Potential Antifungal Effect of Copper Oxide Nanoparticles Combined with Fungicides against Botrytis cinerea and Fusarium oxysporum
Abstract
1. Introduction
2. Results and Discussion
2.1. Checkerboard Assay
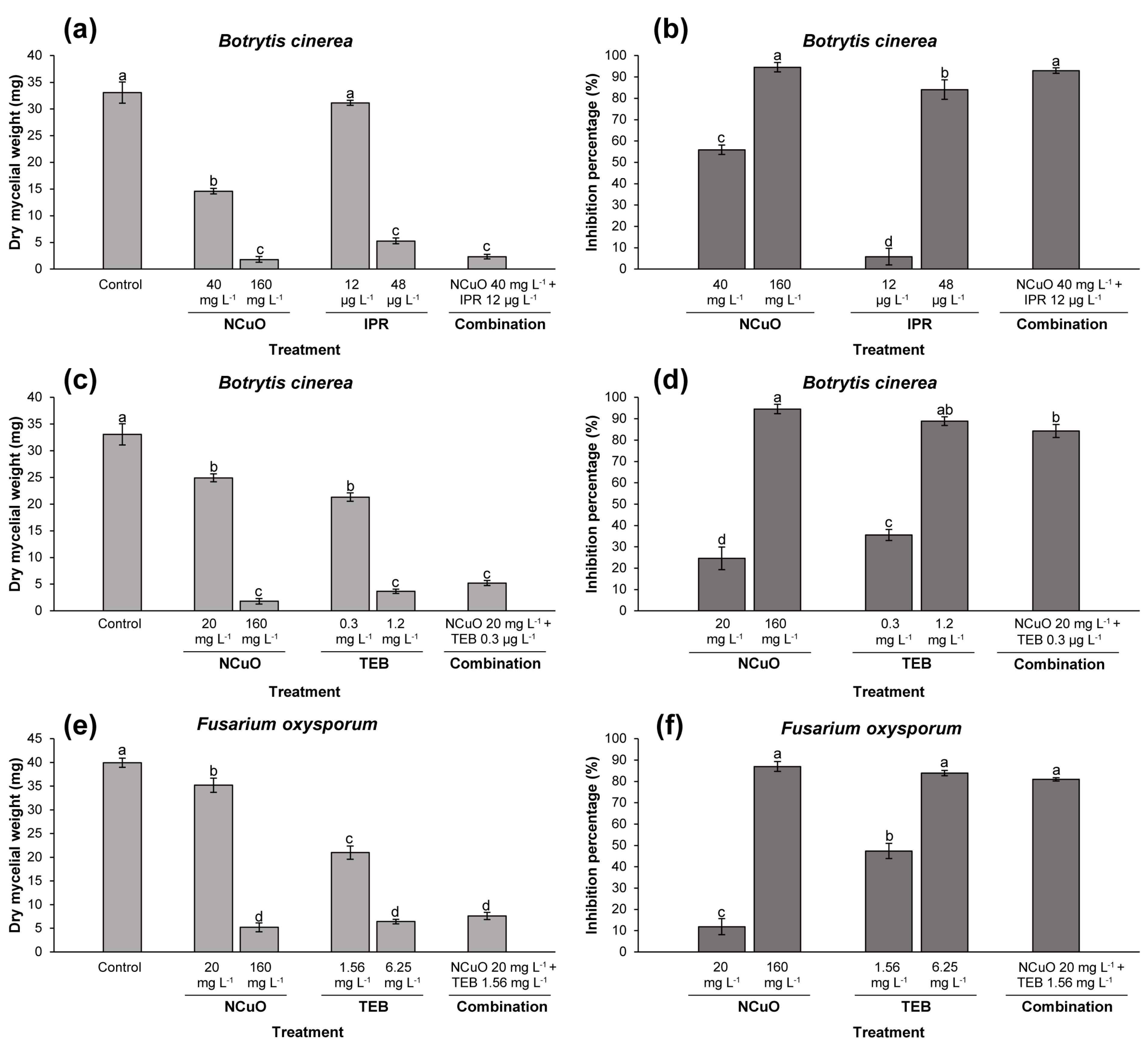
2.2. Effect of Synergistic Combinations against Mycelial Biomass
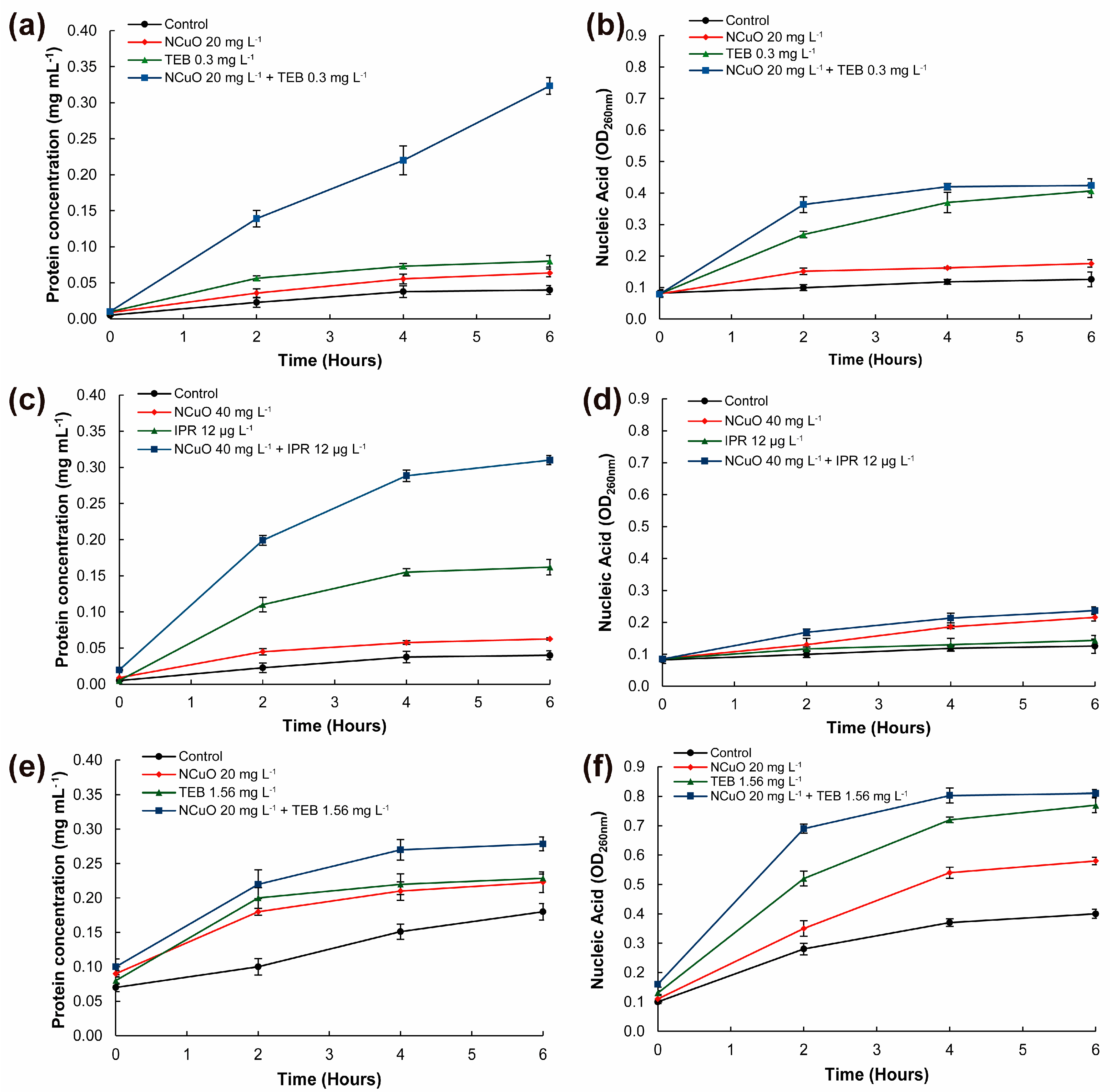
2.3. Synergistic Combined Treatments inducing Cellular Leakage on B. cinerea and F. oxysporum
3. Materials and Methods
3.1. Chemicals and Microbial Strains
3.2. Antifungal Activity
3.2.1. Fractional Inhibitory Concentration
3.2.2. Inhibition of Mycelial Biomass
3.2.3. Cytoplasmic and Nucleic Acid Leakage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Mancini, F.; Woodcock, B.A.; Isaac, N.J.B. Agrochemicals in the wild: Identifying links between pesticide use and declines of nontarget organisms. Curr. Opin. Environ. Sci. Health 2019, 11, 53–58. [Google Scholar] [CrossRef]
- Agrimonti, C.; Lauro, M.; Visioli, G. Smart agriculture for food quality: Facing climate change in the 21st century. Crit. Rev. Food Sci. Nutr. 2021, 61, 971–981. [Google Scholar] [CrossRef]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A.T. Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Dermauw, W.; Mavridis, K.; Vontas, J. Significance and interpretation of molecular diagnostics for insecticide resistance management of agricultural pests. Curr. Opin. Insect Sci. 2020, 39, 69–76. [Google Scholar] [CrossRef]
- Athukorala, W.; Lee, B.L.; Wilson, C.; Fujii, H.; Managi, S. Measuring the impact of pesticide exposure on farmers’ health and farm productivity. Econ. Anal. Policy 2023, 77, 851–862. [Google Scholar] [CrossRef]
- Lee, R.; den Uyl, R.; Runhaar, H. Assessment of policy instruments for pesticide use reduction in Europe; Learning from a systematic literature review. Crop Prot. 2019, 126, 104929. [Google Scholar] [CrossRef]
- Silva, V.; Yang, X.; Fleskens, L.; Ritsema, C.J.; Geissen, V. Environmental and human health at risk—Scenarios to achieve the Farm to Fork 50% pesticide reduction goals. Environ. Int. 2022, 165, 107296. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Ibarra-Laclette, E.; Blaz, J.; Pérez-Torres, C.A.; Villafán, E.; Lamelas, A.; Rosas-Saito, G.; Ibarra-Juárez, L.A.; García-Ávila, C.d.J.; Martínez-Enriquez, A.I.; Pariona, N. Antifungal Effect of Copper Nanoparticles against Fusarium kuroshium, an Obligate Symbiont of Euwallacea kuroshio Ambrosia Beetle. J. Fungi 2022, 8, 347. [Google Scholar] [CrossRef]
- Sadek, M.E.; Shabana, Y.M.; Sayed-Ahmed, K.; Abou Tabl, A.H. Antifungal Activities of Sulfur and Copper Nanoparticles against Cucumber Postharvest Diseases Caused by Botrytis cinerea and Sclerotinia sclerotiorum. J. Fungi 2022, 8, 412. [Google Scholar] [CrossRef]
- Flores-Rábago, K.M.; Rivera-Mendoza, D.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Castro-Longoria, E. Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile. Antibiotics 2023, 12, 1251. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-Agents: The Copper Age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef] [PubMed]
- Keiblinger, K.M.; Schneider, M.; Gorfer, M.; Paumann, M.; Deltedesco, E.; Berger, H.; Jöchlinger, L.; Mentler, A.; Zechmeister-Boltenstern, S.; Soja, G.; et al. Assessment of Cu applications in two contrasting soils—Effects on soil microbial activity and the fungal community structure. Ecotoxicology 2018, 27, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Sousa, D.Z.; Martínez, M.; Fernández-Baldo, M.A.; Tortella, G.R. Short term changes in the abundance of nitrifying microorganisms in a soil-plant system simultaneously exposed to copper nanoparticles and atrazine. Sci. Total Environ. 2019, 670, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Lima Oliveira, E.G.; Vieira, S.A.; Da Silva, F.A.G.; Da Costa, M.M.; Gomes, A.S.L.; De Oliveira, H.P. Synergistic Antibacterial Activity of Green Gold Nanoparticles and Tannin-Based Derivatives. BioChem 2022, 2, 269–279. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Melnikov, M.Y. Hybrid Nanosystems of Antibiotics with Metal Nanoparticles—Novel Antibacterial Agents. Molecules 2023, 28, 1603. [Google Scholar] [CrossRef]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020, 9535432. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Metal nanoparticles against fungicide resistance: Alternatives or partners? Pest Manag. Sci. 2022, 78, 3953–3956. [Google Scholar] [CrossRef]
- Poveda, J.; Barquero, M.; González-Andrés, F. Insight into the Microbiological Control Strategies against Botrytis cinerea Using Systemic Plant Resistance Activation. Agronomy 2020, 10, 1822. [Google Scholar] [CrossRef]
- Hassan, M.A.A.; El-Saadony, M.T.; Mostafa, N.G.; El-Tahan, A.M.; Mesiha, P.K.; El-Saadony, F.M.A.; Hassan, A.M.; El-Shehawi, A.M.; Ashry, N.M. The use of previous crops as sustainable and eco-friendly management to fight Fusarium oxysporum in sesame plants. Saudi J. Biol. Sci. 2021, 28, 5849–5859. [Google Scholar] [CrossRef] [PubMed]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Notte, A.-M.; Plaza, V.; Marambio-Alvarado, B.; Olivares-Urbina, L.; Poblete-Morales, M.; Silva-Moreno, E.; Castillo, L. Molecular identification and characterization of Botrytis cinerea associated to the endemic flora of semi-desert climate in Chile. Curr. Res. Microb. Sci. 2021, 2, 100049. [Google Scholar] [CrossRef] [PubMed]
- Plaza, V.; Silva-Moreno, E.; Castillo, L. Breakpoint: Cell Wall and Glycoproteins and their Crucial Role in the Phytopathogenic Fungi Infection. Curr. Protein Pept. Sci. 2020, 21, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Vargas-Macías, A.P.; García-Carnero, L.C.; Martínez-Duncker, I.; Mora-Montes, H.M. Role of Protein Glycosylation in Interactions of Medically Relevant Fungi with the Host. J. Fungi 2021, 7, 875. [Google Scholar] [CrossRef]
- Li, J.; Xi, T.; Yan, B.; Guan, Y.; Yang, M.; Song, J.; Ma, H. Synergistic actions between ligand tebuconazole and Cu(II) cation: Reasons for the enhanced antifungal activity of four Cu(II) complexes based on fungicide tebuconazole. New J. Chem. 2015, 39, 9550–9556. [Google Scholar] [CrossRef]
- Letelier, M.E.; Faúndez, M.; Jara-Sandoval, J.; Molina-Berríos, A.; Cortés-Troncoso, J.; Aracena-Parks, P.; Marín-Catalán, R. Mechanisms underlying the inhibition of the cytochrome P450 system by copper ions. J. Appl. Toxicol. 2009, 29, 695–702. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef]
- El-Abeid, S.E.; Ahmed, Y.; Daròs, J.A.; Mohamed, M.A. Reduced graphene oxide nanosheet-decorated copper oxide nanoparticles: A potent antifungal nanocomposite against fusarium root rot and wilt diseases of tomato and pepper plants. Nanomaterials 2020, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Anjum, T.; Riaz, S.; Ahmad, I.S.; Irudayaraj, J.; Javed, S.; Qaiser, U.; Naseem, S. Inhibition mechanism of green-synthesized copper oxide nanoparticles from Cassia fistula towards Fusarium oxysporum by boosting growth and defense response in tomatoes. Environ. Sci. Nano 2021, 8, 1729–1748. [Google Scholar] [CrossRef]
- Lopez-Lima, D.; Mtz-Enriquez, A.I.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The bifunctional role of copper nanoparticles in tomato: Effective treatment for Fusarium wilt and plant growth promoter. Sci. Hortic. 2021, 277, 109810. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, T.; Xu, Y.; Tian, S. Antifungal effects of hinokitiol on development of Botrytis cinerea in vitro and in vivo. Postharvest Biol. Technol. 2020, 159, 111038. [Google Scholar] [CrossRef]
- Srivastava, P.; Kim, Y.; Cho, H.; Kim, K.S. Synergistic Action between Copper Oxide (CuO) Nanoparticles and Anthraquinone-2-Carboxylic Acid (AQ) against Staphylococcus aureus. J. Compos. Sci. 2023, 7, 135. [Google Scholar] [CrossRef]
- Arul Selvaraj, R.C.; Rajendran, M.; Nagaiah, H.P. Re-Potentiation of β-Lactam Antibiotic by Synergistic Combination with Biogenic Copper Oxide Nanocubes against Biofilm Forming Multidrug-Resistant Bacteria. Molecules 2019, 24, 3055. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Synergy between Cu-NPs and fungicides against Botrytis cinerea. Sci. Total Environ. 2020, 703, 135557. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef]
- Díaz-Blancas, V.; Medina, D.; Padilla-Ortega, E.; Bortolini-Zavala, R.; Olvera-Romero, M.; Luna-Bárcenas, G. Nanoemulsion Formulations of Fungicide Tebuconazole for Agricultural Applications. Molecules 2016, 21, 1271. [Google Scholar] [CrossRef]
- Jaklová Dytrtová, J.; Bělonožníková, K.; Jakl, M.; Ryšlavá, H. Triazoles and aromatase: The impact of copper cocktails. Environ. Pollut. 2020, 266, 115201. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, X.; Li, Y.; Duan, Y.; Zheng, Z.; Wang, J.; Zhou, M. F240 of β(2)—Tubulin Explains why Fusarium graminearum is Less Sensitive to Carbendazim than Botrytis cinerea. Phytopathology 2018, 108, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy assessed by checkerboard a critical analysis. Diagn. Microbiol. Infect. Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Gisi, U. Synergistic interaction of fungicides in mixtures. Phytopathology 1996, 86, 1273–1279. [Google Scholar]
- Fernandez-San Millan, A.; Gamir, J.; Larraya, L.; Farran, I.; Veramendi, J. Towards understanding of fungal biocontrol mechanisms of different yeasts antagonistic to Botrytis cinerea through exometabolomic analysis. Biol. Control 2022, 174, 105033. [Google Scholar] [CrossRef]
- Feng, S.; Lu, W.; Jian, Y.; Chen, Y.; Meng, R.; Deng, J.; Liu, Q.; Yu, T.; Jin, L.; Yang, X.; et al. Biocontrol Effect and Possible Mechanism of Food-Borne Sulfide 3-Methylthio-1-Propanol against Botrytis cinerea in Postharvest Tomato. Front. Plant Sci. 2021, 12, 763755. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
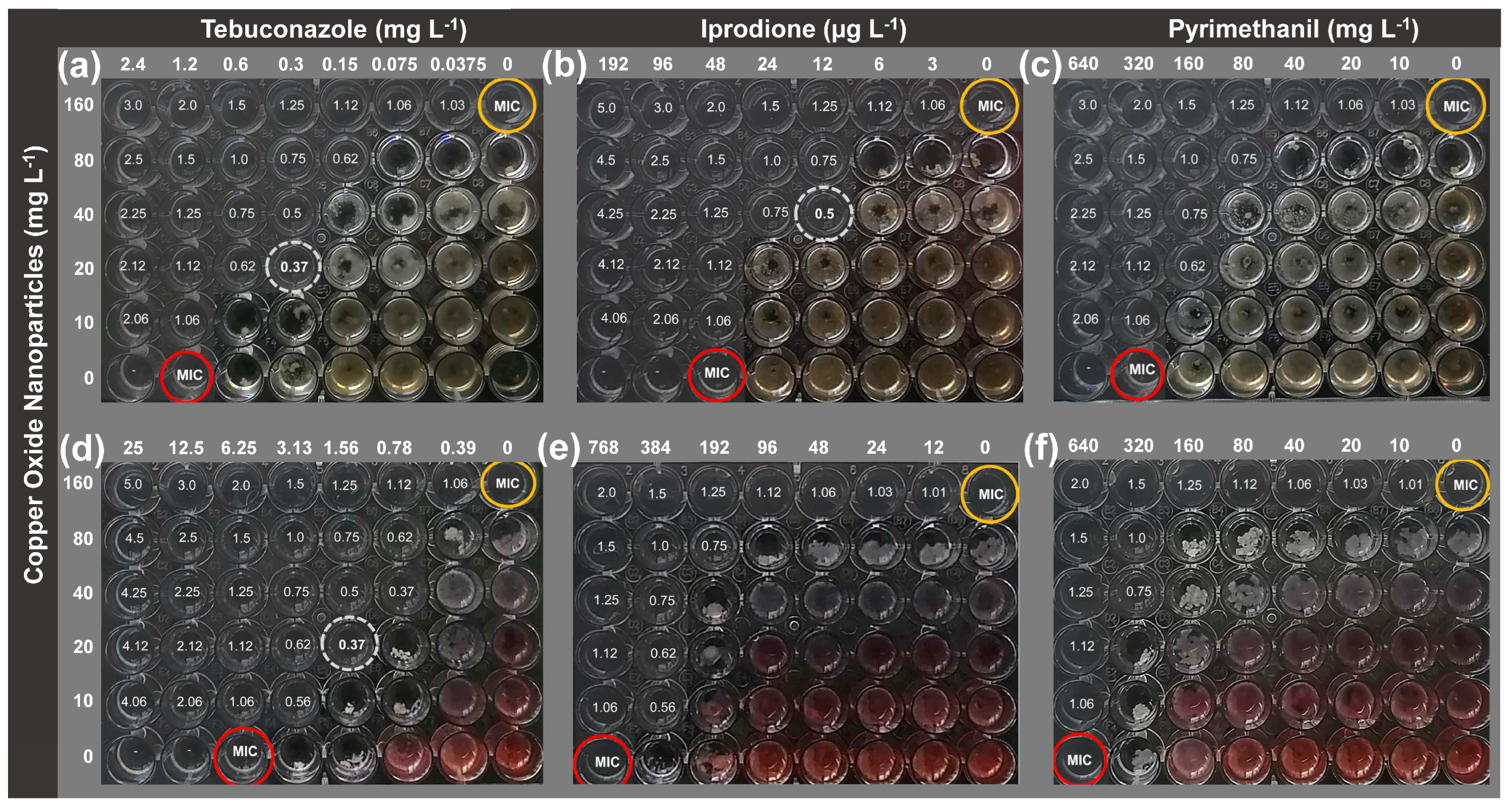
| Synergistic Combinations | Inhibition (%) | Synergy Factor | |||
|---|---|---|---|---|---|
| Alone Compounds | Combined Compounds | ||||
| NCuO (Ia) | Fungicide (Ib) | Obtained (Iab) | Expected (Ciexp) | ||
| Against Botrytis cinerea | |||||
| NCuO 20 mg L−1 + 0.3 mg L−1 TEB | 24.6 ± 5.2 | 35.5 ± 2.6 | 84.3 ± 3.1 | 51.4 | 1.63 |
| NCuO 40 mg L−1 + 12 µg L−1 IPR | 55.8 ± 2.1 | 5.8 ± 3.8 | 93 ± 1.3 | 58.4 | 1.59 |
| Against Fusarium oxysporum | |||||
| NCuO 20 mg L−1 + 1.56 mg L−1 TEB | 11.8 ± 3.7 | 47.41 ± 3.5 | 80.9 ± 0.7 | 53.6 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parada, J.; Tortella, G.; Seabra, A.B.; Fincheira, P.; Rubilar, O. Potential Antifungal Effect of Copper Oxide Nanoparticles Combined with Fungicides against Botrytis cinerea and Fusarium oxysporum. Antibiotics 2024, 13, 215. https://doi.org/10.3390/antibiotics13030215
Parada J, Tortella G, Seabra AB, Fincheira P, Rubilar O. Potential Antifungal Effect of Copper Oxide Nanoparticles Combined with Fungicides against Botrytis cinerea and Fusarium oxysporum. Antibiotics. 2024; 13(3):215. https://doi.org/10.3390/antibiotics13030215
Chicago/Turabian StyleParada, Javiera, Gonzalo Tortella, Amedea B. Seabra, Paola Fincheira, and Olga Rubilar. 2024. "Potential Antifungal Effect of Copper Oxide Nanoparticles Combined with Fungicides against Botrytis cinerea and Fusarium oxysporum" Antibiotics 13, no. 3: 215. https://doi.org/10.3390/antibiotics13030215
APA StyleParada, J., Tortella, G., Seabra, A. B., Fincheira, P., & Rubilar, O. (2024). Potential Antifungal Effect of Copper Oxide Nanoparticles Combined with Fungicides against Botrytis cinerea and Fusarium oxysporum. Antibiotics, 13(3), 215. https://doi.org/10.3390/antibiotics13030215









