi, A.; Hassanien, A.; Wali, G. Efficacy of ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. BMC Infect. Dis. 2019, 19, 772. [Google Scholar] [CrossRef]
- Chen, F.; Zhong, H.; Yang, T.; Shen, C.; Deng, Y.; Han, L.; Chen, X.; Zhang, H.; Qian, Y. Ceftazidime-avibactam as salvage treatment for infections due to carbapenem-resistant Klebsiella pneumoniae in liver transplantation recipients. Infect. Drug Resist. 2021, 14, 5603–5612. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-avibactam use for Klebsiella pneumoniae carbapenemase-producing K. pneumoniae infections: A retrospective observational multicenter study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef] [PubMed]
- Nagvekar, V.; Shah, A.; Unadkat, V.P.; Chavan, A.; Kohli, R.; Hodgar, S.; Ashpalia, A.; Patil, N.; Kamble, R. Clinical outcome of patients on ceftazidime-avibactam and combination therapy in carbapenem-resistant enterobacteriaceae. Indian J. Crit. Care Med. Peer-Rev. 2021, 25, 780–784. [Google Scholar]
- Chen, Y.; Huang, H.B.; Peng, J.M.; Weng, L.; Du, B. Efficacy and safety of ceftazidime-avibactam for the treatment of carbapenem-resistant enterobacterales bloodstream infection: A systematic review and meta-analysis. Microbiol. Spectr. 2022, 10, e0260321. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Q.; Chen, X.; Wu, J.; Wu, Y.; Teng, G.; Huang, M. Ceftazidime/avibactam versus polymyxin b in the challenge of carbapenem-resistant pseudomonas aeruginosa infection. Infect. Drug Resist. 2022, 15, 655–667. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
| Variables | Total Admitted Patients (n = 114) | Intervention (n = 64) | Control (n = 50) | p-Values |
|---|---|---|---|---|
| Age (years) * | 71 (20.0–102.0) | 75 (20.0–102.0) | 69 (27.0–97.0) | 0.094 |
| Sex (Female) | 48 (42.1%) | 25 (39.1%) | 23 (46.0%) | 0.556 |
| ICU admissions | 85 (74.6%) | 42 (65.6%) | 43 (86.0%) | 0.017 |
| Inotropes ** | 56 (49.1%) | 27 (42.2%) | 29 (58.0%) | 0.131 |
| Sites of infections | ||||
| Multi-site infection *** | 28 (24.6%) | 11 (17.20%) | 17 (34.0%) | 0.663 |
| Bloodstream | 30 (26.3%) | 18 (28.1%) | 12 (24.0%) | 0.053 |
| Respiratory tract | 31 (27.2%) | 14 (21.9%) | 17 (34.0%) | 1.000 |
| Soft tissues | 21 (18.4%) | 18 (28.1%) | 3 (6.0%) | 0.003 |
| Urinary tract | 4 (3.5%) | 3 (4.7%) | 1 (2.0%) | 0.318 |
| Co-morbidities | ||||
| Respiratory diseases | 37 (32.5%) | 20 (31.3%) | 17 (34.0%) | 0.841 |
| Cardiovascular diseases | 83 (72.8%) | 46 (71.9%) | 37 (74.0%) | 0.835 |
| Diabetes mellitus | 69 (60.5%) | 40 (62.5%) | 29 (58.0%) | 0.701 |
| Kidney diseases | 46 (40.4%) | 26 (40.6%) | 20 (40.0%) | 1.000 |
| Central nervous system diseases | 16 (14.0%) | 8 (12.5%) | 8 (16.0%) | 0.594 |
| Cerebrovascular diseases | 23 (20.2%) | 10 (15.6%) | 13 (26.0%) | 0.240 |
| Gastrointestinal diseases | 5 (4.4%) | 3 (4.7%) | 2 (4.0%) | 1.000 |
| Septic shock/sepsis | 29 (25.4%) | 15 (23.4%) | 14 (28.0%) | 0.666 |
| Tumors | 2 (1.7%) | 1 (1.6%) | 1 (2.0%) | 1.000 |
| COVID-19 infections | 21 (18.4%) | 13 (20.3%) | 8 (16.0%) | 0.631 |
| Antibiotic usage | ||||
| Duration time of treatment (days) * | 14.0 (±0.7) | 14.2 (±1.0) | 13.7 (±0.9) | 0.881 |
| Monotherapy | 24 (21.1%) | 13 (20.3%) | 11 (22.0%) | 0.825 |
| Combinations of two agents | 49 (43.0%) | 28 (43.8%) | 21 (42.0%) | 1.000 |
| Combinations of ≥triple agents | 41 (36.0%) | 23 (35.9%) | 18 (36.0%) | 1.000 |
| Aminoglycosides | 9 (7.9%) | 6 (9.4%) | 3 (6.0%) | 0.729 |
| Aztreonam | 4 (3.5%) | 3 (4.7%) | 1 (2.0%) | 0.630 |
| Colistin | 61 (53.5%) | 32 (50.0%) | 29 (58.0%) | 0.452 |
| Meropenem | 56 (49.1%) | 8 (12.5%) | 48 (96.0%) | <0.0001 |
| Tigecycline | 45 (39.5%) | 23 (35.9%) | 22 (44.0%) | 0.442 |
| Lab and clinical signs | ||||
| WBC counts (×109/L) * | 12.0 (±0.7) | 11.3 (±0.7) | 13.0 (±1.2) | 0.355 |
| Neutrophil counts (×109/L) * | 12.7 (±2.0) | 12.7 (±2.1) | 12.6 (±1.9) | 0.261 |
| C-reactive protein (mg/L) * | 121.0 (±11.7) | 80.1 (±8.7) | 169.3 (±21.4) | 0.0011 |
| Fervescence **** | 62 (54.4%) | 27 (42.2%) | 35 (70.0%) | 0.004 |
| Outcomes * | Total Admitted Patients (n = 114) | Intervention (n = 64) | Control (n = 50) | p-Values |
|---|---|---|---|---|
| Clinical success | 38 (33.3%) | 27 (42.2%) | 11 (22.0%) | 0.028 |
| Microbial eradication | 65 (57.0%) | 44 (68.8%) | 21 (42.0%) | 0.007 |
| Bacterial recurrence | 17 (14.9%) | 5 (7.8%) | 12 (24.0%) | 0.019 |
| 30-day all-cause mortality | 67 (58.7%) | 32 (50.0%) | 35 (70.0%) | 0.036 |
| Total Monotherapy Patients (n = 24) | Intervention Monotherapy (n = 13) | Control Monotherapy (n = 11) | p-Values | |
| Clinical success | 9 (37.5%) | 6 (46.2%) | 3 (27.3%) | 0.423 |
| Microbial eradication | 14 (58.3%) | 10 (76.9%) | 4 (36.4%) | 0.095 |
| Bacterial recurrence | 7 (29.2%) | 3 (23.1%) | 4 (36.4%) | 0.659 |
| 30-day all-cause mortality | 15 (62.5%) | 9 (69.2%) | 6 (54.5%) | 0.675 |
| Total Combined therapy Patients (n = 90) | Intervention Add-on Therapy (n = 51) | Control Combined Therapy (n = 39) | p-Values | |
| Clinical success | 29 (32.2%) | 21 (41.2%) | 8 (20.5%) | 0.043 |
| Microbial eradication | 51 (56.7%) | 34 (66.7%) | 17 (43.6%) | 0.034 |
| Bacterial recurrence | 10 (11.11%) | 2 (3.9%) | 8 (20.5%) | 0.018 |
| 30-day all-cause mortality | 52 (57.8%) | 23 (45.1%) | 29 (74.4%) | 0.009 |
| Variables | Clinical Efficiency | 30-Day All-Cause Mortality | ||||
|---|---|---|---|---|---|---|
| CAZ-AVI Treatment Success (n = 27) | CAZ-AVI Treatment Failure (n = 37) | p-Values | CAZ-AVI Patient Survived (n = 32) | CAZ-AVI Patient Deceased (n = 32) | p-Values | |
| Age (years) * | 71 (27.0–97.0) | 67 (31.0–93.0) | 0.960 | 69 (27.0–97.0) | 70 (39.0–95.0) | 0.333 |
| Sex (Female) | 7 (25.9%) | 18 (48.6%) | 0.026 | 8 (25.0%) | 17 (53.1%) | 0.039 |
| ICU admissions | 13 (48.1%) | 29 (78.4%) | 0.017 | 14 (43.8%) | 28 (87.5%) | 0.017 |
| Inotropes | 4 (14.8%) | 23 (62.2%) | <0.001 | 4 (12.5%) | 23 (71.9%) | <0.0001 |
| Sites of infections | ||||||
| Multi-site infection | 5 (18.5%) | 6 (16.2%) | 1.000 | 7 (21.9%) | 4 (12.5%) | 0.509 |
| Bloodstream | 6 (22.2%) | 12 (32.4%) | 0.413 | 8 (25%) | 10 (31.3%) | 0.782 |
| Respiratory tract | 5 (18.5%) | 9 (24.3%) | 0.761 | 6 (18.8%) | 8 (25.0%) | 0.763 |
| Soft tissues | 12 (44.4%) | 6 (16.2%) | 0.023 | 13 (40.6%) | 5 (15.6%) | 0.049 |
| Urinary tract | 1 (3.7%) | 2 (5.4%) | 1.000 | 0 (0.0%) | 3 (9.4%) | 0.238 |
| Co-morbidities | ||||||
| Respiratory diseases | 8 (29.6%) | 12 (32.4%) | 1.000 | 8 (25.0%) | 12 (37.5%) | 0.419 |
| Cardiovascular diseases | 21 (77.8%) | 25 (67.6%) | 0.413 | 22 (68.8%) | 24 (75.0%) | 0.782 |
| Diabetes mellitus | 17 (63.0%) | 23 (62.1%) | 1.000 | 18 (56.3%) | 22 (68.8%) | 0.439 |
| Kidney diseases | 11 (40.7%) | 15 (40.5%) | 1.000 | 12 (37.5%) | 14 (43.75%) | 0.799 |
| Central nervous system diseases | 3 (11.1%) | 5 (13.5%) | 1.000 | 4 (12.5%) | 4 (12.5%) | 1.000 |
| Cerebrovascular diseases | 4 (14.8%) | 6 (16.2%) | 1.000 | 4 (12.5%) | 6 (18.8%) | 0.732 |
| Gastrointestinal diseases | 1 (3.7%) | 2 (5.4%) | 1.000 | 1 (3.1%) | 2 (6.3%) | 1.000 |
| Septic shock/sepsis | 4 (14.8%) | 11 (29.7%) | 0.235 | 3 (9.4%) | 12 (37.5%) | 0.016 |
| Tumors | 0 (0.0%) | 1 (2.7%) | 1.000 | 0 (0.0%) | 1 (3.1%) | 1.000 |
| COVID-19 infections | 5 (18.5%) | 8 (21.6%) | 1.000 | 3 (9.4%) | 10 (31.3%) | 0.060 |
| Antibiotic usage | ||||||
| CAZ-AVI duration therapy (days) * | 16.8 (±1.7) | 11.6 (±1.0) | 0.003 | 16.1 (±1.6) | 11.4 (±0.8) | 0.037 |
| CAZ-AVI monotherapy | 6 (22.2%) | 7 (18.9%) | 0.763 | 5 (15.6%) | 8 (25%) | 0.536 |
| Combinations of two agents | 13 (48.1%) | 15 (40.5%) | 0.615 | 16 (50.0%) | 12 (37.5%) | 0.450 |
| Combinations of ≥ triple agents | 10 (37.0%) | 13 (35.1%) | 1.000 | 13 (40.6%) | 10 (31.3%) | 0.603 |
| Aminoglycosides | 2 (7.4%) | 4 (10.8%) | 1.000 | 5 (15.6%) | 1 (3.1%) | 0.105 |
| Aztreonam | 1 (3.7%) | 2 (5.4%) | 1.000 | 1 (3.1%) | 2 (6.3%) | 1.000 |
| Colistin | 13 (50.0%) | 19 (51.4%) | 1.000 | 14 (43.8%) | 18 (56.3%) | 0.454 |
| Meropenem | 3 (48.1%) | 5 (13.5%) | 1.000 | 5 (15.6%) | 3 (9.4%) | 0.708 |
| Tigecycline | 12 (44.4%) | 11 (29.7%) | 0.294 | 11 (34.4%) | 12 (37.5%) | 1.000 |
| CAZ-AVI first dosage (g) | 2.5 (0.94–2.5) | 0.94 (0.94–2.5) | 0.223 | 2.5 (0.94–2.5) | 0.94 (0.94–2.5) | 0.023 |
| CAZ-AVI average dosage (g/day) | 4.4 (1.9–7.5) | 2.2 (0.94–7.5) | 0.100 | 7.5 (0.94–7.5) | 2.2 (0.94–7.5) | 0.016 |
| CAZ-AVI cumulative dosage (g) | 60.0 (9.4–360.0) | 37.1 (4.7–157.5) | 0.002 | 66.7 (6.6–360.0) | 29.7 (4.7–157.5) | <0.001 |
| Lab and clinical signs | ||||||
| WBC counts (×109/L) * | 7.6 (±0.5) | 13.5 (±1.2) | <0.001 | 8.5 (±0.7) | 13.1 (±1.3) | 0.016 |
| Neutrophil counts (×109/L) * | 7.12 (±2.1) | 15.0 (±2.9) | <0.001 | 9.2 (±2.1) | 13.3 (±2.7) | 0.007 |
| C-reactive protein (mg/L) * | 25.1 (±5.6) | 112.0 (±9.7) | <0.0001 | 47.5 (±9.4) | 108.2 (±11.5) | <0.0001 |
| Fervescence | 2 (7.4%) | 25 (67.6%) | <0.0001 | 4 (12.5%) | 23 (71.9%) | <0.0001 |
| Variables | Microbial Eradication | Bacterial Recurrence | ||||
|---|---|---|---|---|---|---|
| CAZ-AVI Infection Eradicated (n = 44) | CAZ-AVI Infection Persistent (n = 20) | p-Values | CAZ-AV Infection Relapsed (n = 5) | CAZ-AVI Infection Receded (n = 59) | p-Values | |
| Age (years) * | 69 (27.0–97.0) | 70 (39.0–95.0) | 0.300 | 69 (49.0–77.0) | 70 (27.0–97.0) | 0.582 |
| Sex (Female) | 8 (18.2%) | 17 (85.0%) | 0.011 | 0 (0.0%) | 25 (42.4%) | 0.147 |
| ICU admissions | 24 (54.5%) | 18 (90.0%) | 0.009 | 2 (40.0%) | 40 (67.8%) | 0.329 |
| Inotropes | 11 (25.0%) | 16 (80.0%) | <0.0001 | 0 (0.0%) | 27 (45.8%) | 0.068 |
| Sites of infections | ||||||
| Multi-site infection | 8 (18.2%) | 3 (15.0%) | 1.000 | 2 (40.0%) | 9 (15.3%) | 0.201 |
| Bloodstream | 10 (22.7%) | 8 (40.0%) | 0.230 | 2 (40.0%) | 16 (27.1%) | 0.615 |
| Respiratory tract | 10 (22.7%) | 4 (20.0%) | 1.000 | 1 (20.0%) | 13 (22.0%) | 1.000 |
| Soft tissues | 15 (34.1%) | 3 (15.0%) | 0.143 | 0 (0.0%) | 18 (30.5%) | 0.310 |
| Urinary tract | 2 (4.5%) | 1 (5.0%) | 1.000 | 0 (0.0%) | 3 (5.1%) | 1.000 |
| Co-morbidities | ||||||
| Respiratory diseases | 10 (22.7%) | 10 (50.0%) | 0.042 | 0 (0.0%) | 20 (33.9%) | 0.314 |
| Cardiovascular diseases | 32 (72.7%) | 14 (70.0%) | 1.000 | 4 (80.0%) | 42 (71.2%) | 1.000 |
| Diabetes mellitus | 29 (65.9%) | 11 (55.0%) | 0.419 | 2 (40.0%) | 38 (64.4%) | 0.355 |
| Kidney diseases | 17 (38.6%) | 9 (45.0%) | 0.784 | 2 (40.0%) | 24 (40.7%) | 1.000 |
| Central nervous system diseases | 6 (13.6%) | 2 (10.0%) | 1.000 | 1 (20.0%) | 7 (11.9%) | 0.499 |
| Cerebrovascular diseases | 7 (15.9%) | 3 (15.0%) | 0.728 | 1 (20.0%) | 9 (15.3%) | 1.000 |
| Gastrointestinal diseases | 1 (2.3%) | 2 (10.0%) | 0.228 | 0 (0.0%) | 3 (5.1%) | 1.000 |
| Septic shock/sepsis | 7 (15.9%) | 8 (40.0%) | 0.207 | 0 (0.0%) | 15 (25.4%) | 0.329 |
| Tumors | 0 (0.0%) | 1 (5.0%) | 0.313 | 0 (0.0%) | 1 (1.7%) | 1.000 |
| COVID-19 infections | 4 (9.1%) | 9 (45.0%) | 0.002 | 0 (0.0%) | 13 (22.0%) | 0.574 |
| Antibiotic usage | ||||||
| CAZ-AVI duration therapy (days) * | 16.0 (±1.2) | 8.7 (±0.8) | <0.0001 | 17.4 (±2.5) | 13.4 (±1.0) | 0.100 |
| CAZ-AVI Monotherapy | 10 (22.7%) | 3 (15.0%) | 0.739 | 0 (0.0%) | 13 (22.0%) | 0.574 |
| Combinations of two agents | 20 (45.5%) | 8 (40.0%) | 0.789 | 4 (80.0%) | 24 (40.7%) | 0.159 |
| Combinations of ≥triple agents | 15 (34.1%) | 8 (40.0%) | 0.780 | 1 (20.0%) | 22 (37.3%) | 0.646 |
| Aminoglycosides | 5 (11.4%) | 1 (5.0%) | 0.656 | 1 (20.0%) | 5 (8.5%) | 0.399 |
| Aztreonam | 1 (2.3%) | 2 (10.0%) | 0.228 | 0 (0.0%) | 3 (5.1%) | 1.000 |
| Colistin | 21 (47.7%) | 11 (55.0%) | 0.788 | 3 (60.0%) | 29 (49.2%) | 1.000 |
| Meropenem | 6 (13.6%) | 2 (10.0%) | 1.000 | 0 (0.0%) | 8 (13.6%) | 1.000 |
| Tigecycline | 16 (36.4%) | 7 (35.0%) | 1.000 | 2 (40.0%) | 21 (35.6%) | 1.000 |
| CAZ-AVI first dosage (g) | 1.25 (0.94–2.5) | 0.94 (0.94–2.5) | 0.217 | 2.5 (0.94–2.5) | 1.25 (0.94–2.5) | 0.346 |
| CAZ-AVI average dosage (g/day) | 3.8 (0.94–7.5) | 2.2 (0.94–7.5) | 0.133 | 5.0 (1.9–7.5) | 3.8 (0.94–7.5) | 0.434 |
| CAZ-AVI cumulative dosage (g) | 54.4 (6.1–360.0) | 19.4 (4.7–127.5) | <0.001 | 105.0 (18.8–127.5) | 39.5 (4.7–360.0) | 0.080 |
| Lab and clinical signs | ||||||
| WBC counts (×109/L) * | 8.2 (±0.5) | 16.5 (±1.6) | <0.0001 | 7.1 (±0.7) | 11.1 (±0.8) | 0.154 |
| Neutrophil counts (×109/L) * | 8.0 (±1.8) | 18.2 (±4.0) | <0.0001 | 3.9 (±0.7) | 11.9 (±2.0) | 0.035 |
| C-reactive protein (mg/L) * | 59.8 (±9.8) | 114.3 (±11.7) | <0.001 | 21.1 (±9.6) | 80.9 (±8.7) | 0.025 |
| Fervescence | 9 (20.5%) | 18 (90.0%) | 0.017 | 1 (20.0%) | 26 (44.1%) | 0.387 |
| Variables * | 30-Day All-Cause Mortality | Clinical Efficiency | ||||||
| B | ORs (95% CI) | p-Values | VIF (TI) | B | ORs (95% CI) | p-Values | VIF (TI) | |
| Sex | −2.252 | 0.105 (–4.200; −0.305) | 0.023 | 1.78 (0.56) | −1.598 | 0.202 (–4.229; 1.033) | 0.234 | 1.95 (0.51) |
| ICU admissions | −1.957 | 0.141 (–3.518; −0.397) | 0.014 | 1.45 (0.69) | 3.053 | 21.183 (–0.102; 6.208) | 0.048 | 3.13 (0.32) |
| Inotropes | −0.801 | 0.449 (–2.827; 1.223) | 0.438 | 1.92 (0.52) | −2.974 | 0.051 (–4.795; −1.153) | 0.001 | 1.91 (0.53) |
| Soft tissues | 0.002 | 1.002 (–2.166; 2.169) | 0.999 | 1.73 (0.58) | ||||
| Septic shock/sepsis | −0.750 | 0.473 (–2.970; 1.470) | 0.508 | 1.55 (0.65) | ||||
| CAZ-AVI duration | 0.049 | 0.952 (–0.219; 0.121) | 0.572 | 1.70 (0.59) | ||||
| CAZ-AVI cumulative | 0.005 | 1.005 (–0.013; 0.024) | 0.563 | 1.31 (0.77) | 0.008 | 1.008 (–0.020; 0.036) | 0.583 | 1.93 (0.52) |
| WBC counts | 0.005 | 1.005 (–0.164; 0.175) | 0.951 | 1.52 (0.66) | −0.235 | 0.790 (–0.564; 0.093) | 0.160 | 1.73 (0.58) |
| Neutrophil counts | 0.030 | 1.031 (–0.025; 0.085) | 0.282 | 1.27 (0.79) | −0.020 | 0.981 (–0.125; 0.086) | 0.715 | 1.33 (0.75) |
| C-reactive protein | −0.002 | 0.998 (–0.016; 0.012) | 0.766 | 1.85 (0.54) | −0.013 | 0.987 (–0.026; −0.000) | 0.043 | 1.81 (0.55) |
| Fervescence | −2.524 | 0.080 (–4.465; −0.582) | 0.011 | 1.70 (0.59) | −5.596 | 0.004 (–9.507; −1.685) | 0.004 | 2.46 (0.41) |
| Variables * | Microbial Eradication | Bacterial Recurrence | ||||||
| B | ORs (95% CI) | p-Values | VIF (TI) | B | ORs (95% CI) | p-Values | VIF (TI) | |
| Sex | −2.866 | 0.057 (–6.318; 0.586) | 0.104 | 3.50 (0.29) | ||||
| ICU admissions | −1.952 | 0.142 (–4.441; 0.536) | 0.124 | 1.08 (0.93) | ||||
| Inotropes | −1.620 | 0.198 (–3.434; 0.195) | 0.080 | 1.36 (0.74) | ||||
| Respiratory diseases | 1.373 | 3.949 (–0989; 3.736) | 0.255 | 1.56 (0.64) | ||||
| COVID-19 infections | 0.476 | 1.609 (–2.066; 3.018) | 0.714 | 1.47 (0.68) | ||||
| CAZ-AVI duration | 0.368 | 1.446 (0.101; 0.636) | 0.007 | 1.26 (0.79) | ||||
| CAZ-AVI cumulative | 0.002 | 1.002 (–0.028; 0.031) | 0.920 | 1.32 (0.76) | ||||
| WBC counts | −0.292 | 0.747 (–0.563; −0.021) | 0.034 | 1.48 (0.67) | ||||
| Neutrophil counts | −0.004 | 0.996 (–0.063; 0.055) | 0.903 | 1.34 (0.75) | −0.298 | 0.742 (–0.842; 0.247) | 0.284 | 1.05 (0.95) |
| C-reactive protein | −0.004 | 0.996 (–0.018; 0.010) | 0.598 | 1.09 (0.92) | −0.022 | 0.978 (–0.060; 0.016) | 0.257 | 1.05 (0.95) |
| Fervescence | −4.353 | 0.013 (–7.499; −1.206) | 0.007 | 2.75 (0.36) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Jaber, A.M.R.; Basgut, B.; Hawan, A.A.; Al Shehri, A.A.; AlKahtani, S.A.; Ahmed, N.J.; Abdi, A. The Clinical Efficacy of Adding Ceftazidime/Avibactam to Standard Therapy in Treating Infections Caused by Carbapenem-Resistant Klebsiella pneumonia with blaOXA-48-like Genes. Antibiotics 2024, 13, 265. https://doi.org/10.3390/antibiotics13030265
Abu Jaber AMR, Basgut B, Hawan AA, Al Shehri AA, AlKahtani SA, Ahmed NJ, Abdi A. The Clinical Efficacy of Adding Ceftazidime/Avibactam to Standard Therapy in Treating Infections Caused by Carbapenem-Resistant Klebsiella pneumonia with blaOXA-48-like Genes. Antibiotics. 2024; 13(3):265. https://doi.org/10.3390/antibiotics13030265
Chicago/Turabian StyleAbu Jaber, Al Maamon R., Bilgen Basgut, Ali Abdullah Hawan, Ali Amer Al Shehri, Sultan Ahmad AlKahtani, Nehad J. Ahmed, and Abdikarim Abdi. 2024. "The Clinical Efficacy of Adding Ceftazidime/Avibactam to Standard Therapy in Treating Infections Caused by Carbapenem-Resistant Klebsiella pneumonia with blaOXA-48-like Genes" Antibiotics 13, no. 3: 265. https://doi.org/10.3390/antibiotics13030265
APA StyleAbu Jaber, A. M. R., Basgut, B., Hawan, A. A., Al Shehri, A. A., AlKahtani, S. A., Ahmed, N. J., & Abdi, A. (2024). The Clinical Efficacy of Adding Ceftazidime/Avibactam to Standard Therapy in Treating Infections Caused by Carbapenem-Resistant Klebsiella pneumonia with blaOXA-48-like Genes. Antibiotics, 13(3), 265. https://doi.org/10.3390/antibiotics13030265






