Conundrums in the Management of Febrile Infants under Three Months of Age and Future Research
Abstract
1. Introduction
2. Methods
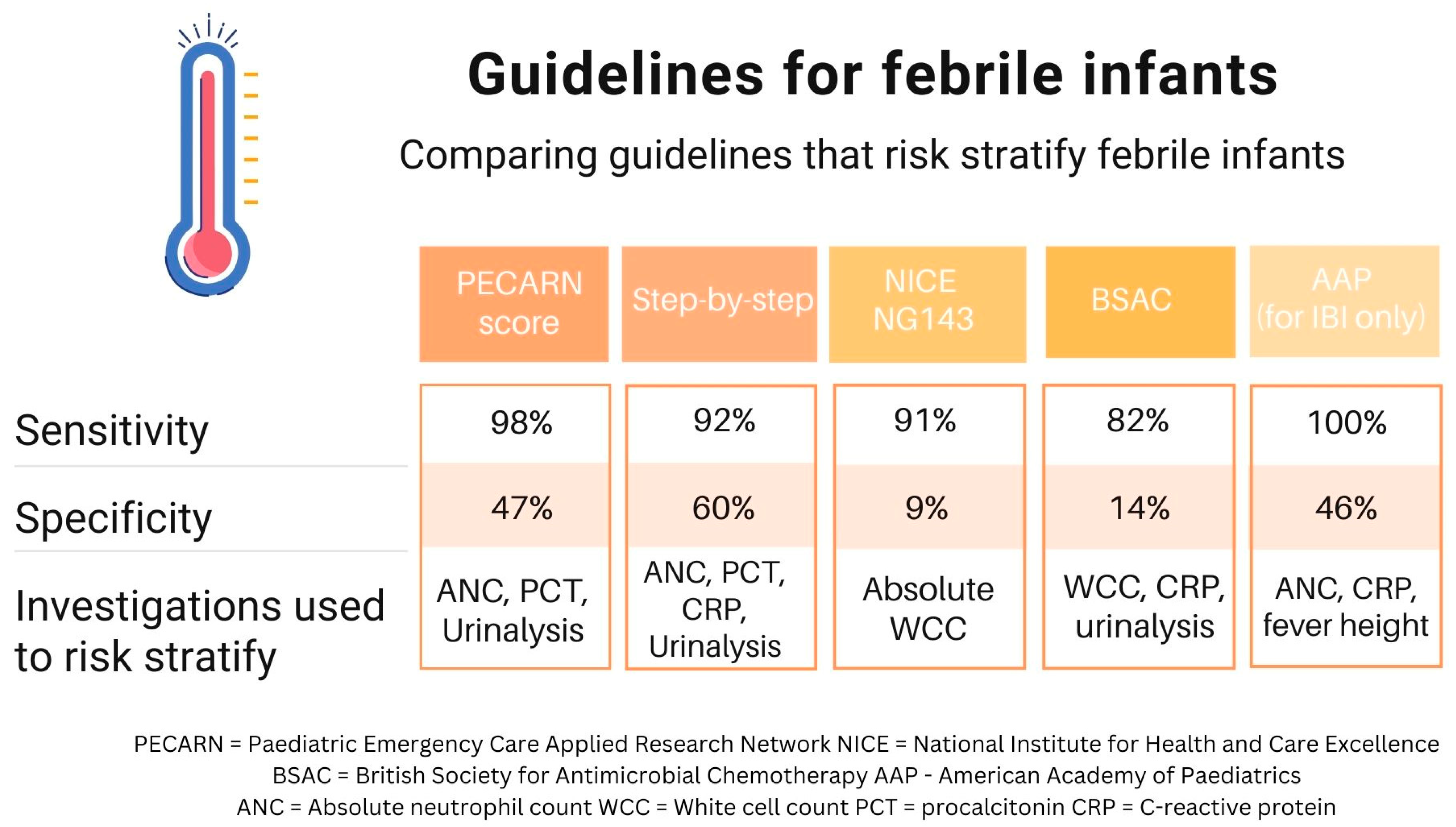
3. Clinical Practice Guidelines and the Variation in Practice Worldwide
4. The Variation in Invasive Bacterial Infection Rate According to Age
5. Febrile Infant Conundrums
5.1. They “Felt Hot at Home” but Have No Fever Now—Should I Worry?
5.2. The Baby’s Urine Dip Is Positive, How Reliable Is This?
5.3. Do Infants with Urinary Tract Infection Routinely Require a Lumbar Puncture to Exclude Meningitis?
5.4. Do I Need to Worry about Fever in an Infant following Vaccination?
5.5. Should a Positive Viral Respiratory Swab Alter the Management of Febrile Infants?
6. Future Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dagan, R.; Sofer, S.; Phillip, M.; Shachak, E. Ambulatory care of febrile infants younger than 2 months of age classified as being at low risk for having serious bacterial infections. J. Pediatr. 1988, 112, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Pantell, R.H.; Roberts, K.B.; Adams, W.G.; Dreyer, B.P.; Kuppermann, N.; O’leary, S.T.; Okechukwu, K.; Woods, C.R.; Infants, S.O.F. Clinical Practice Guideline: Evaluation and Management of Well-Appearing Febrile Infants 8 to 60 Days Old. Pediatrics 2021, 148, e2021052228. [Google Scholar] [CrossRef] [PubMed]
- Aronson, P.L.; Thurm, C.; Alpern, E.R.; Alessandrini, E.A.; Williams, D.J.; Shah, S.S.; Nigrovic, L.E.; McCulloh, R.J.; Schondelmeyer, A.; Tieder, J.S.; et al. Variation in Care of the Febrile Young Infant <90 Days in US Pediatric Emergency Departments. Pediatrics 2014, 134, 667–677. [Google Scholar] [PubMed]
- Kuppermann, N.; Dayan, P.S.; Levine, D.A.; Vitale, M.; Tzimenatos, L.; Tunik, M.G.; Saunders, M.; Ruddy, R.M.; Roosevelt, G.; Rogers, A.J.; et al. A Clinical Prediction Rule to Identify Febrile Infants 60 Days and Younger at Low Risk for Serious Bacterial Infections. JAMA Pediatr. 2019, 173, 342–351. [Google Scholar] [CrossRef]
- Tan, C.D.; van der Walle, E.E.P.L.; Vermont, C.L.; von Both, U.; Carrol, E.D.; Eleftheriou, I.; Emonts, M.; van der Flier, M.; de Groot, R.; Herberg, J.; et al. Guideline adherence in febrile children below 3 months visiting European Emergency Departments: An observational multicenter study. Eur. J. Pediatr. 2022, 181, 4199–4209. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef]
- The South Australian Paediatric Clinical Practice Guideline; Fever in Children Aged 1–2 Months [Internet]. Available online: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/resources/policies/fever+in+children+aged+1-2+months+-+sa+paediatric+clinical+guideline (accessed on 12 November 2023).
- NICE: Fever in under 5s: Assessment and Initial Management [Internet]. Available online: https://www.nice.org.uk/guidance/ng143/chapter/Recommendations#management-by-the-paediatric-specialist (accessed on 2 February 2023).
- British Society for Antimicrobial Chemotherapy (BSAC): Paediatric Pathways. Infant <90 Days of Age with Fever and No Source. [Internet]. Available online: https://bsac.org.uk/paediatricpathways/febrile-infant-aged-90-days.php (accessed on 4 January 2023).
- Gomez, B.; Mintegi, S.; Bressan, S.; Da Dalt, L.; Gervaix, A.; Lacroix, L. Validation of the “Step-by-Step” Approach in the Management of Young Febrile Infants. Pediatrics 2016, 138, e20154381. [Google Scholar] [CrossRef]
- Fisher, K.A.; Landyn, V.; Lindenauer, P.K.; Walkey, A.J. Procalcitonin Test Availability: A Survey of Acute Care Hospitals in Massachusetts. Ann. Am. Thorac. Soc. 2017, 14, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Burstein, B.; Gravel, J.; Aronson, P.L.; Neuman, M.I.; Pediatric Emergency Research Canada (PERC). Emergency department and inpatient clinical decision tools for the management of febrile young infants among tertiary paediatric centres across Canada. Paediatr. Child Health 2019, 24, e142–e154. [Google Scholar] [CrossRef]
- Waterfield, T.; Lyttle, M.D.; Munday, C.; Foster, S.; McNulty, M.; Platt, R.; Barrett, M.; Rogers, E.; Durnin, S.; Jameel, N.; et al. Validating clinical practice guidelines for the management of febrile infants presenting to the emergency department in the UK and Ireland. Arch. Dis. Child. 2022, 107, 329. [Google Scholar] [CrossRef] [PubMed]
- IMCI Chart Booklet. Integrated Management of Childhood Illness: Management of the Sick Young Infant Aged up to 2 Months. [Internet]; World Health Organization: Geneva, Switzerland, 2019; License: CC BY-NC-SA 3.0 IGO; Available online: www.who.int/publications/i/item/9789241516365 (accessed on 2 February 2023).
- Gera, T.; Shah, D.; Garner, P.; Richardson, M.; Sachdev, H.S. Integrated management of childhood illness (IMCI) strategy for children under five. Cochrane Database Syst. Rev. 2016, 2016, CD010123. [Google Scholar] [CrossRef] [PubMed]
- Lishman, J.; Smit, L.; Redfern, A. Infants 21–90 days presenting with a possible serious bacterial infection—Are evaluation algorithms from high income countries applicable in the South African public health sector? Afr. J. Emerg. Med. 2021, 11, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Procalcitonin Testing for Diagnosing and Monitoring Sepsis, 2016. [Internet]. NICE Guideline. Available online: https://www.nice.org.uk/guidance/dg18 (accessed on 2 February 2023).
- Esposito, S.; Rinaldi, V.E.; Argentiero, A.; Farinelli, E.; Cofini, M.; D’alonzo, R.; Mencacci, A.; Principi, N. Approach to Neonates and Young Infants with Fever without a Source Who Are at Risk for Severe Bacterial Infection. Mediat. Inflamm. 2018, 2018, 4869329. [Google Scholar] [CrossRef] [PubMed]
- Mintegi, S.; Bressan, S.; Gomez, B.; Da Dalt, L.; Blázquez, D.; Olaciregui, I.; de la Torre, M.; Palacios, M.; Berlese, P.; Benito, J. Accuracy of a sequential approach to identify young febrile infants at low risk for invasive bacterial infection. Emerg. Med. J. 2014, 31, e19. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, N. The PECARN Rule for Low Risk Febrile Infants 29–60 Days Old [Internet]. Available online: https://www.mdcalc.com/calc/10204/pecarn-rule-low-risk-febrile-infants-29-60-days-old#evidence (accessed on 4 February 2023).
- Burstein, B.; Alathari, N.; Papenburg, J. Guideline-Based Risk Stratification for Febrile Young Infants Without Procalcitonin Measurement. Pediatrics 2022, 149, e2021056028. [Google Scholar] [CrossRef] [PubMed]
- NICE. NICE Guideline [NG51] Sepsis: Recognition, Diagnosis and Early Management [Internet]. NICE Guideline. 2017. Available online: https://www.nice.org.uk/guidance/ng51 (accessed on 5 February 2023).
- Alonso-Ojembarrena, A.; Martínez-Díaz, J.V.; Lechuga-Sancho, A.M.; Galán-Sánchez, F.; Lubián-López, S.P. Broad spectrum antibiotics in newborns increase multi-drug resistant infections. J. Chemother. 2019, 31, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Tsai, J.D.; Ku, M.S.; Chen, S.M.; Liao, P.F.; Hung, T.W.; Tsai, M.-L.; Sheu, J.-N. Antimicrobial Resistance and Diagnostic Imaging in Infants Younger than 2 Months Old Hospitalized with a First Febrile Urinary Tract Infection: A Population-based Comparative Study. Pediatr. Infect. Dis. J. 2016, 35, 840–845. Available online: https://journals.lww.com/pidj/fulltext/2016/08000/antimicrobial_resistance_and_diagnostic_imaging_in.3.aspx (accessed on 5 March 2023). [CrossRef]
- Pantell, R.H.; Newman, T.B.; Bernzweig, J.; Bergman, D.A.; Takayama, J.I.; Segal, M.; Finch, S.A.; Wasserman, R.C. Management and Outcomes of Care of Fever in Early Infancy. JAMA 2004, 291, 1203–1212. [Google Scholar] [CrossRef]
- Umana, E.; Norman-Bruce, H.; Mills, C.; Mitchell, H.; McFetridge, L.; Waterfield, T.; Febrile Infants Diagnostic Assessment and Outcome Study Group. Applying the American Academy of Pediatrics guideline to a cohort of febrile infants attending emergency departments in the UK and Ireland. Eur. J. Emerg. Med. Off. J. Eur. Soc. Emerg. Med. 2023, 30, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, L.; Gomez, B.; Pintos, C.; Benito, J.; Mintegi, S. Prevalence of Bacterial Infection in Febrile Infant 61–90 Days Old Compared with Younger Infants. Pediatr. Infect. Dis. J. 2019, 38, 1163–1167. Available online: https://journals.lww.com/pidj/Fulltext/2019/12000/Prevalence_of_Bacterial_Infection_in_Febrile.2.aspx (accessed on 5 March 2023). [CrossRef]
- Mintegi, S.; Gomez, B.; Carro, A.; Diaz, H.; Benito, J. Invasive bacterial infections in young afebrile infants with a history of fever. Arch. Dis. Child. 2018, 103, 665. [Google Scholar] [CrossRef] [PubMed]
- Katz-Sidlow, R.J.; Rowberry, J.P.; Ho, M. Fever Determination in Young Infants: Prevalence and Accuracy of Parental Palpation. Pediatr. Emerg. Care 2009, 25, 12–14. Available online: https://journals.lww.com/pec-online/fulltext/2009/01000/fever_determination_in_young_infants__prevalence.3.aspx (accessed on 6 March 2023). [CrossRef]
- Callanan, D. Detecting Fever in Young Infants: Reliability of Perceived, Pacifier, and Temporal Artery Temperatures in Infants Younger than 3 Months of Age. Pediatr. Emerg. Care 2003, 19, 240–243. Available online: https://journals.lww.com/pec-online/fulltext/2003/08000/detecting_fever_in_young_infants__reliability_of.4.aspx (accessed on 3 April 2023). [CrossRef]
- Edwards, G.; Fleming, S.; Verbakel, J.Y.; Bruel, A.v.D.; Hayward, G. Accuracy of parents’ subjective assessment of paediatric fever with thermometer measured fever in a primary care setting. BMC Prim. Care 2022, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.R.; Chang, P.W.; Shen, M.W.; Biondi, E.A.; Greenhow, T.L. Diagnostic Accuracy of the Urinalysis for Urinary Tract Infection in Infants <3 Months of Age. Pediatrics 2015, 135, 965–971. [Google Scholar] [CrossRef] [PubMed]
- NICE Guideline NG224. Urinary Tract Infection in under 16s: Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2022. [Google Scholar]
- Roberts, K.B. Subcommittee on Urinary Tract Infection SC on QI and M. Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months. Pediatrics 2011, 128, 595–610. [Google Scholar] [CrossRef]
- Society, C.P. Urinary Tract Infection in Infants and Children: Diagnosis and Management|Canadian Paediatric Society [Internet]. Available online: https://cps.ca/en/documents/position/urinary-tract-infections-in-children (accessed on 23 October 2023).
- Velasco, R.; Benito, H.; Mozun, R.; E Trujillo, J.; A Merino, P.; de la Torre, M.; Gomez, B.; on behalf of the Group for the Study of Febrile Infant of the RiSEUP-SPERG Network. Using a urine dipstick to identify a positive urine culture in young febrile infants is as effective as in older patients. Acta Paediatr. 2015, 104, e39–e44. [Google Scholar] [CrossRef] [PubMed]
- Tzimenatos, L.; Mahajan, P.; Dayan, P.S.; Vitale, M.; Linakis, J.G.; Blumberg, S.; Borgialli, D.; Ruddy, R.M.; Van Buren, J.; Ramilo, O.; et al. Accuracy of the Urinalysis for Urinary Tract Infections in Febrile Infants 60 Days and Younger. Pediatrics 2018, 141, e20173068. [Google Scholar] [CrossRef]
- Waterfield, T.; Foster, S.; Platt, R.; Barrett, M.J.; Durnin, S.; Maney, J.-A.; Roland, D.; McFetridge, L.; Mitchell, H.; Umana, E.; et al. Diagnostic test accuracy of dipstick urinalysis for diagnosing urinary tract infection in febrile infants attending the emergency department. Arch. Dis. Child. 2022, 107, 1095–1099. [Google Scholar] [CrossRef]
- Mahajan, P.; VanBuren, J.M.; Tzimenatos, L.; Cruz, A.T.; Vitale, M.; Powell, E.C.; Leetch, A.N.; Pickett, M.L.; Brayer, A.; Nigrovic, L.E.; et al. Serious Bacterial Infections in Young Febrile Infants with Positive Urinalysis Results. Pediatrics 2022, 150, e2021055633. [Google Scholar] [CrossRef]
- Burstein, B.; Sabhaney, V.; Bone, J.N.; Doan, Q.; Mansouri, F.F.; Meckler, G.D. Prevalence of Bacterial Meningitis Among Febrile Infants Aged 29-60 Days with Positive Urinalysis Results: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e214544. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Lejarzegi, A.; Gomez, B.; de la Torre, M.; Duran, I.; Camara, A.; de la Rosa, D.; Manzano, S.; Rodriguez, J.; González, A.; et al. Febrile young infants with abnormal urine dipstick at low risk of invasive bacterial infection. Arch. Dis. Child. 2020, 106, 758–763. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Riordan, A. The yin and yang of fever after meningococcal B vaccination. Arch. Dis. Child. 2017, 102, 881. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Bourke, T.; Maney, J.-A.; Moriarty, P. Emergency department attendance following 4-component meningococcal B vaccination in infants. Arch. Dis. Child. 2017, 102, 899. [Google Scholar] [CrossRef]
- Wolff, M.; Bachur, R. Serious Bacterial Infection in Recently Immunized Young Febrile Infants. Acad. Emerg. Med. 2009, 16, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-Parrado, A.; Lopez, E.; Gomez, B.; Lejarzegi, A.; Fernandez-Uria, A.; Benito, J.; Mintegi, S. Rate of invasive bacterial infection in recently vaccinated young infants with fever without source. Arch. Dis. Child. 2022, 107, 995. [Google Scholar] [CrossRef]
- Channon-Wells, S.W.; Tough, E.; So, N.; O’Connor, D.; Snape, M.D. Differentiating vaccine reactions from invasive bacterial infections in young infants presenting to the emergency department in the 4CMenB era: A retrospective observational comparison. BMJ Paediatr. Open 2022, 6, e001559. [Google Scholar] [CrossRef]
- Doan, Q.; Enarson, P.; Kissoon, N.; Klassen, T.P.; Johnson, D.W. Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Browne, L.R.; Levine, D.A.; Cohen, D.M.; Gattu, R.; Linakis, J.G.; Anders, J.; Borgialli, D.; Vitale, M.; Dayan, P.S.; et al. Risk of Bacterial Coinfections in Febrile Infants 60 Days Old and Younger with Documented Viral Infections. J. Pediatr. 2018, 203, 86–91.e2. [Google Scholar] [CrossRef] [PubMed]
- Byington, C.L.; Enriquez, F.R.; Hoff, C.; Tuohy, R.; Taggart, E.W.; Hillyard, D.R.; Carroll, K.C.; Christenson, J.C. Serious Bacterial Infections in Febrile Infants 1 to 90 Days Old with and Without Viral Infections. Pediatrics 2004, 113, 1662–1666. [Google Scholar] [CrossRef]
- Blaschke, A.J.; Korgenski, E.K.; Wilkes, J.; Presson, A.P.; Thorell, E.A.; Pavia, A.T.; Knackstedt, E.D.; Reynolds, C.; Schunk, J.E.; Daly, J.A.; et al. Rhinovirus in Febrile Infants and Risk of Bacterial Infection. Pediatrics 2018, 141, e20172384. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.; Hill, V.; Waters, A. Occult Serious Bacterial Infection in Infants Younger Than 60 to 90 Days with Bronchiolitis: A Systematic Review. Arch. Pediatr. Adolesc. Med. 2011, 165, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Aronson, P.L.; Louie, J.P.; Kerns, E.; Jennings, B.; Magee, S.; Wang, M.E.; Gupta, N.; Kovaleski, C.; McDaniel, L.M.; McDaniel, C.E. Prevalence of Urinary Tract Infection, Bacteremia, and Meningitis Among Febrile Infants Aged 8 to 60 Days with SARS-CoV-2. JAMA Netw. Open 2023, 6, e2313354. [Google Scholar] [CrossRef]
- Bressan, S.; Andreola, B.; Cattelan, F.; Zangardi, T.; Perilongo, G.; Da Dalt, L. Predicting Severe Bacterial Infections in Well-Appearing Febrile Neonates: Laboratory Markers Accuracy and Duration of Fever. Pediatr. Infect Dis. J. 2010, 29, 227–232. Available online: https://journals.lww.com/pidj/fulltext/2010/03000/predicting_severe_bacterial_infections_in.9.aspx (accessed on 8 September 2023). [CrossRef]
- Pratt, A.; Attia, M.W. Duration of fever and markers of serious bacterial infection inyoung febrile children. Pediatr. Int. 2007, 49, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Kuppermann, N.; Mejias, A.; Suarez, N.; Chaussabel, D.; Casper, T.C.; Smith, B.; Alpern, E.R.; Anders, J.; Atabaki, S.M.; et al. Association of RNA Biosignatures With Bacterial Infections in Febrile Infants Aged 60 Days or Younger. JAMA 2016, 316, 846–857. [Google Scholar] [CrossRef]
- CORDIS: European Commission. Personalised Risk Assessment in Febrile Illness to Optimise Real-Life Management across the European Union (PERFORM Project) [Internet]. Available online: https://cordis.europa.eu/project/id/668303 (accessed on 9 September 2023).
- Umana, E.; Mills, C.; Norman-Bruce, H.; Wilson, K.; Mitchell, H.; McFetridge, L.; Woolfall, K.; A Lynn, F.; McKeeman, G.; Foster, S.; et al. Applying clinical decision aids for the assessment and management of febrile infants presenting to emergency care in the UK and Ireland: Febrile Infant Diagnostic Assessment and Outcome (FIDO) Study protocol. BMJ Open 2023, 13, e075823. [Google Scholar] [CrossRef]
- DIAMONDS: Making Diagnosis Personal [Internet]. DIAMONDS: Personalised Molecular Testing for Serious Illness. Available online: https://www.diamonds2020.eu/ (accessed on 8 August 2023).
| Conundrum | Response |
|---|---|
| They “felt hot at home” but have no fever now—should I worry? | Infants with measured fever at home are still at risk of IBI even if afebrile in the emergency department. |
| The baby’s urine dip is positive, how reliable is this? | Urinalysis is reliable screening test for UTI but should be interpreted in line with the method of urine collection. |
| Do infants with urinary tract infection routinely require a lumbar puncture to exclude meningitis? | Infants with urinary tract infection and low risk for IBI do not require routine lumbar puncture. |
| Do I need to worry about fever in an infant following vaccination? | Infants post vaccination may only require observation and urinalysis for their evaluation in the emergency department if they present with fever. |
| Should a positive viral respiratory swab alter the management of febrile infants? | Infants with positive viral swab have lower risk of IBI and UTI. May still require investigation based on age and clinical appearance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilcox, H.; Umana, E.; Fauteux-Lamarre, E.; Velasco, R.; Waterfield, T. Conundrums in the Management of Febrile Infants under Three Months of Age and Future Research. Antibiotics 2024, 13, 88. https://doi.org/10.3390/antibiotics13010088
Wilcox H, Umana E, Fauteux-Lamarre E, Velasco R, Waterfield T. Conundrums in the Management of Febrile Infants under Three Months of Age and Future Research. Antibiotics. 2024; 13(1):88. https://doi.org/10.3390/antibiotics13010088
Chicago/Turabian StyleWilcox, Helena, Etimbuk Umana, Emmanuelle Fauteux-Lamarre, Roberto Velasco, and Thomas Waterfield. 2024. "Conundrums in the Management of Febrile Infants under Three Months of Age and Future Research" Antibiotics 13, no. 1: 88. https://doi.org/10.3390/antibiotics13010088
APA StyleWilcox, H., Umana, E., Fauteux-Lamarre, E., Velasco, R., & Waterfield, T. (2024). Conundrums in the Management of Febrile Infants under Three Months of Age and Future Research. Antibiotics, 13(1), 88. https://doi.org/10.3390/antibiotics13010088






