The Synthetic Peptide LyeTx I mn∆K, Derived from Lycosa erythrognatha Spider Toxin, Is Active against Methicillin-Resistant Staphylococcus aureus (MRSA) In Vitro and In Vivo
Abstract
1. Introduction
2. Results
2.1. LyeTx I mn∆K Shows Good In Vitro Activity against MRSA
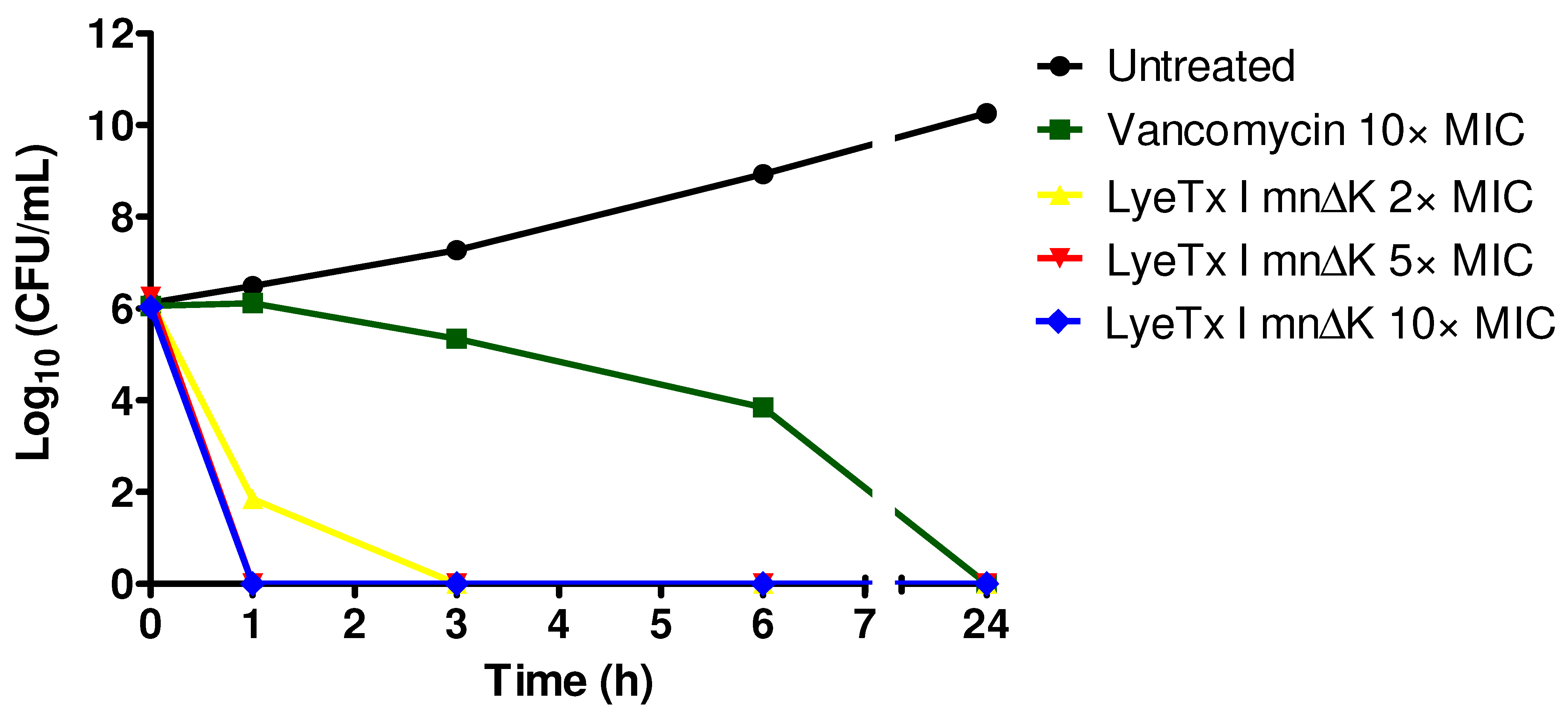
2.2. LyeTx I mn∆K Exhibits Rapid Bactericidal Effects on Cells in Logarithmic Growth of MRSA
2.3. LyeTx I mn∆K Reduces Preformed Biofilms of MRSA
2.4. Behavior of LyeTx I mnΔK after Combination with Conventional Antimicrobial Agents against MRSA
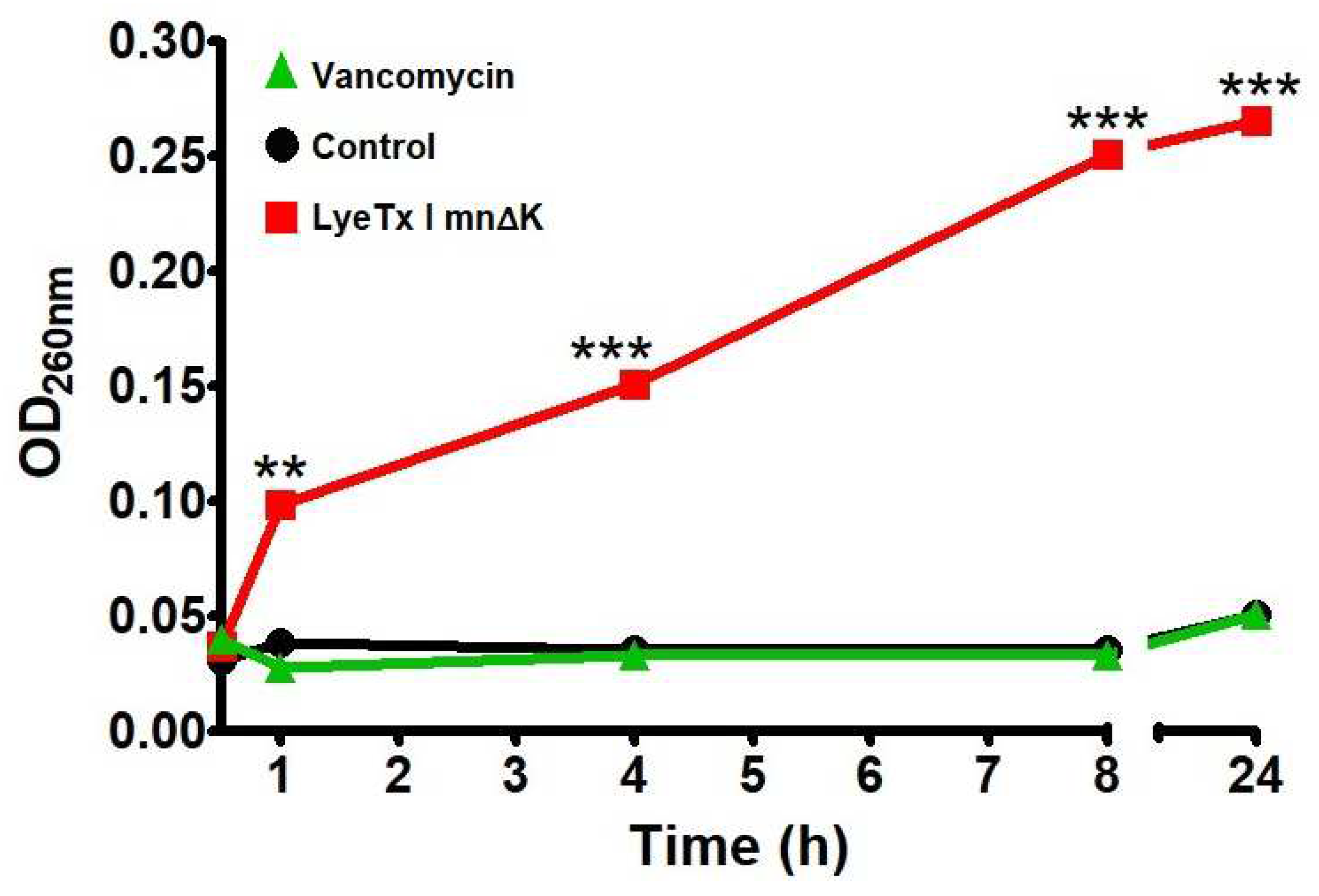
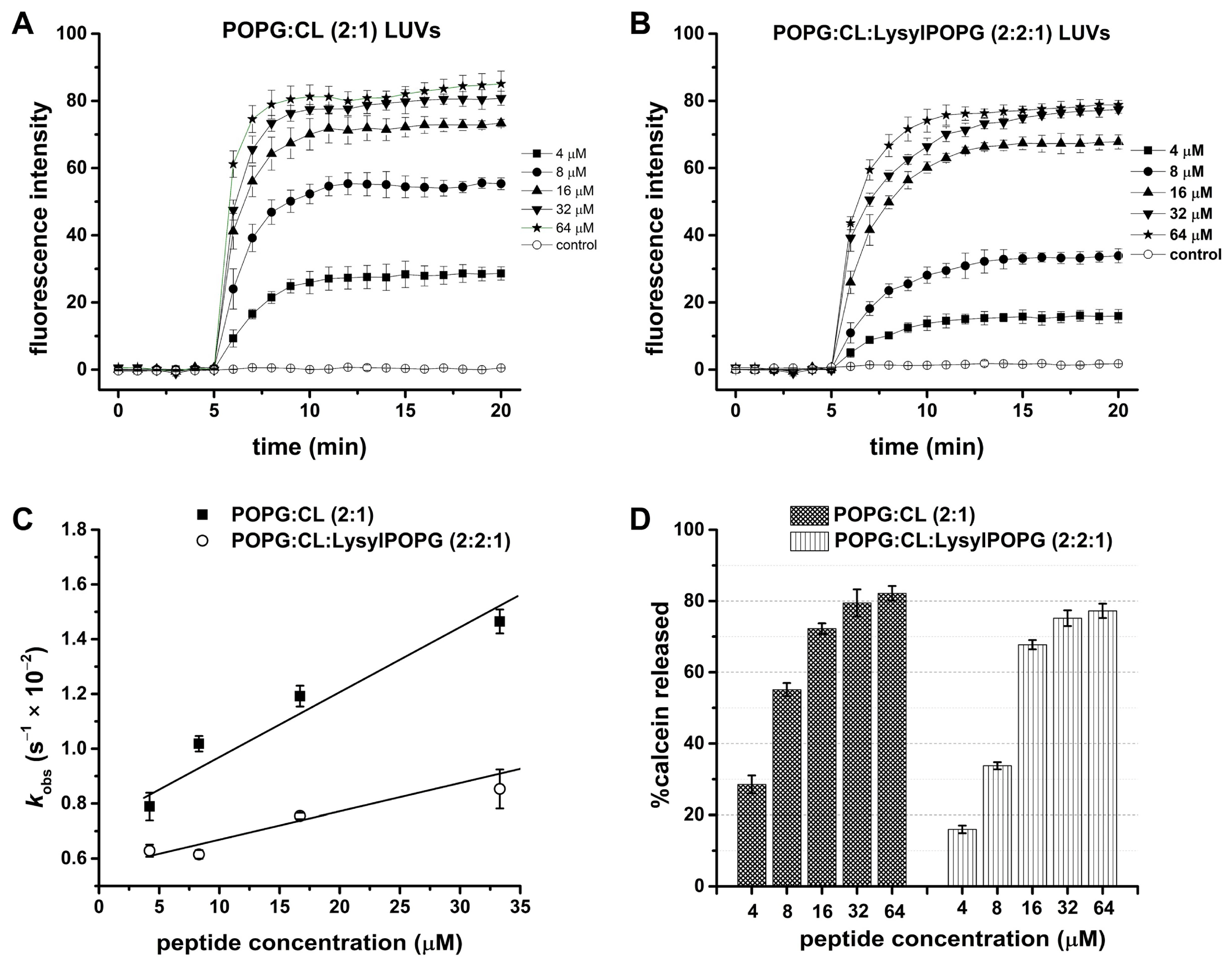
2.5. LyeTx I mn∆K Induces Membranolytic Effect on MRSA Cells
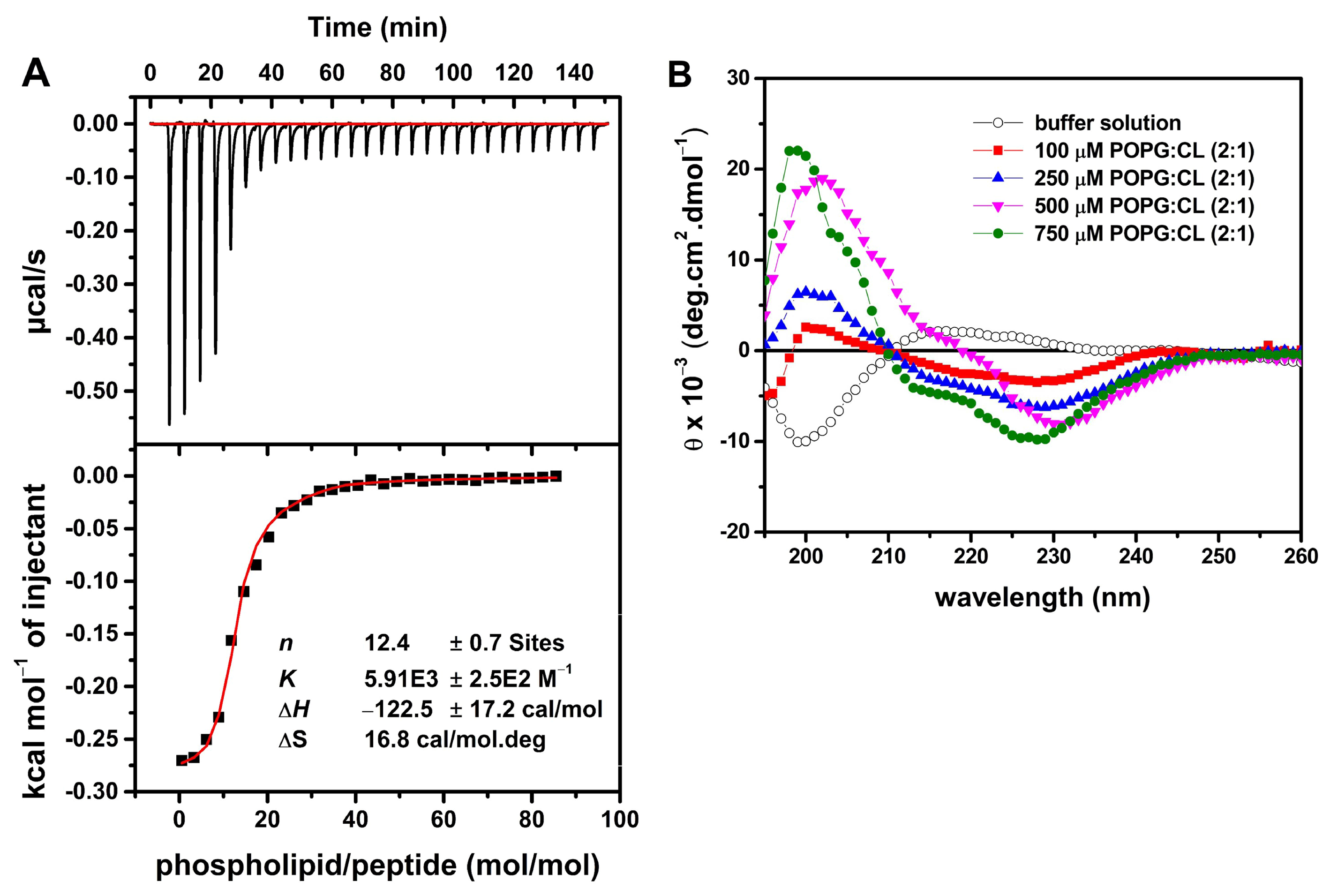
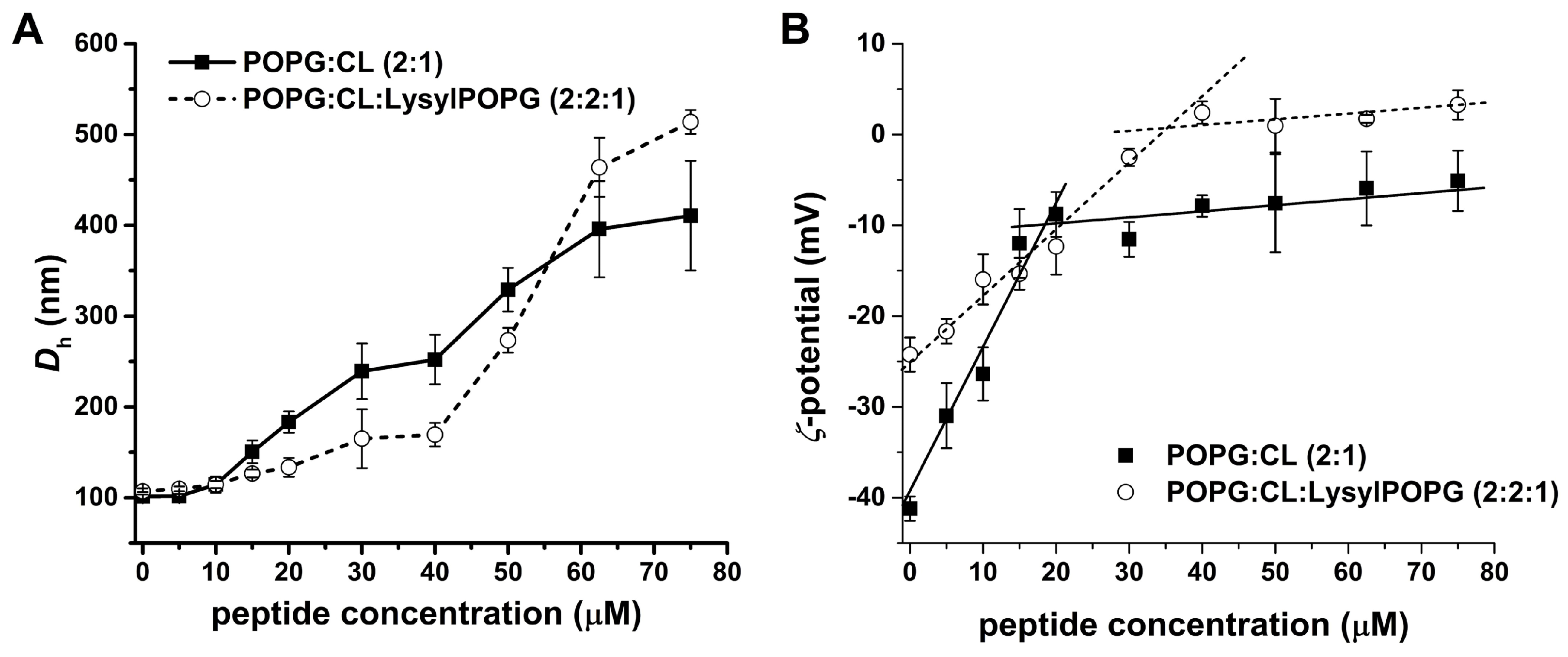
2.6. LyeTx I mn∆K Interacts with POPG:CL Membranes
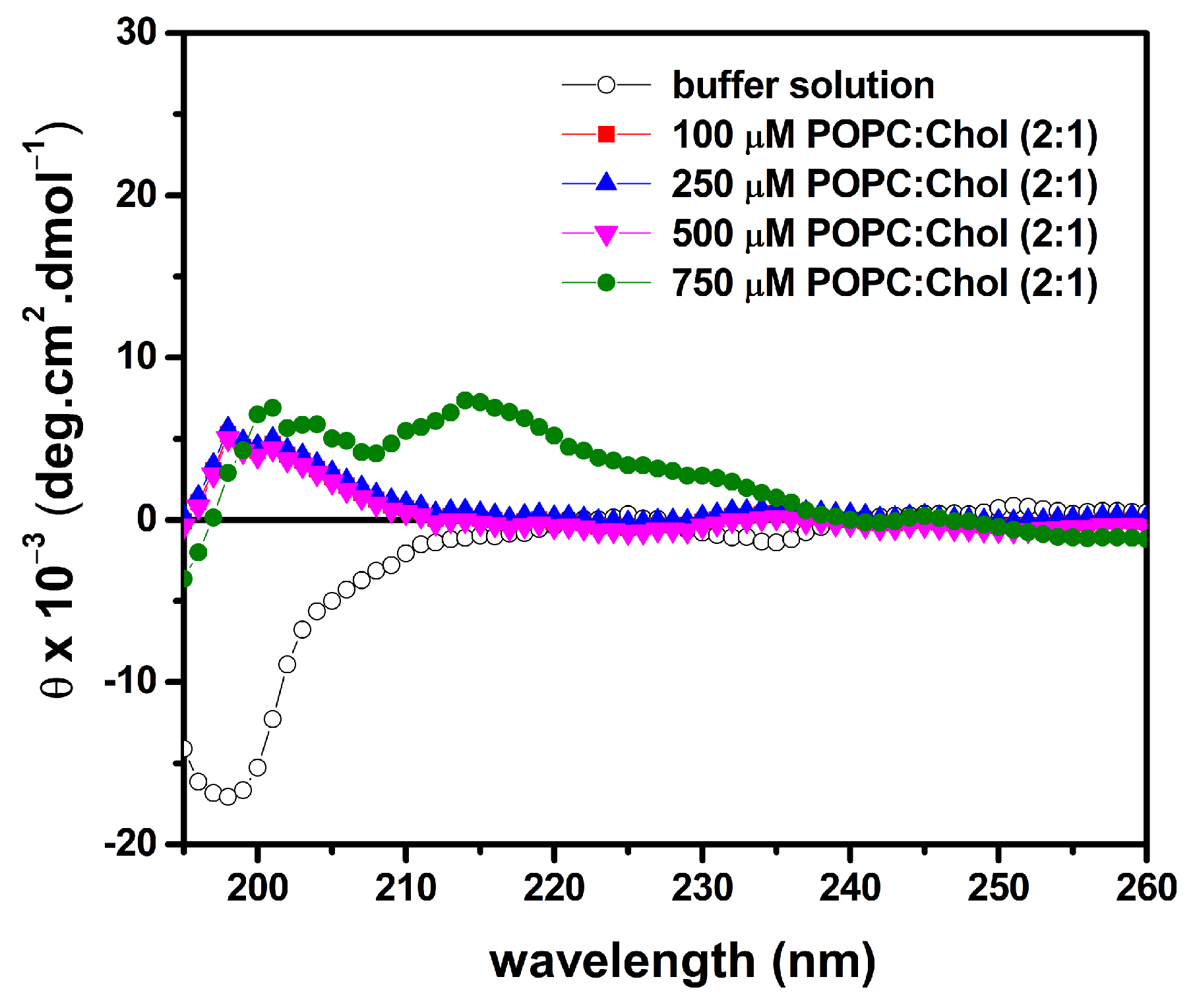
2.7. LyeTx I mn∆K Has Low Interaction with Artificial Vesicles That Mimic Eukaryotic Membranes
2.8. Effect of LysylPOPG on the Peptide–Membrane Interaction
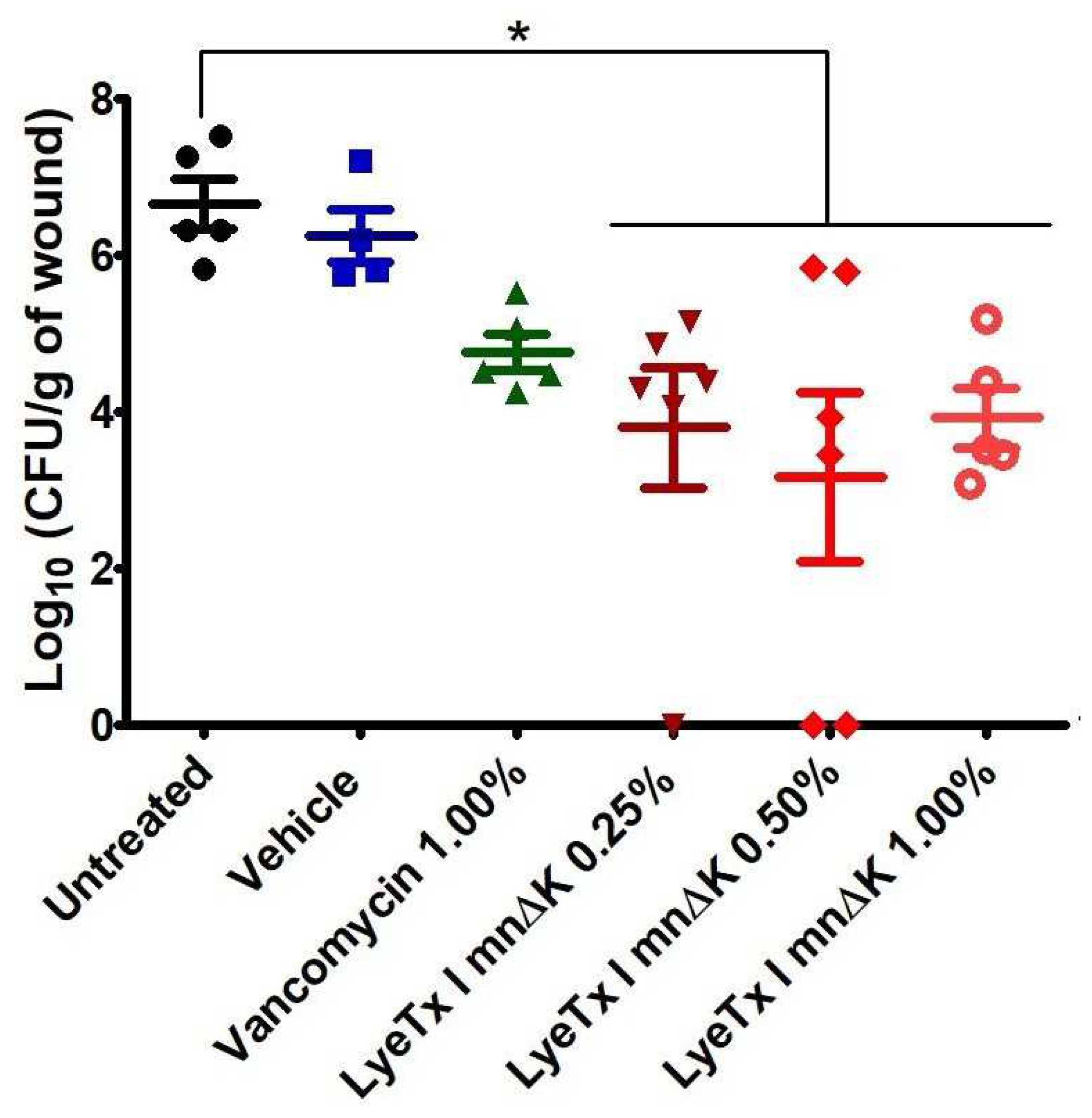
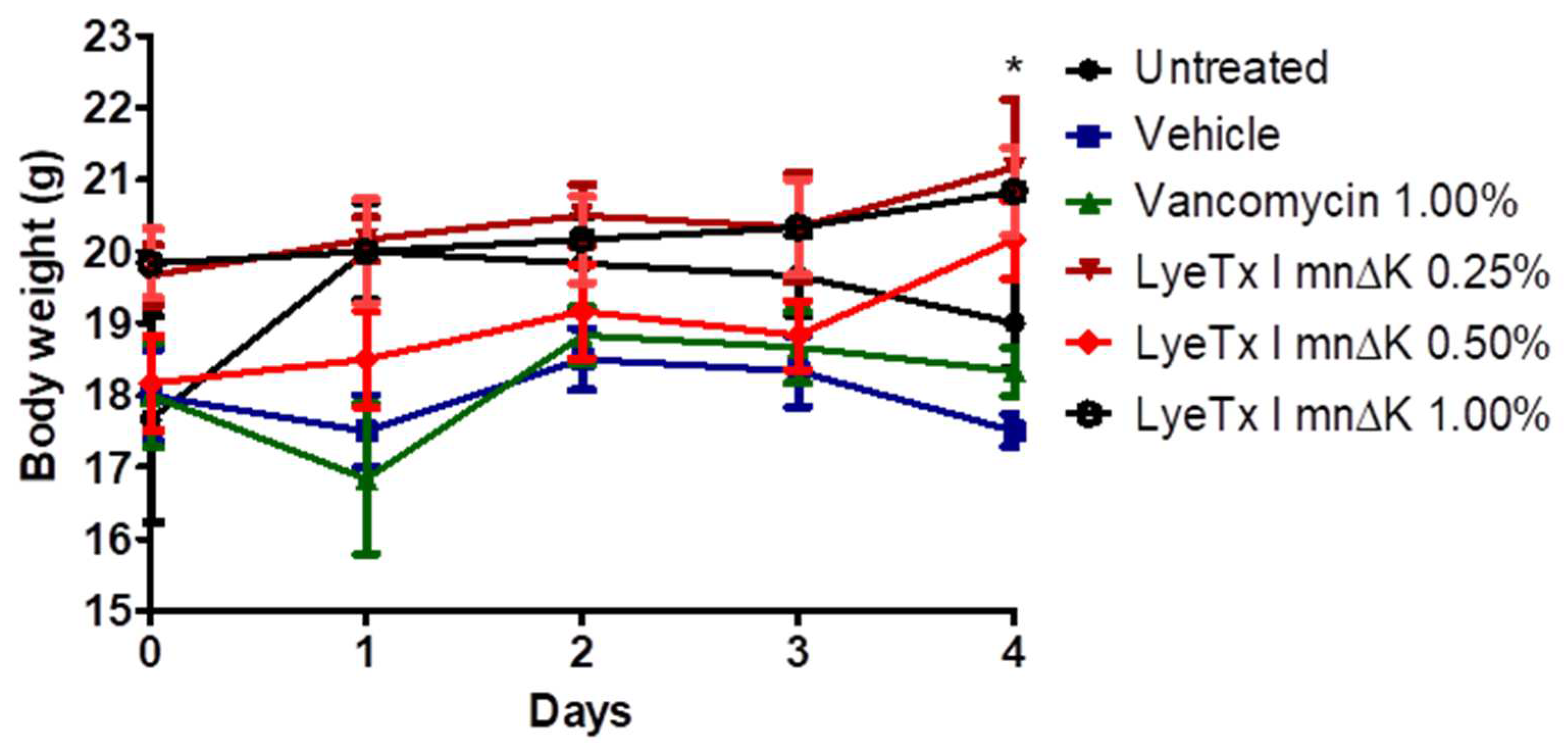
2.9. Gel Containing LyeTx I mnΔK Is Effective in MRSA-Induced Wounds in Mice
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Reagents
4.3. Antimicrobial Activity
4.3.1. Preparation of the Inoculum
4.3.2. Determination of Minimum Inhibitory Concentration (MIC)
4.3.3. Determination of Minimum Bactericidal Concentration (MBC)
4.4. Time-Kill Curve
4.5. Anti-Biofilm Activity
4.6. Membranolytic Effect
4.7. Combination Assays
4.7.1. Synergism
4.7.2. Resensitization
4.8. Preparation of Large Unilamellar Vesicles (LUVS)
4.9. Isothermal Titration Calorimetry (ITC)
4.10. Dynamic Light Scattering (DLS)
4.11. Calcein Release
4.12. Peptide Formulation
4.13. In Vivo Assays
4.13.1. Animals
4.13.2. Murine Model of Non-Surgical MRSA-Infected Wounds
4.13.3. Treatment of Animals
4.14. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, N.K.; Garg, R.; Baliga, S.; Bhat, G. Nosocomial infections and drug susceptibility patterns in methicillin sensitive and methicillin resistant Staphylococcus aureus. J. Clin. Diagn. Res. 2013, 7, 2178. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Mahjabeen, F.; Saha, U.; Mostafa, M.N.; Siddique, F.; Ahsan, E.; Fathma, S.; Tasnim, A.; Rahman, T.; Faruq, R.; Sakibuzzaman, M.; et al. An Update on Treatment Options for Methicillin-Resistant Staphylococcus aureus (MRSA) Bacteremia: A Systematic Review. Cureus 2022, 14, e31486. [Google Scholar] [CrossRef]
- Oliveira, C.F.d.; Morey, A.T.; Biasi-Garbin, R.P.; Perugini, M.R.E.; Yamauchi, L.M.; Yamada-Ogatta, S.F. Emergência de Staphylococcus aureus resistentes aos antimicrobianos: Um desafio contínuo. Rev. Ciênc. Méd. Biol. 2014, 13, 242–247. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Focus: Antimicrobial Resistance: Antimicrobial Peptides Therapy: An Emerging Alternative for Treating Drug-Resistant Bacteria. Yale J. Biol. Med. 2022, 95, 445. [Google Scholar]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.-Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488. [Google Scholar] [CrossRef]
- Vale, N.; Aguiar, L.; Gomes, P. Antimicrobial peptides: A new class of antimalarial drugs? Front. Pharmacol. 2014, 5, 275. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.-U. Antimicrobial peptides: Mechanisms of action and resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Charron, N.E. Understanding membrane-active antimicrobial peptides. Q. Rev. Biophys. 2017, 50, e10. [Google Scholar] [CrossRef]
- Espeche, J.C.; Varas, R.; Maturana, P.; Cutro, A.C.; Maffía, P.C.; Hollmann, A. Membrane permeability and antimicrobial peptides: Much more than just making a hole. Pept. Sci. 2023, 116, e24305. [Google Scholar] [CrossRef]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E.W. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, J.; Tong, Z.; Jia, Y.; Yang, K.; Wang, Z. Potent broad-spectrum antibacterial activity of amphiphilic peptides against multidrug-resistant bacteria. Microorganisms 2020, 8, 1398. [Google Scholar] [CrossRef]
- Bordon, K.d.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From animal poisons and venoms to medicines: Achievements, challenges and perspectives in drug discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from venomous animals: An overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Santos, D.; Verly, R.; Piló-Veloso, D.; De Maria, M.; De Carvalho, M.; Cisalpino, P.; Soares, B.; Diniz, C.; Farias, L.; Moreira, D.F.F.; et al. LyeTx I, a potent antimicrobial peptide from the venom of the spider Lycosa erythrognatha. Amino Acids 2010, 39, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Fuscaldi, L.L.; de Avelar Júnior, J.T.; Dos Santos, D.M.; Boff, D.; de Oliveira, V.L.S.; Gomes, K.A.G.G.; Cruz, R.d.C.; de Oliveira, P.L.; Magalhães, P.P.; Cisalpino, P.S.; et al. Shortened derivatives from native antimicrobial peptide LyeTx I: In vitro and in vivo biological activity assessment. Exp. Biol. Med. 2021, 246, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.G.; de Lima, W.G.; Fernandes, S.O.A.; de Lima, M.E.; Cardoso, V.N. Antifungal activity of a shortened analogue of the natural peptide LyeTx I isolated from the venom of the spider Lycosa erythrognatha. Nat. Prod. Res. 2023, 37, 759–763. [Google Scholar] [CrossRef]
- Lima, W.G.; Brito, J.C.M.; de Lima, M.E.; Pizarro, A.C.S.T.; Vianna, M.A.M.d.M.; de Paiva, M.C.; de Assis, D.C.S.; Cardoso, V.N.; Fernandes, S.O.A. A short synthetic peptide, based on LyeTx I from Lycosa erythrognatha venom, shows potential to treat pneumonia caused by carbapenem-resistant Acinetobacter baumannii without detectable resistance. J. Antibiot. 2021, 74, 425–434. [Google Scholar] [CrossRef]
- Santos Neto, N.A.d. United We Stand, Divided We Fall: Antibiofilm Activity and Mechanisms of Action of Synthetic Peptides in Combination with Ciprofloxacin against Staphylococcus aureus Biofilm. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2023. [Google Scholar]
- Moreira Brito, J.C.; Carvalho, L.R.; Neves de Souza, A.; Carneiro, G.; Magalhães, P.P.; Farias, L.M.; Guimarães, N.R.; Verly, R.M.; Resende, J.M.; Elena de Lima, M. PEGylation of the antimicrobial peptide LyeTx Ib maintains structure-related biological properties and improves selectivity. Front. Mol. Biosci. 2022, 9, 1001508. [Google Scholar] [CrossRef]
- Wiseman, T.; Williston, S.; Brandts, J.F.; Lin, L.-N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 1989, 179, 131–137. [Google Scholar] [CrossRef]
- Seelig, J. Thermodynamics of lipid–peptide interactions. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1666, 40–50. [Google Scholar] [CrossRef]
- Abrunhosa, F.; Faria, S.; Gomes, P.; Tomaz, I.; Pessoa, J.C.; Andreu, D.; Bastos, M. Interaction and lipid-induced conformation of two cecropin− melittin hybrid peptides depend on peptide and membrane composition. J. Phys. Chem. B 2005, 109, 17311–17319. [Google Scholar] [CrossRef]
- Niu, Z.; Prade, E.; Malideli, E.; Hille, K.; Jussupow, A.; Mideksa, Y.G.; Yan, L.M.; Qian, C.; Fleisch, M.; Messias, A.C.; et al. Structural Insight into IAPP-Derived Amyloid Inhibitors and Their Mechanism of Action. Angew. Chem. 2020, 132, 5820–5830. [Google Scholar] [CrossRef]
- Junior, E.F.; Guimarães, C.F.; Franco, L.L.; Alves, R.J.; Kato, K.C.; Martins, H.R.; de Souza Filho, J.D.; Bemquerer, M.P.; Munhoz, V.H.; Resende, J.M.; et al. Glycotriazole-peptides derived from the peptide HSP1: Synergistic effect of triazole and saccharide rings on the antifungal activity. Amino Acids 2017, 49, 1389–1400. [Google Scholar] [CrossRef]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, P.N.K.; Kumbukgolla, W.W.; Jayaweera, J.A.A.S.; Rawat, D. Review on usage of vancomycin in livestock and humans: Maintaining its efficacy, prevention of resistance and alternative therapy. Vet. Sci. 2017, 4, 6. [Google Scholar] [CrossRef]
- Wu, K.-C.; Hua, K.-F.; Yu, Y.-H.; Cheng, Y.-H.; Cheng, T.-T.; Huang, Y.-K.; Chang, H.-W.; Chen, W.-J. Antibacterial and Antibiofilm Activities of Novel Antimicrobial Peptides against Multidrug-Resistant Enterotoxigenic Escherichia coli. Int. J. Mol. Sci. 2021, 22, 3926. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef]
- Tolker-Nielsen, T. Biofilm development. Microb. Biofilms 2015, 51–66. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Futur. Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- de Avelar Júnior, J.T. Estudo de três Peptídeos Sintéticos com Atividade Antimicrobiana, Derivados da Toxina LyeTx I da Aranha Lycosa Erythrognatha (Lucas, 1836). Master’s Theis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2015. [Google Scholar]
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016, 17, 603. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; de Brito, J.C.M.; Cardoso, V.N.; Fernandes, S.O.A. In-depth characterization of antibacterial activity of melittin against Staphylococcus aureus and use in a model of non-surgical MRSA-infected skin wounds. Eur. J. Pharm. Sci. 2021, 156, 105592. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Hayami, M.; Okabe, A.; Kariyama, R.; Abe, M.; Kanemasa, Y. Lipid composition of Staphylococcus aureus and its derived L-forms. Microbiol. Immunol. 1979, 23, 435–442. [Google Scholar] [CrossRef]
- Hernández-Villa, L.; Manrique-Moreno, M.; Leidy, C.; Jemioła-Rzemińska, M.; Ortíz, C.; Strzałka, K. Biophysical evaluation of cardiolipin content as a regulator of the membrane lytic effect of antimicrobial peptides. Biophys. Chem. 2018, 238, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.I.; Trier, S.M.; Bernal, A.; Vargas, J.C.; Herrfurth, C.; Feussner, I.; Gonzalez, J.M.; Leidy, C. Aureus adapt to growth conditions by changing membrane order. Biophys. J. 2014, 106, 580a. [Google Scholar] [CrossRef]
- Verly, R.M.; Rodrigues, M.A.; Daghastanli, K.R.P.; Denadai, A.M.L.; Cuccovia, I.M.; Bloch, C., Jr.; Frézard, F.; Santoro, M.M.; Piló-Veloso, D.; Bemquerer, M.P. Effect of cholesterol on the interaction of the amphibian antimicrobial peptide DD K with liposomes. Peptides 2008, 29, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Sanchez, S.P.; Ocampo-Ibáñez, I.D.; Liscano, Y.; Martínez, N.; Muñoz, I.; Manrique-Moreno, M.; Martinez-Martinez, L.; Oñate-Garzon, J. Integrating In Vitro and In Silico Analysis of a Cationic Antimicrobial Peptide Interaction with Model Membranes of Colistin-Resistant Pseudomonas aeruginosa Strains. Pharmaceutics 2022, 14, 1248. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Verly, R.M.; Resende, J.M.; Junior, E.F.; de Magalhães, M.T.; Guimarães, C.F.; Munhoz, V.H.; Bemquerer, M.P.; Almeida, F.C.; Santoro, M.M.; Piló-Veloso, D.; et al. Structure and membrane interactions of the homodimeric antibiotic peptide homotarsinin. Sci. Rep. 2017, 7, 40854. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Cazenave-Gassiot, A.; Gao, I.H.; Nair, Z.J.; Kumar, J.K.; Gao, L.; Kline, K.A.; Wenk, M.R. Comprehensive analysis of phospholipids and glycolipids in the opportunistic pathogen Enterococcus faecalis. PLoS ONE 2017, 12, e0175886. [Google Scholar] [CrossRef] [PubMed]
- Van Bambeke, F.; Tulkens, P.M.; Brasseur, R.; Mingeot-Leclercq, M.-P. Aminoglycoside antibiotics induce aggregation but not fusion of negatively-charged liposomes. Eur. J. Pharmacol. Mol. Pharmacol. 1995, 289, 321–333. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Mingeot-Leclercq, M.-P.; Brasseur, R.; Tulkens, P.M.; Schanck, A. Aminoglycoside antibiotics prevent the formation of non-bilayer structures in negatively-charged membranes. Comparative studies using fusogenic (bis (β-diethylaminoethylether) hexestrol) and aggregating (spermine) agents. Chem. Phys. Lipids 1996, 79, 123–135. [Google Scholar] [CrossRef]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current treatment strategies against multidrug-resistant bacteria: A review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef]
- Tängdén, T. Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Upsala J. Med Sci. 2014, 119, 149–153. [Google Scholar] [CrossRef]
- Hatlen, T.J.; Miller, L.G. Staphylococcal skin and soft tissue infections. Infect. Dis. Clin. North Am. 2021, 35, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Marasca, S.; Candelora, M.; Rizzetto, G.; Radi, G.; Molinelli, E.; Brescini, L.; Cirioni, O.; Offidani, A. Methicillin-resistant Staphylococcus aureus as a cause of chronic wound infections: Alternative strategies for management. AIMS Microbiol. 2022, 8, 125. [Google Scholar] [CrossRef]
- Santana, H.J.A.; Caseli, L. A bactericide peptide changing the static and dilatational surface elasticity properties of zwitterionic lipids at the air-water interface: Relationship with the thermodynamic, structural and morphological properties. Biophysic. Chem. 2021, 277, 1. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Committee on Antimicrobial Susceptibility Testing (BrCAST). Orientações do EUCAST para a Detecção de Mecanismos de Resistência e Resistências Específicas de Importância Clínica e/ou Epidemiológica; BrCAST: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Latka, A.; Drulis-Kawa, Z. Advantages and limitations of microtiter biofilm assays in the model of antibiofilm activity of Klebsiella phage KP34 and its depolymerase. Sci. Rep. 2020, 10, 20338. [Google Scholar] [CrossRef] [PubMed]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Oroojalian, F.; Kasra-Kermanshahi, R.; Azizi, M. Synergistic antibacterial activity of Bunium persicum and Cuminum cyminum essential oils. Planta Medica 2008, 74, PI36. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Abdelkhalek, A.; Seleem, M.N. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 29707. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- ANVISA—Agencia Nacional de Vigilância Sanitária. Farmacopéia Brasileira, 6th ed.; ANVISA: Brasilia, Brazil, 2019.


| Microorganism | Antibacterial Activity (µM) | |||
|---|---|---|---|---|
| LyeTx I mnΔK | Vancomycin | |||
| MIC | MBC | MIC | MBC | |
| S. aureus 11 | 8 | 32 | 1 | 8 |
| S. aureus 29 | 8 | 16 | 1 | 2 |
| S. aureus 130 | 8 | 32 | 1 | 2 |
| S. aureus 366 | 16 | 32 | 1 | 8 |
| S. aureus 524 | 16 | 32 | 1 | 2 |
| S. aureus 526 | 16 | 32 | 1 | 4 |
| MRSA USA300 | 8 | 16 | 1 | 2 |
| MIC50 | 8 | 1 | ||
| MBC50 | 32 | 2 | ||
| Class | Antimicrobials | FIC | FICI (ΣFIC) | Effect | |
|---|---|---|---|---|---|
| LyeTx I mnΔK | Antimicrobial | ||||
| Glycopeptide | Vancomycin | 1.00 | 0.50 | 1.50 | Indifferent |
| β-lactam | Oxacillin | 1.00 | 0.02 | 1.02 | Indifferent |
| Antibacterial | MICs (µg/mL) | Fold Resensitization | |
|---|---|---|---|
| Not Exposed to LyeTx I mnΔK | Exposed to LyeTx I mnΔK | ||
| Vancomycin | 1.0 | 0.5 | 2 |
| Oxacillin | 128 | 64 | 2 |
| LyeTx I mn∆K (mM) | POPG:CL | POPG:CL:Lysyl:POPG | ||||||
|---|---|---|---|---|---|---|---|---|
| %Calcein Released | SD * | kobs (s−1) × 10−2 | SD * ×10−4 | %Calcein Released | SD * | kobs (s−1) × 10−2 | SD * ×10−4 | |
| 4 | 28.51 | 2.50 | 0.78 | 5.01 | 15.98 | 1.06 | 0.629 | 2.23 |
| 8 | 55.11 | 1.83 | 1.01 | 2.82 | 33.74 | 1.01 | 0.615 | 1.56 |
| 18 | 72.23 | 1.49 | 1.19 | 3.81 | 67.73 | 1.30 | 0.755 | 1.13 |
| 32 | 79.45 | 3.82 | 1.46 | 4.36 | 75.17 | 2.20 | 0.853 | 7.10 |
| 64 | 82.17 | 2.04 | 2.16 | 12.5 | 77.23 | 1.99 | 1.225 | 4.89 |
| Sample | Vpep (µL) | VLUVs (µL) | Vbuffer (µL) | [LyeTx I mn∆K] (µM) |
|---|---|---|---|---|
| 1 | 0 | 400 | 400 | 0 |
| 2 | 4 | 400 | 396 | 5 |
| 3 | 8 | 400 | 392 | 10 |
| 4 | 12 | 400 | 388 | 15 |
| 5 | 16 | 400 | 384 | 20 |
| 6 | 24 | 400 | 376 | 30 |
| 7 | 32 | 400 | 368 | 40 |
| 8 | 40 | 400 | 360 | 50 |
| 9 | 50 | 400 | 350 | 62.5 |
| 10 | 60 | 400 | 340 | 75 |
| Sample | Vpep (µL) | VLUVs (µL) | Vbuffer (µL) | [LyeTx I mn∆K] (µM) |
|---|---|---|---|---|
| 1 | 0 | 150 | 150 | 0 |
| 2 | 5 | 150 | 145 | 4,2 |
| 3 | 10 | 150 | 140 | 8,3 |
| 4 | 20 | 150 | 130 | 16,7 |
| 5 | 40 | 150 | 110 | 33,3 |
| 6 | 80 | 150 | 70 | 66,7 |
| Component | Concentration |
|---|---|
| Hydroxyethylcellulose (Natrosol®) | 2.2% |
| Sodium metabisulfite | 0.6% |
| Methylparaben (Nipagin®) | 0.2% |
| Distilled water | q.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, A.P.G.C.; de Souza, A.N.; Lima, W.G.; Brito, J.C.M.; Simião, D.C.; Gonçalves, L.V.R.; Cordeiro, L.P.B.; de Oliveira Scoaris, D.; Fernandes, S.O.A.; Resende, J.M.; et al. The Synthetic Peptide LyeTx I mn∆K, Derived from Lycosa erythrognatha Spider Toxin, Is Active against Methicillin-Resistant Staphylococcus aureus (MRSA) In Vitro and In Vivo. Antibiotics 2024, 13, 248. https://doi.org/10.3390/antibiotics13030248
Vieira APGC, de Souza AN, Lima WG, Brito JCM, Simião DC, Gonçalves LVR, Cordeiro LPB, de Oliveira Scoaris D, Fernandes SOA, Resende JM, et al. The Synthetic Peptide LyeTx I mn∆K, Derived from Lycosa erythrognatha Spider Toxin, Is Active against Methicillin-Resistant Staphylococcus aureus (MRSA) In Vitro and In Vivo. Antibiotics. 2024; 13(3):248. https://doi.org/10.3390/antibiotics13030248
Chicago/Turabian StyleVieira, Ana Paula Gonçalves Coelho, Amanda Neves de Souza, William Gustavo Lima, Julio Cesar Moreira Brito, Daniela Carolina Simião, Lucas Vinícius Ribeiro Gonçalves, Lídia Pereira Barbosa Cordeiro, Denise de Oliveira Scoaris, Simone Odília Antunes Fernandes, Jarbas Magalhães Resende, and et al. 2024. "The Synthetic Peptide LyeTx I mn∆K, Derived from Lycosa erythrognatha Spider Toxin, Is Active against Methicillin-Resistant Staphylococcus aureus (MRSA) In Vitro and In Vivo" Antibiotics 13, no. 3: 248. https://doi.org/10.3390/antibiotics13030248
APA StyleVieira, A. P. G. C., de Souza, A. N., Lima, W. G., Brito, J. C. M., Simião, D. C., Gonçalves, L. V. R., Cordeiro, L. P. B., de Oliveira Scoaris, D., Fernandes, S. O. A., Resende, J. M., Bechinger, B., Verly, R. M., & de Lima, M. E. (2024). The Synthetic Peptide LyeTx I mn∆K, Derived from Lycosa erythrognatha Spider Toxin, Is Active against Methicillin-Resistant Staphylococcus aureus (MRSA) In Vitro and In Vivo. Antibiotics, 13(3), 248. https://doi.org/10.3390/antibiotics13030248










