The Impact of COVID-19 on Multidrug-Resistant Bacteria at a Slovenian Tertiary Medical Center
Abstract
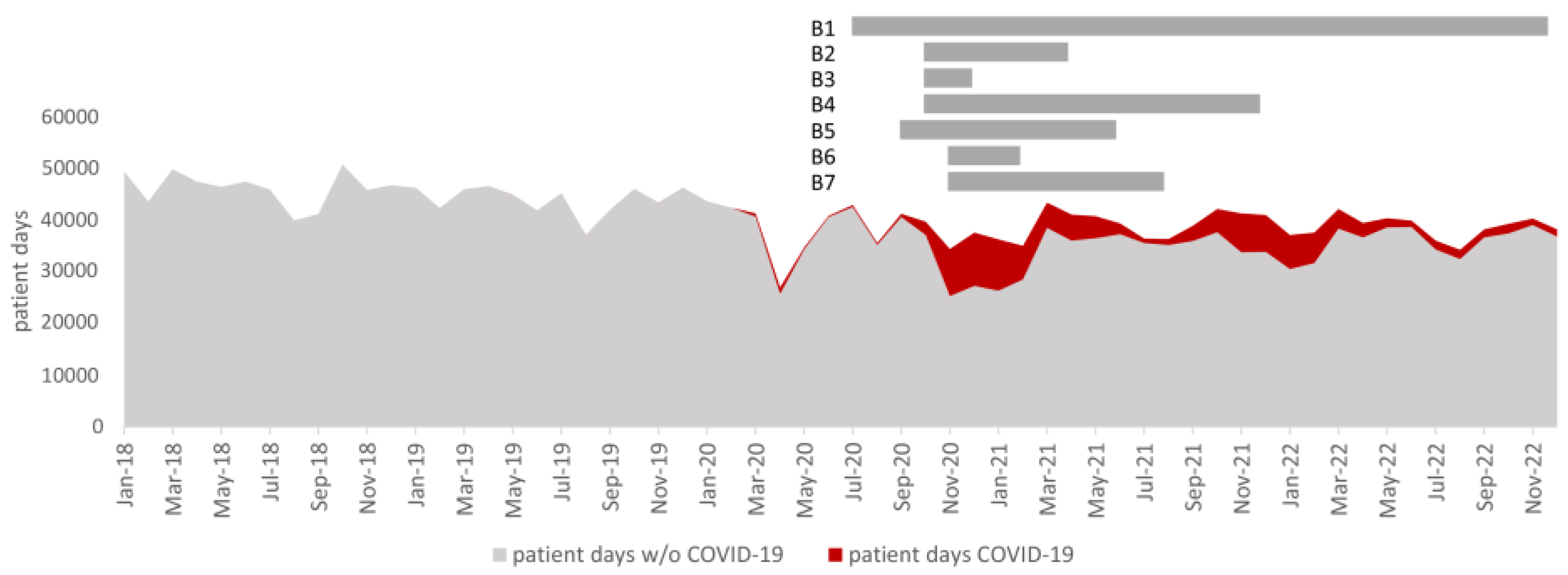
1. Introduction
2. Results
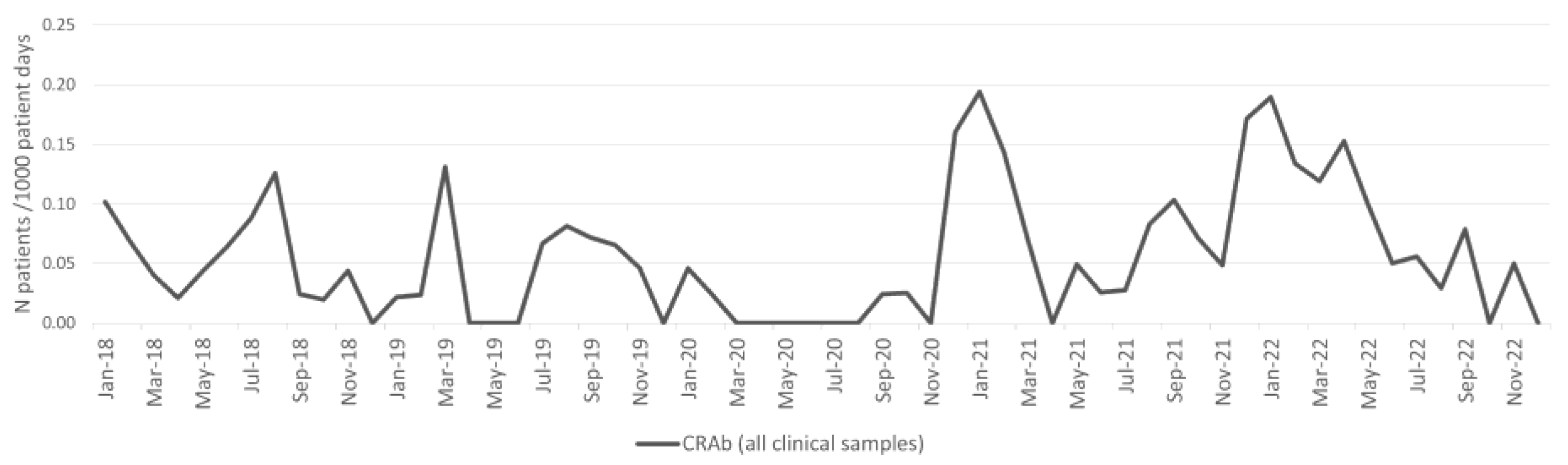
2.1. Acinetobacter baumannii
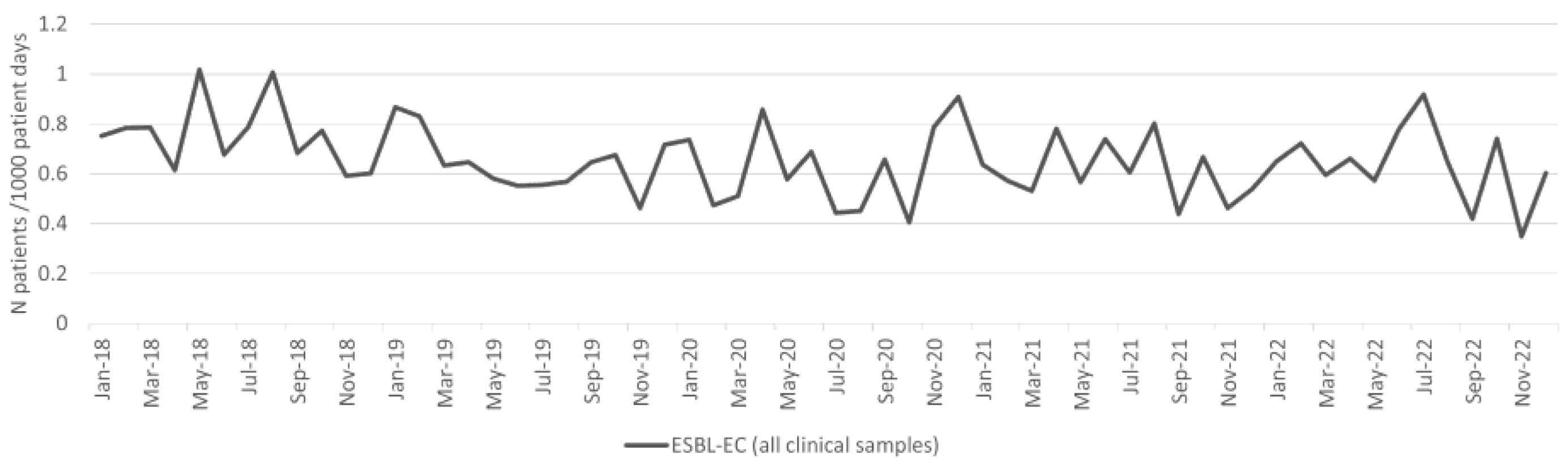
2.2. Esherichia coli
2.3. Enterococcus faecium
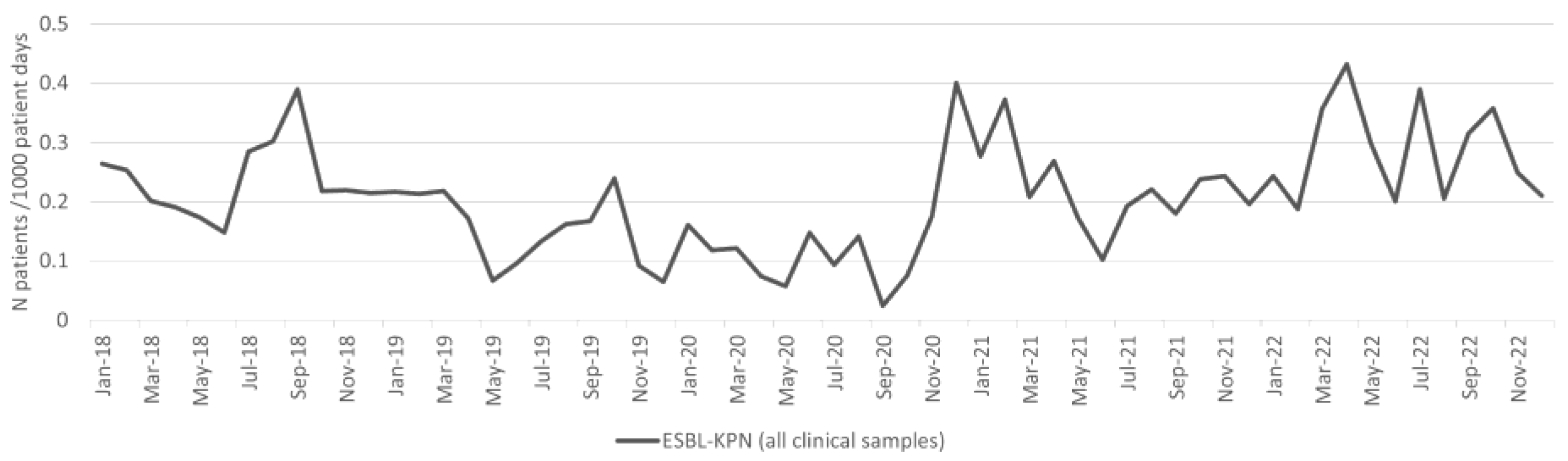
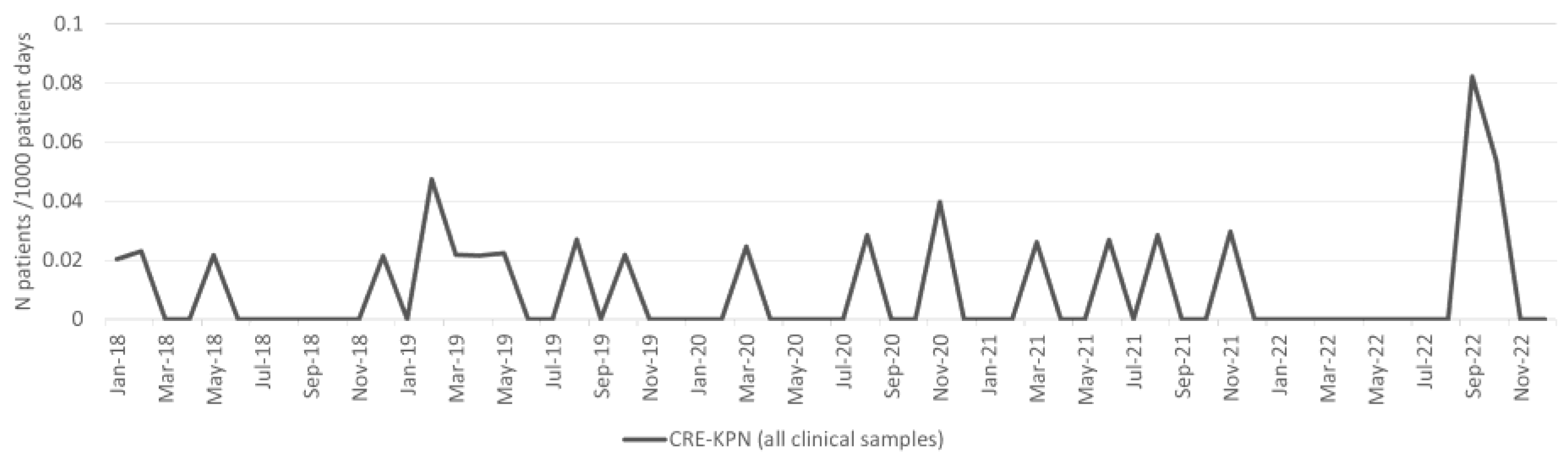
2.4. Klebsiella pneumoniae
2.5. Pseudomonas aeruginosa
2.6. Staphylococcus aureus
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. ECDC Guidance for Health System Contingency Planning during Widespread Transmission of SARS-CoV-2 with High Impact on Healthcare; ECDC: Stockholm, Sweden, 2020.
- Garcia Godoy, L.R.; Jones, A.E.; Anderson, T.N.; Fisher, C.L.; Seeley, K.M.L.; Beeson, E.A.; Zane, H.K.; Peterson, J.W.; Sullivan, P.D. Facial Protection for Healthcare Workers during Pandemics: A Scoping Review. BMJ Glob. Health 2020, 5, e002553. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Rational Use of Personal Protective Equipment for Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baño, J. Impact of COVID-19 Pandemic on Multidrug Resistant Gram Positive and Gram Negative Pathogens: A Systematic Review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef] [PubMed]
- CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; National Center for Emerging and Zoonotic Infectious Diseases, CDC: Atlanta, GA, USA, 2022. [CrossRef]
- Langford, B.J.; Soucy, J.-P.R.; Leung, V.; So, M.; Kwan, A.T.H.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.R.; Daneman, N. Antibiotic Resistance Associated with the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2023, 29, 302–309. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control; World Health Organization. Antimicrobial Resistance Surveillance in Europe 2023: 2021 Data; ECDC: Stockholm, Sweden, 2023.
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2023.
- European Centre for Disease Prevention and Control. Carbapenem-Resistant Acinetobacter baumannii in Healthcare Settings; ECDC: Stockholm, Sweden, 2017.
- Pirš, M.; Štrumbelj, I.; Lejko Zupanc, T. Enterobakterije, Acinetobacter baumannii in Pseudomonas aeruginosa—Označevanje večkratno odpornih izolatov in okrajšave preiskav nadzornih kužnin—2 izdaja. In Dokument SKUOPZ 003; Izdaja 2; Slovenska komisija za ugotavljanje občutljivosti za protimikrobna zdravila: Ljubljana, Slovenia, 2022. (In Slovene) [Google Scholar]
- European Centre for Disease Prevention and Control. Safe Use of Personal Protective Equipment in the Treatment of Infectious Diseases of High Consequence: A Tutorial for Healthcare Settings; Version 2; ECDC: Stockholm, Sweden, 2014.
- European Centre for Disease Prevention and Control. Infection Prevention and Control and Preparedness for COVID-19 in Healthcare Settings. Available online: https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings (accessed on 12 November 2023).
- Monnet, D.L.; Harbarth, S. Will Coronavirus Disease (COVID-19) Have an Impact on Antimicrobial Resistance? Eurosurveillance 2020, 25, 2001886. [Google Scholar] [CrossRef] [PubMed]
- Kinross, P.; Gagliotti, C.; Merk, H.; Plachouras, D.; Monnet, D.L.; Högberg, L.D.; EARS-Net Study Group. Large Increase in Bloodstream Infections with Carbapenem-Resistant Acinetobacter Species during the First 2 Years of the COVID-19 Pandemic, EU/EEA, 2020 and 2021. Eurosurveillance 2022, 27, 2200845. [Google Scholar] [CrossRef]
- Bar-Yoseph, H.; Hussein, K.; Braun, E.; Paul, M. Natural History and Decolonization Strategies for ESBL/Carbapenem-Resistant Enterobacteriaceae Carriage: Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2016, 71, 2729–2739. [Google Scholar] [CrossRef]
- Shenoy, E.S.; Paras, M.L.; Noubary, F.; Walensky, R.P.; Hooper, D.C. Natural History of Colonization with Methicillin-Resistant Staphylococcus Aureus (MRSA) and Vancomycin-Resistant Enterococcus (VRE): A Systematic Review. BMC Infect. Dis. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Despotovic, A.; Milosevic, B.; Cirkovic, A.; Vujovic, A.; Cucanic, K.; Cucanic, T.; Stevanovic, G. The Impact of COVID-19 on the Profile of Hospital-Acquired Infections in Adult Intensive Care Units. Antibiotics 2021, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.-J.; Song, W.; Kim, H.-S.; Kim, H.S.; Kim, J.-S. Impact of COVID-19 on Antimicrobial Consumption and Spread of Multidrug-Resistance in Bacterial Infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef]
- Polemis, M.; Mandilara, G.; Pappa, O.; Argyropoulou, A.; Perivolioti, E.; Koudoumnakis, N.; Pournaras, S.; Vasilakopoulou, A.; Vourli, S.; Katsifa, H.; et al. COVID-19 and Antimicrobial Resistance: Data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance—WHONET-Greece (January 2018–March 2021). Life 2021, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Porto, A.P.M.; Borges, I.C.; Buss, L.; Machado, A.; Bassetti, B.R.; Cocentino, B.; Bicalho, C.S.; Carrilho, C.M.D.M.; Rodrigues, C.; Neto, E.A.S.; et al. Healthcare-Associated Infections on the Intensive Care Unit in 21 Brazilian Hospitals during the Early Months of the Coronavirus Disease 2019 (COVID-19) Pandemic: An Ecological Study. Infect. Control Hosp. Epidemiol. 2023, 44, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Lötsch, F.; Albiger, B.; Monnet, D.L.; Struelens, M.J.; Seifert, H.; Kohlenberg, A.; European Antimicrobial Resistance Genes Surveillance Network (EURGen-Net) carbapenem-resistant Acinetobacter baumannii capacity survey group. Epidemiological Situation, Laboratory Capacity and Preparedness for Carbapenem-Resistant Acinetobacter Baumannii in Europe, 2019. Eurosurveillance 2020, 25, 2001735. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of Multidrug-Resistant (MDR) Bacterial Infections during the COVID-19 Pandemic: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.I.; Conceicao, E.P.; Tan, J.Y.; Magesparan, K.D.; Amin, I.B.M.; Ismail, B.B.S.; Toh, H.X.; Jin, P.; Zhang, J.; Wee, E.G.L.; et al. Unintended Consequences of Infection Prevention and Control Measures during COVID-19 Pandemic. Am. J. Infect. Control 2021, 49, 469–477. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Checklist for Hospitals Preparing for the Reception and Care of Coronavirus 2019 (COVID-19) Patients; ECDC: Stockholm, Sweden, 2020.
- EUCAST Clinical Breakpoints—Breakpoints and Guidance. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 1 December 2023).
- Giske, C.; MartInez-MartInez, L.; Canton, R.; Stefani, S.; Skov, R.; Glupczynski, Y.; Nordmann, P.; Wootton, M.; Miriagou, V.; Simonsen, G.S.; et al. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance; EUCAST: Växjö, Sweden, 2017. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]



| Pre-Pandemic Total vs. Pandemic Total—All Patients | Pre-Pandemic Total vs. Pandemic Non-COVID-19 Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Pre-Pandemic Total | Pandemic Total—All Patients | Percentage Change | p-Value | Pre-Pandemic Total | Pandemic—Non-COVID-19 Patients | Percentage Change | p-Value |
| Changes in the incidence density of patients with bacterial isolate from clinical samples per 1000 patient days | ||||||||
| A. baumannii | 4.0 | 5.8 | 44.0% | 0.698089 | 4.0 | 6.6 | 64.7% | 0.317535 |
| E. coli | 242.8 | 335.8 | 38.3% | 0.024283 | 242.8 | 372.5 | 53.4% | 0.000130 |
| E. faecium | 30.4 | 51.2 | 68.7% | 0.000142 | 30.4 | 57.4 | 89.0% | 0.000008 |
| K. pneumoniae | 54.3 | 69.8 | 28.6% | 0.731516 | 54.3 | 77.8 | 43.2% | 0.141717 |
| P. aeruginosa | 61.2 | 95.9 | 56.8% | 0.000653 | 61.2 | 106.6 | 74.2% | 0.000003 |
| S. aureus | 137.7 | 204.0 | 48.2% | 0.000952 | 137.7 | 226.0 | 64.2% | 0.000003 |
| Changes in the incidence density of patients with MDRB isolate from all clinical samples per 1000 patient days | ||||||||
| CRAb | 15.5 | 26.3 | 69.3% | 0.243931 | 15.5 | 30.0 | 93.5% | 0.178420 |
| ESBL-EC | 4.3 | 5.7 | 31.3% | 0.899099 | 4.3 | 6.3 | 45.5% | 0.163077 |
| VRE-EFA | 0.9 | 2.7 | 189.4% | 0.019194 | 0.9 | 3.0 | 221.4% | 0.008451 |
| ESBL-KPN | 5.3 | 9.3 | 74.3% | 0.010516 | 5.3 | 10.4 | 94.9% | 0.001176 |
| CRPs-PA | 2.9 | 4.7 | 63.2% | 0.015988 | 2.9 | 5.3 | 81.1% | 0.001869 |
| MRSA | 4.3 | 5.4 | 24.1% | 0.321187 | 4.3 | 5.9 | 36.9% | 0.887303 |
| Changes in the incidence density of patients with blood culture MDRB isolate per 1000 patient days | ||||||||
| CRAb | 11.2 | 15.2 | 36.3% | 0.814302 | 11.2 | 18.2 | 63.5% | 0.781330 |
| ESBL-EC | 5.4 | 7.5 | 38.6% | 0.737137 | 5.4 | 8.3 | 53.8% | 0.343502 |
| VRE-EFA | 0.9 | 4.1 | 334.2% | 0.230420 | 0.9 | 4.6 | 384.0% | 0.220754 |
| ESBL-KPN | 4.6 | 7.4 | 61.9% | 0.385179 | 4.6 | 8.4 | 84.3% | 0.269986 |
| CRPs-PA | 8.4 | 5.4 | −36.1% | 0.052249 | 8.4 | 5.8 | −31.3% | 0.075986 |
| MRSA | 6.0 | 6.3 | 4.7% | 0.215509 | 6.0 | 6.8 | 13.9% | 0.430595 |
| Changes in the incidence density of patients with MDRB isolate from clinical and surveillance samples per 1000 patient days | ||||||||
| CRAb | 2.4 | 3.0 | 24.8% | 0.590810 | 2.4 | 3.5 | 44.5% | 0.834377 |
| ESBL-EC | 46.7 | 57.5 | 23.0% | 0.172265 | 46.7 | 63.4 | 35.8% | 0.321187 |
| VRE-EFA | 2.8 | 10.9 | 294.4% | 0.000000 | 2.8 | 12.4 | 346.7% | 0.000000 |
| ESBL-KPN | 11.9 | 16.9 | 41.6% | 0.487890 | 11.9 | 18.8 | 57.2% | 0.149995 |
| CRE-KPN | 0.9 | 2.2 | 131.9% | 0.003322 | 0.9 | 2.4 | 153.0% | 0.001745 |
| CRPs-PA | 3.9 | 7.4 | 90.4% | 0.002521 | 3.9 | 8.2 | 111.8% | 0.000265 |
| MRSA | 24.9 | 23.8 | −4.3% | 0.000014 | 24.9 | 26.3 | 5.7% | 0.001965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrvič, T.; Stevanoska, S.; Beović, B.; Logar, M.; Gregorčič, S.; Žnidaršič, B.; Seme, K.; Velimirović, I.; Švent Kučina, N.; Maver Vodičar, P.; et al. The Impact of COVID-19 on Multidrug-Resistant Bacteria at a Slovenian Tertiary Medical Center. Antibiotics 2024, 13, 214. https://doi.org/10.3390/antibiotics13030214
Mrvič T, Stevanoska S, Beović B, Logar M, Gregorčič S, Žnidaršič B, Seme K, Velimirović I, Švent Kučina N, Maver Vodičar P, et al. The Impact of COVID-19 on Multidrug-Resistant Bacteria at a Slovenian Tertiary Medical Center. Antibiotics. 2024; 13(3):214. https://doi.org/10.3390/antibiotics13030214
Chicago/Turabian StyleMrvič, Tatjana, Sintija Stevanoska, Bojana Beović, Mateja Logar, Sergeja Gregorčič, Benica Žnidaršič, Katja Seme, Ivana Velimirović, Nataša Švent Kučina, Polona Maver Vodičar, and et al. 2024. "The Impact of COVID-19 on Multidrug-Resistant Bacteria at a Slovenian Tertiary Medical Center" Antibiotics 13, no. 3: 214. https://doi.org/10.3390/antibiotics13030214
APA StyleMrvič, T., Stevanoska, S., Beović, B., Logar, M., Gregorčič, S., Žnidaršič, B., Seme, K., Velimirović, I., Švent Kučina, N., Maver Vodičar, P., Križan Hergouth, V., Džeroski, S., & Pirs, M. (2024). The Impact of COVID-19 on Multidrug-Resistant Bacteria at a Slovenian Tertiary Medical Center. Antibiotics, 13(3), 214. https://doi.org/10.3390/antibiotics13030214








