A Five-Year Analysis of Antibiotic Resistance Trends among Bacteria Identified in Positive Urine Samples in a Tertiary Care Hospital from Bucharest, Romania
Abstract
1. Introduction
2. Results
2.1. Escherichia coli
2.2. Klebsiella spp.
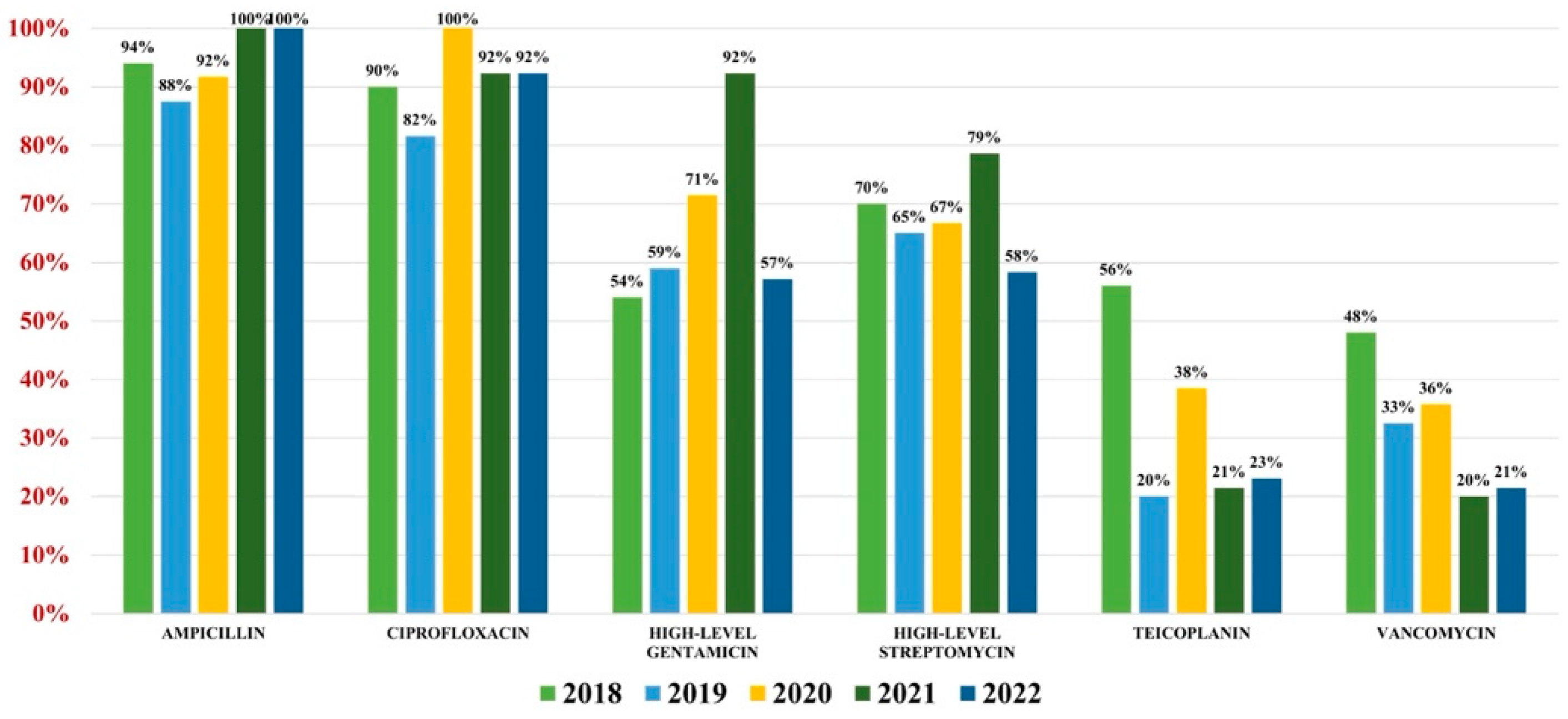
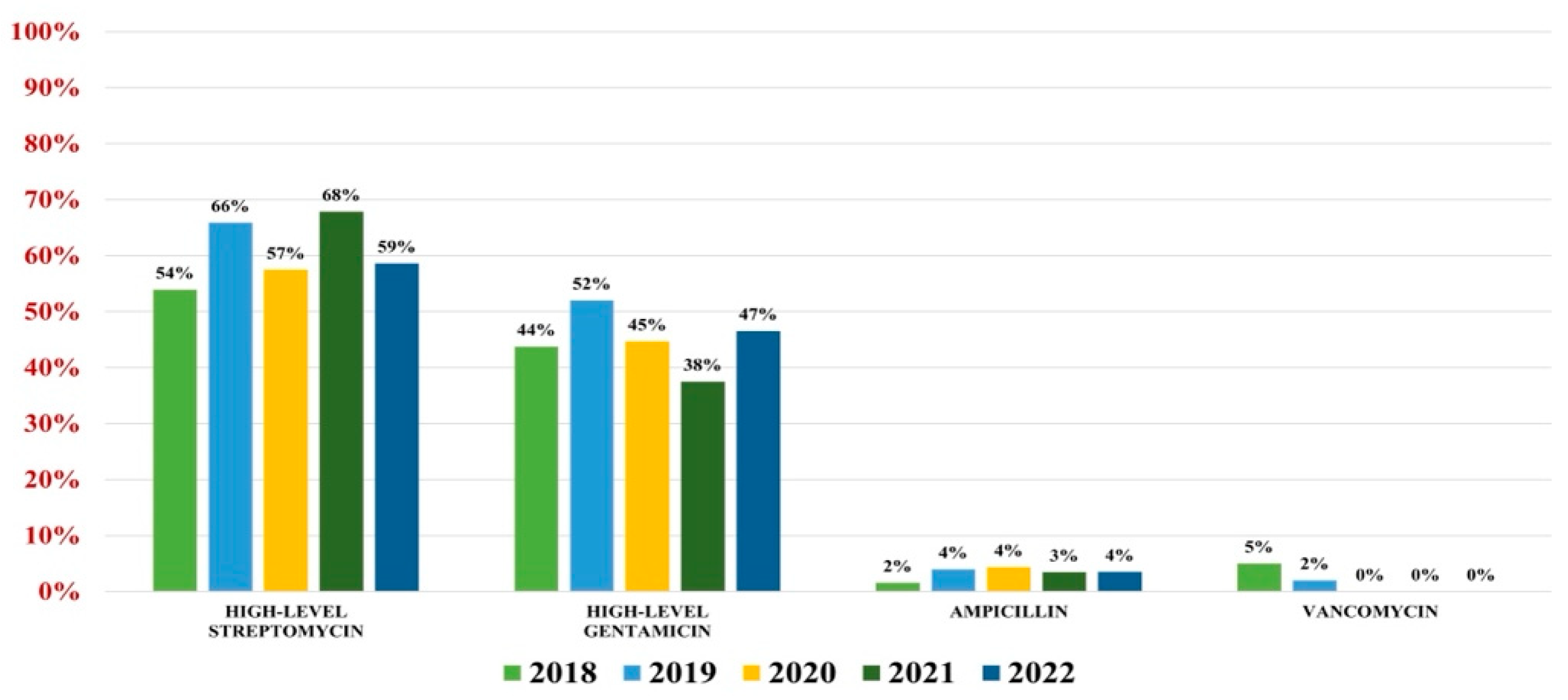
2.3. Enterococcus spp.
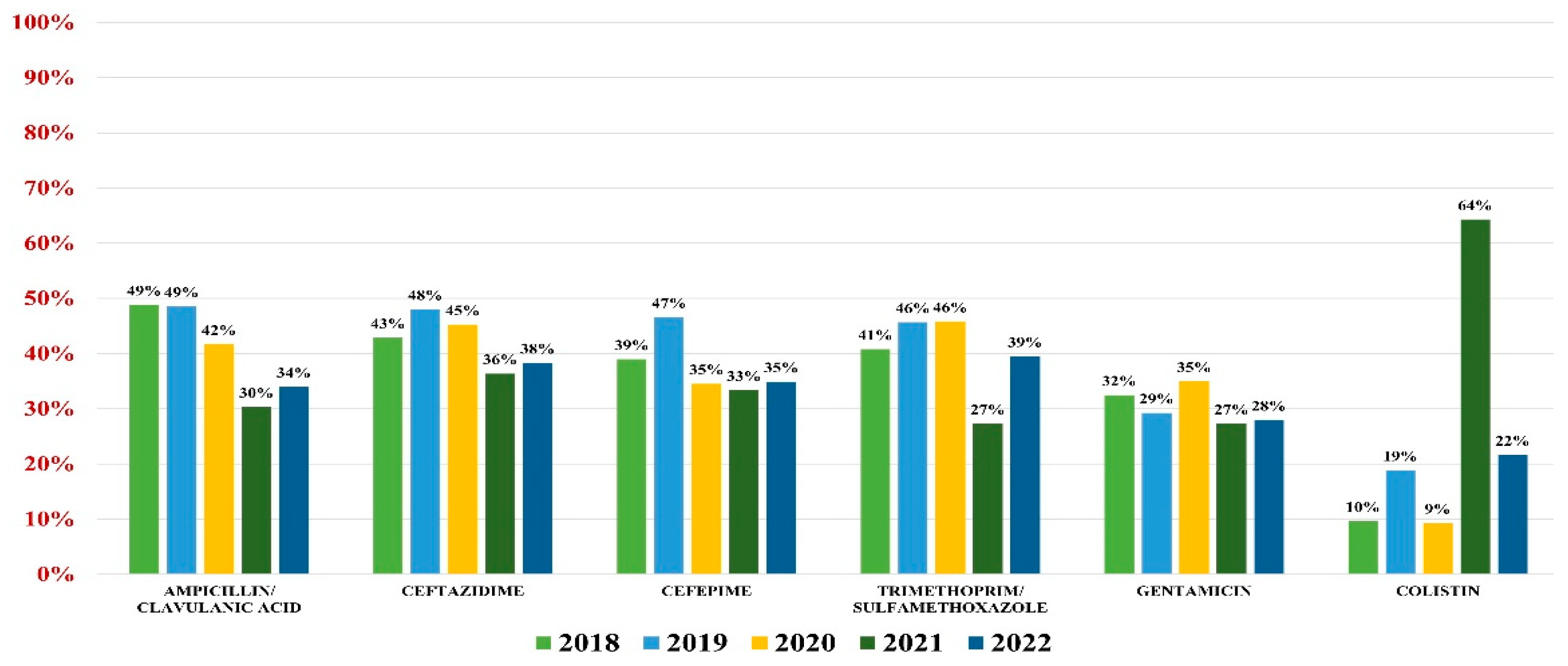
2.4. Pseudomonas aeruginosa
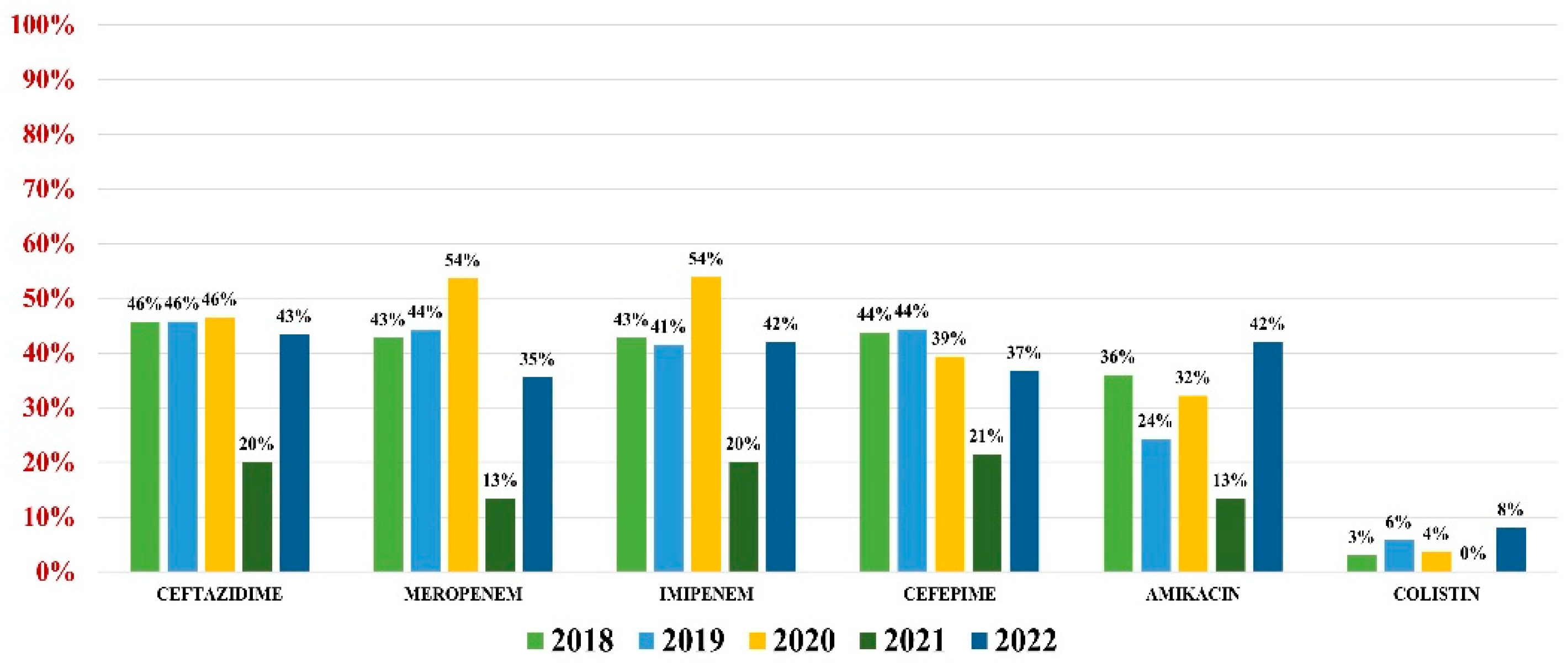
2.5. Acinetobacter baumannii
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Culture Procedure
4.2. Antibiotic Susceptibility Testing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foxman, B.; Barlow, R.; D’Arcy, H.; Gillespie, B.; Sobel, J.D. Urinary Tract Infection. Ann. Epidemiol. 2000, 10, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Codelia-Anjum, A.; Lerner, L.B.; Elterman, D.; Zorn, K.C.; Bhojani, N.; Chughtai, B. Enterococcal Urinary Tract Infections: A Review of the Pathogenicity, Epidemiology, and Treatment. Antibiotics 2023, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Reza Mortazavi-Tabatabaei, S.; Ghaderkhani, J.; Nazari, A.; Sayehmiri, K.; Sayehmiri, F.; Pakzad, I. Pattern of antibacterial resistance in urinary tract infections: A systematic review and meta-analysis. Int. J. Prev. Med. 2019, 10, 169. [Google Scholar]
- Li, J.; Jiang, F.; Xie, A.; Jiang, Y. Analysis of the Distribution and Drug Resistance of Pathogens in Patients with Urinary Tract Infection in the Eastern Chongming Area of Shanghai from 2018 to 2020. Infect. Drug Resist. 2022, 15, 6413–6422. [Google Scholar] [CrossRef] [PubMed]
- Sendra, E.; López Montesinos, I.; Rodriguez-Alarcón, A.; Du, J.; Siverio-Parés, A.; Arenas-Miras, M.; Cañas-Ruano, E.; Prim, N.; Durán-Jordà, X.; Blasco-Hernando, F.; et al. Comparative Analysis of Complicated Urinary Tract Infections Caused by Extensively Drug-Resistant Pseudomonas aeruginosa and Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae. Antibiotics 2022, 11, 1511. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Kattula, D.; Burkert, F. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://policycommons.net/artifacts/1818147/global-priority-list-of-antibiotic-resistant-bacteria-to-guide-research-discovery-and-development/2555608/ (accessed on 1 June 2023).
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Hasan, M.R.; Vincent, Y.M.; Leto, D.; Almohri, H. Trends in the Rates of Extended-Spectrum-β-Lactamase-Producing Enterobacterales Isolated from Urine Cultures during the COVID-19 Pandemic in Ontario, Canada. Microbiol. Spectr. 2023, 11, e03124-22. [Google Scholar] [CrossRef]
- Lee, D.S.; Choe, H.-S.; Lee, S.J.; Bae, W.J.; Cho, H.J.; Yoon B il Cho, Y.-H.; Han, C.H.; Jang, H.; Park, S.B.; Cho, W.J.; et al. Antimicrobial susceptibility pattern and epidemiology of female urinary tract infections in South Korea, 2010–2011. Antimicrob. Agents Chemother. 2013, 57, 5384–5393. [Google Scholar] [CrossRef]
- Bouza, E.; Juan, R.S.; Muñoz, P.; Voss, A.; Kluytmans, J. A European perspective on nosocomial urinary tract infections I. Report on the microbiology workload, etiology and antimicrobial susceptibility (ESGNI–003 study). Clin. Microbiol. Infect. 2001, 7, 523–531. [Google Scholar] [CrossRef]
- Linhares, I.; Raposo, T.; Rodrigues, A.; Almeida, A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: A ten-year surveillance study (2000–2009). BMC Infect. Dis. 2013, 13, 19. [Google Scholar] [CrossRef]
- Galani, I.; Karaiskos, I.; Giamarellou, H. Multidrug-resistant Klebsiella pneumoniae: Mechanisms of resistance including updated data for novel β-lactam-β-lactamase inhibitor combinations. Expert. Rev. Anti-Infect. Ther. 2021, 19, 1457–1468. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: An approach based on the mechanisms of resistance to carbapenems. Infection 2020, 48, 835–851. [Google Scholar] [CrossRef]
- Walkty, A.; Karlowsky, J.A.; Lagace-Wiens, P.; Baxter, M.R.; Adam, H.J.; Zhanel, G.G. Antimicrobial resistance patterns of bacterial pathogens recovered from the urine of patients at Canadian hospitals from 2009 to 2020. JAC Antimicrob. Resist. 2022, 4, dlac122. [Google Scholar] [CrossRef]
- Denisuik, A.J.; Karlowsky, J.A.; Adam, H.J.; Baxter, M.R.; Lagacé-Wiens, P.R.S.; Mulvey, M.R.; Hoban, D.J.; Zhanel, G.G. Dramatic rise in the proportion of ESBL-producing Escherichia coli and Klebsiella pneumoniae among clinical isolates identified in Canadian hospital laboratories from 2007 to 2016. J. Antimicrob. Chemother. 2019, 74, IV64–IV71. [Google Scholar] [CrossRef]
- Mills, E.G.; Martin, M.J.; Luo, T.L.; Ong, A.C.; Maybank, R.; Corey, B.W.; Harless, C.; Preston, L.N.; Rosado-Mendez, J.A.; Preston, S.B.; et al. A one-year genomic investigation of Escherichia coli epidemiology and nosocomial spread at a large US healthcare network. Genome Med. 2022, 14, 147. [Google Scholar] [CrossRef]
- Al Benwan, K.; Jamal, W. Etiology and Antibiotic Susceptibility Patterns of Urinary Tract Infections in Children in a General Hospital in Kuwait: A 5-Year Retrospective Study. Med. Princ. Pract. 2022, 31, 562–569. [Google Scholar] [CrossRef]
- Abalkhail, A.; AlYami, A.S.; Alrashedi, S.F.; Almushayqih, K.M.; Alslamah, T.; Alsalamah, Y.A.; Elbehiry, A. The Prevalence of Multidrug-Resistant Escherichia coli Producing ESBL among Male and Female Patients with Urinary Tract Infections in Riyadh Region, Saudi Arabia. Healthcare 2022, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, B.; Labi, A.-K.; Gupte, H.A.; Davtyan, H.; Peprah, G.M.; Adu-Gyan, F.; Nair, D.; Muradyan, K.; Jessani, N.S.; Sekyere-Nyantakyi, P. High Resistance to Antibiotics Recommended in Standard Treatment Guidelines in Ghana: A Cross-Sectional Study of Antimicrobial Resistance Patterns in Patients with Urinary Tract Infections between 2017–2021. Int. J. Environ. Res. Public Health 2022, 19, 16556. [Google Scholar] [CrossRef]
- Farfour, E.; Dortet, L.; Guillard, T.; Chatelain, N.; Poisson, A.; Mizrahi, A.; Fournier, D.; Bonnin, R.A.; Degand, N.; Morand, P.; et al. Antimicrobial Resistance in Enterobacterales Recovered from Urinary Tract Infections in France. Pathogens 2022, 11, 356. [Google Scholar] [CrossRef]
- Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections. Pathogens 2022, 12, 34. [Google Scholar] [CrossRef]
- Levitus, M.; Rewane, A.; Perera, T.B. Vancomycin-Resistant Enterococci; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Álvarez-Artero, E.; Campo-Nuñez, A.; García-García, I.; García-Bravo, M.; Cores-Calvo, O.; Galindo-Pérez, I.; Pendones-Ulerio, J.; López-Bernus, A.; Belhassen-García, M.; Pardo-Lledías, J. Urinary tract infection caused by Enterococcus spp.: Risk factors and mortality. An observational study. Rev. Clínica Española (Engl. Ed.) 2021, 221, 375–383. [Google Scholar] [CrossRef]
- Salm, J.; Salm, F.; Arendarski, P.; Kramer, T.S. High frequency of Enterococcus faecalis detected in urinary tract infections in male outpatients—A retrospective, multicenter analysis, Germany 2015 to 2020. BMC Infect. Dis. 2023, 23, 812. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2016, 3, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Nicolau, D.P.; Gill, C.M. Carbapenemase-producing Pseudomonas aeruginosa—An emerging challenge. Emerg. Microbes Infect. 2022, 11, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi Halat, D.; Ayoub Moubareck, C. The Intriguing Carbapenemases of Pseudomonas aeruginosa: Current Status, Genetic Profile, and Global Epidemiology. Yale J. Biol. Med. 2022, 95, 507–515. [Google Scholar] [PubMed]
- Piperaki, E.-T.; Tzouvelekis, L.S.; Miriagou, V.; Daikos, G.L. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019, 25, 951–957. [Google Scholar] [CrossRef]
- Alvarez-Uria, G.; Gandra, S.; Mandal, S.; Laxminarayan, R. Global forecast of antimicrobial resistance in invasive isolates of Escherichia coli and Klebsiella pneumoniae. Int. J. Infect. Dis. 2018, 68, 50–53. [Google Scholar] [CrossRef]
- Bagińska, N.; Cieślik, M.; Górski, A.; Jończyk-Matysiak, E. The Role of Antibiotic Resistant A. baumannii in the Pathogenesis of Urinary Tract Infection and the Potential of Its Treatment with the Use of Bacteriophage Therapy. Antibiotics 2021, 10, 281. [Google Scholar] [CrossRef]
- Stoltidis-Claus, C.; Rosenberger, K.D.; Mandraka, F.; Quante, X.; Gielen, J.; Hoffmann, D.; Wisplinghoff, H.; Jazmati, N. Antimicrobial resistance of clinical Enterobacterales isolates from urine samples, Germany, 2016 to 2021. Eurosurveillance 2023, 28, 2200568. [Google Scholar] [CrossRef]
- Dablool, A.S. An Antibiogram Study for Urine Culture Testing in Makkah Region Hospitals. Cureus 2023, 15, 36012. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef]
- Goodlet, K.J.; Benhalima, F.Z.; Nailor, M.D. A Systematic Review of Single-Dose Aminoglycoside Therapy for Urinary Tract Infection: Is It Time To Resurrect an Old Strategy? Antimicrob. Agents Chemother. 2019, 63, 10-1128. [Google Scholar] [CrossRef]
- Han, S.B.; Lee, S.C.; Lee, S.Y.; Jeong, D.C.; Kang, J.H. Aminoglycoside therapy for childhood urinary tract infection due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae. BMC Infect. Dis. 2015, 15, 414. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Xie, X.; Wu, X.; Li, X.; Zhao, Z.; Luo, S.; Wan, Z.; Liu, J.; Fu, L.; et al. In vitro and in vivo assessment of the antibacterial activity of colistin alone and in combination with other antibiotics against Acinetobacter baumannii and Escherichia coli. J. Glob. Antimicrob. Resist. 2020, 20, 351–359. [Google Scholar] [CrossRef]
- Assis, R.E.; Coelho, I.; Real, A.; França, L.; Araújo, A.; Pereira, T.; Catorze, N. Intra-Vesical Colistin for Pseudomonas aeruginosa Urinary Tract Infections. Eur. J. Case Rep. Intern. Med. 2019, 6, 000996. [Google Scholar] [CrossRef]
- Gardiner, B.J.; Stewardson, A.J.; Abbott, I.J.; Peleg, A.Y. Nitrofurantoin and fosfomycin for resistant urinary tract infections: Old drugs for emerging problems. Aust. Prescr. 2019, 42, 14. [Google Scholar] [CrossRef]
- Fajfr, M.; Balik, M.; Cermakova, E.; Bostik, P. Effective Treatment for Uncomplicated Urinary Tract Infections with Oral Fosfomycin, Single Center Four Year Retrospective Study. Antibiotics 2020, 9, 511. [Google Scholar] [CrossRef]
- Mahdizade Ari, M.; Dashtbin, S.; Ghasemi, F.; Shahroodian, S.; Kiani, P.; Bafandeh, E.; Darbandi, T.; Ghanavati, R.; Darbandi, A. Nitrofurantoin: Properties and potential in treatment of urinary tract infection: A narrative review. Front. Cell Infect. Microbiol. 2023, 13, 1148603. [Google Scholar] [CrossRef] [PubMed]
- Bruyndonckx, R.; Latour, K.; Atud, G.A.; Dubovy, P.; Jaspers, S.; Hens, N.; Catry, B.; Goossens, H.; Coenen, S. Time trend of prevalence and susceptibility to nitrofurantoin of urinary MDR Escherichia coli from outpatients. J. Antimicrob. Chemother. 2019, 74, 3264–3267. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Advani, S.; Rink, A.; Bianco, L.; Van Ness, P.H.; Quagliarello, V.; Juthani-Mehta, M. Increased Fluoroquinolone-Susceptibility and Preserved Nitrofurantoin-Susceptibility among Escherichia coli Urine Isolates from Women Long-Term Care Residents: A Brief Report. Open Access J. Gerontol. Geriatr. Med. 2018, 4, 555636. [Google Scholar] [CrossRef] [PubMed]
- Tariq, T.M. Frequency of uropathogens showing resistance to Nitrofurantoin. J. Pak. Med. Assoc. 2023, 73, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Giedraitiene, A.; Pereckaite, L.; Bredelyte-Gruodiene, E.; Virgailis, M.; Ciapiene, I.; Tatarunas, V. CTX-M-producing Escherichia coli strains: Resistance to temocillin, fosfomycin, nitrofurantoin and biofilm formation. Future Microbiol. 2022, 17, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Bielec, F.; Wenecka, M.; Brauncajs, M.; Pastuszak-Lewandoska, D. Analysis of Cumulative Antibiogram Reports in Search for Optimal Empirical Urinary Tract Infection Treatment at the Central Teaching Hospital of the Medical University of Lodz, Poland: Results of a 3-Year Surveillance. J. Clin. Med. 2023, 12, 6270. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borcan, A.M.; Radu, G.; Simoiu, M.; Costea, E.L.; Rafila, A. A Five-Year Analysis of Antibiotic Resistance Trends among Bacteria Identified in Positive Urine Samples in a Tertiary Care Hospital from Bucharest, Romania. Antibiotics 2024, 13, 160. https://doi.org/10.3390/antibiotics13020160
Borcan AM, Radu G, Simoiu M, Costea EL, Rafila A. A Five-Year Analysis of Antibiotic Resistance Trends among Bacteria Identified in Positive Urine Samples in a Tertiary Care Hospital from Bucharest, Romania. Antibiotics. 2024; 13(2):160. https://doi.org/10.3390/antibiotics13020160
Chicago/Turabian StyleBorcan, Alina Maria, Georgiana Radu, Mădălina Simoiu, Elena Liliana Costea, and Alexandru Rafila. 2024. "A Five-Year Analysis of Antibiotic Resistance Trends among Bacteria Identified in Positive Urine Samples in a Tertiary Care Hospital from Bucharest, Romania" Antibiotics 13, no. 2: 160. https://doi.org/10.3390/antibiotics13020160
APA StyleBorcan, A. M., Radu, G., Simoiu, M., Costea, E. L., & Rafila, A. (2024). A Five-Year Analysis of Antibiotic Resistance Trends among Bacteria Identified in Positive Urine Samples in a Tertiary Care Hospital from Bucharest, Romania. Antibiotics, 13(2), 160. https://doi.org/10.3390/antibiotics13020160



