Abstract
Purpose: Our aim was to investigate risk factors, clinical characteristics, and antibiotic susceptibility patterns of cornea-isolated Streptococcus species collected at a tertiary hospital in China over 18 years. Methods: This retrospective study reviewed data from 350 patients diagnosed with Streptococcal keratitis at Beijing Tongren Hospital between January 2006 and December 2023, including demographics, risk factors, clinical signs, in vivo confocal microscopy (IVCM) imaging, and antibiotic susceptibility testing. Results: The predominant type was Streptococcus pneumoniae (n = 108, 29.8%), followed by Streptococcus mitis (n = 90, 24.9%) and Streptococcus oralis (n = 85, 23.5%). Main risk factors included previous ocular surface disease (24.6%), ocular surgery (21.4%), and trauma (16.3%). Significant differences in clinical characteristics were observed among S. pneumoniae, S. oralis, and S. mitis regarding infiltration location (p = 0.038) and size (p = 0.037), as well as hypopyon presence (p = 0.006). IVCM revealed deeper inflammatory cell distribution and structural disruption as the disease progressed. Resistance rates of aminoglycosides, β-lactams, and fluoroquinolones have increased, with significant differences among species for amikacin (p = 0.010), gentamicin (p = 0.007), and others. Poor outcomes correlated with disease duration over one month, central corneal ulcers, dense infiltrations, hypopyon, and scar tissue presence on IVCM. Conclusions: Streptococcal keratitis is a complex ocular infection with multiple risk factors. S. pneumoniae, S. mitis, and S. oralis are the primary causative agents, exhibiting varying clinical features and antibiotic resistance patterns. Key factors associated with poor outcomes include long disease duration, central corneal ulcers, and severe infiltration.
1. Introduction
Microbial keratitis (MK) is a serious, sight-threatening ophthalmological condition characterized by symptoms such as pain, redness, inflammation, and corneal opacity. It is the fourth leading cause of blindness worldwide, with 1.5–2.0 million new cases occurring yearly [1,2]. As one of the top three pathogens responsible for bacterial keratitis, Streptococcal keratitis typically presents with a deep, round central stromal ulcer with a progressive edge and anterior chamber hypopyon, and can result in corneal perforation, severe endophthalmitis, and vision loss [3,4,5,6,7].
Streptococcal keratitis is attributed to three distinct species of Streptococcus: S. pneumoniae, S. mitis, and S. oralis. Among these, S. pneumoniae is the most prevalent and is characterized by its high virulence, which can result in rapid disease progression and corneal perforation [8]. In contrast, S. mitis and S. oralis exhibit lower virulence, generally leading to milder clinical presentations [9]. Despite the significance of these pathogens, there is a paucity of studies addressing the clinical features and risk factors associated with Streptococcal species isolated from corneas, particularly with respect to the differences among infections caused by these three specific Streptococcus species. Furthermore, the treatment regimens and prognoses for these infections are not uniform, underscoring the necessity for comprehensive research in this area.
This retrospective study aimed to investigate the risk factors, clinical characteristics, and antibiotic susceptibility patterns of Streptococcal keratitis over an 18-year period. Additionally, we evaluated the clinical manifestations and antibiotic resistance rates of different species of cornea-isolated Streptococcus.
2. Results
2.1. Demographics and Predisposing Factors for Streptococcal Keratitis
In this retrospective study, a total of 350 patients diagnosed with Streptococcal keratitis were included. The demographic data are summarized in Table 1, indicating that 47.1% of the patients were women, and 56.0% of the cases involved the right eye. The participants’ ages ranged from 2 months to 89 years, with a mean age of 49.1 years (SD = 23.1 years). A total of 350 strains were isolated from corneal scrapings and were all culture-positive. Species identification demonstrated that S. pneumoniae was the most frequently isolated organism, accounting for 29.8% (n = 108) of cases. This was followed by S. mitis at 24.9% (n = 90) and S. oralis at 23.5% (n = 85), collectively representing 78.0% (n = 273) of all Streptococcal isolates identified in this cohort. In terms of treatment outcomes, it was found that 87.7% (n = 307) of patients received antibiotic therapy alone, while 12.3% (n = 43) required surgical intervention to address the infection. These findings underscore the importance of timely diagnosis and appropriate management strategies in the treatment of Streptococcal keratitis.

Table 1.
Demographic and predisposing factors of Streptococcus keratitis.
The most prevalent risk factor identified was a history of previous ocular surface disease, which accounted for 24.6% of cases. Among these, the most common conditions included glaucoma (6.0%), uveitis (5.1%), blepharitis (3.2%), and scleritis (2.0%). In addition to ocular surface diseases, previous ocular surgery was reported in 21.4% of patients, while ocular trauma was noted in 16.3%, both of which are critical risk factors for the development of keratitis. Systemic diseases were present in 9.1% of the cohort, and co-infection with other pathogens was observed in 8.3% of cases. Furthermore, recent corticosteroid use was identified in 4.0% of patients, indicating a potential immunosuppressive risk. Timeliness of medical intervention varied among patients; 43.1% sought care at an outpatient clinic within two weeks of the onset of symptoms, while 27.1% delayed seeking medical attention for one month or more. These findings emphasize the importance of recognizing predisposing conditions and the need for prompt treatment to mitigate the risk of developing Streptococcal keratitis.
2.2. Clinical Characteristics of Streptococcal Keratitis Subtypes
Patients with Streptococcal keratitis commonly presented with a constellation of clinical features, including corneal ulceration (99.0% of cases), dense infiltration (94.0%), corneal thinning (55.0%), edema (54.0%), and early-stage hypopyon (63.0%). Other notable findings were neovascularization and Descemet’s membrane folds (Figure 1). This distinct clinical presentation can aid in the prompt recognition and management of this sight-threatening corneal infection.
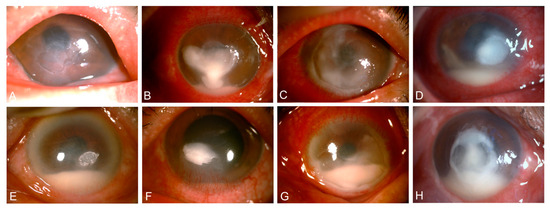
Figure 1.
Slit-lamp images showing typical signs of Streptococcal keratitis. (A) Ulceration, (B) dense infiltrate, (C) corneal thinning, (D) edema, (E) Descemet’s membrane folds, (F) neovascularization, (G) conjunctival hyperemia, and (H) hypopyon.
As shown in Table 2, significant differences were observed in the clinical presentation of keratitis caused by various Streptococcal species, specifically with respect to the location (p = 0.038) and size (p = 0.037) of dense infiltrations, as well as the presence of hypopyon (p = 0.006) among cases attributed to S. pneumoniae, S. oralis, and S. mitis. Patients with S. pneumoniae keratitis were more likely to have central corneal involvement (59.0%) compared to S. oralis (26.0%, p = 0.011). Small dense infiltrations (<3 mm) were more common in S. mitis keratitis (35.5%) than in S. pneumoniae (17.9%, p = 0.041). Hypopyon was more frequently observed in S. pneumoniae keratitis (82.0%) compared to S. oralis (47.0%, p = 0.002) and S. mitis (55.0%, p = 0.014). There was no statistically significant difference in the time from symptom onset to first clinical visit among the three Streptococcal species. These findings highlight the importance of identifying specific Streptococcal species, as they may inform clinical management and treatment strategies.

Table 2.
Comparison of clinical signs of S. pneumoniae, S. oralis, and S. mitis keratitis.
2.3. In Vivo Confocal Microscopy Findings in Streptococcal Keratitis Progression
In the mild stage of Streptococcal keratitis, IVCM revealed inflammatory cells confined to the epithelial layer, along with dendriform cells near the basement membrane (Figures S1A, S2A and S3A). As the disease progressed, enlarged dense infiltration, hypopyon, and Descemet’s membrane folds emerged (Figure S1B). IVCM showed inflammatory cells in the epithelium and anterior stroma, with dendriform cells near the basement membrane (Figures S2B, S3B and S4B). In the severe stage, large dense infiltration, neovascularization, and extensive hypopyon were observed (Figure S1C). IVCM detected inflammatory cells from the epithelium to the posterior stroma, with loss of normal keratocyte structure (Figures S2C, S3C, S4C, S5C and S6C). These IVCM findings provide detailed insights into the cellular changes associated with the progression of Streptococcal keratitis, which may guide clinical management.
Progression of the disease was marked by increased densities of inflammatory and dendritiform cells. Inflammatory cell density rose from 1049 ± 142 cells/mm2 in the mild stage to 3207 ± 436 cells/mm2 in the severe stage (Figure S7A), while dendritiform cell density increased from 238 ± 37 cells/mm2 to 657 ± 78 cells/mm2 (Figure S7B). There were 23 eyes (50.0%) presenting honeycomb-shaped inflammatory cells, 44 eyes (95.7%) inflammatory cell infiltration in nonspecific distribution, 30 eyes (65.2%) normal keratocytes, 40 eyes (87.0%) activated keratocytes with nuclei, and 29 eyes (63.0%) activated keratocytes without nuclei. In addition, dendritiform cells could be seen in 34 eyes (73.9%), epithelial bullae were presented in 25 eyes (54.3%), stromal bullae appeared in 23 eyes (50.0%), “spindles” were seen in 30 eyes (65.2%), and scar tissue could be found in 17 eyes (37.0%).
2.4. Antibiotic Resistance Profiles of Streptococcus Species
A total of 277 Streptococcus isolates underwent antimicrobial susceptibility testing and were included in this part of the study (Table S2). The resistant rates of vancomycin, amikacin, gentamicin, ciprofloxacin, ofloxacin, rifampin, levofloxacin, tobramycin, ceftazidime, moxifloxacin, and benzalkonium chloride were evaluated separately.
Subsequently, we conducted an analysis to trace the trends of resistance rates of S. pneumoniae, S. oralis, and S. mitis. The resistance rate for rifamycin (0–14.2%) and glycopeptides (0–7.1%) among Streptococcus species remained notably low, with no resistance rate observed over the past decade (Figure 2A). In contrast, the resistance rate for aminoglycosides increased significantly, rising from 64.9% in 2006 to 91.6% in 2014. Furthermore, from 2015 to 2023, there was a marked increase in the resistance rates of fluoroquinolones, which rose from 12.0% to 41.6%, as well as β-lactam antibiotics, which increased from 48.2% to 66.7%.
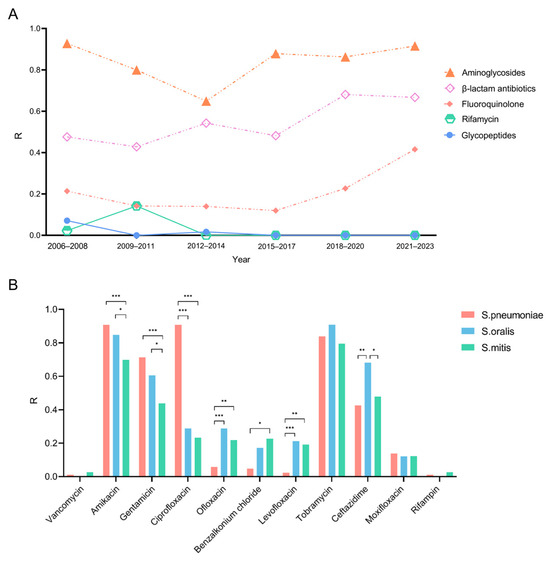
Figure 2.
Changes in resistance rates of cornea-derived Streptococci over the last 18 years and a comparison of resistance rates among S. pneumoniae, S. oralis, and S. mitis. R: the percentage of isolates resistant to each antibiotic. (A) Changes in antimicrobial resistance patterns of cornea-derived Streptococci over the past 18 years. (B) Comparison of resistance rates among S. pneumoniae, S. oralis, and S. mitis. * = p-value < 0.05; ** = p-value < 0.01; *** = p-value < 0.001.
The antibiotic resistance rates among the three Streptococcus species were analyzed (Figure 2B). Amikacin and tobramycin exhibited high resistance rates across all three types of Streptococcal keratitis. Specifically, S. pneumoniae, S. oralis, and S. mitis showed resistance rates of 90%, 84.8%, and 69.8% to amikacin and 83.9%, 90.9%, and 79.5% to gentamicin, respectively. Significantly different antibiotic resistance rates among three species were found in amikacin (p = 0.010), gentamycin (p = 0.007), ciprofloxacin (p < 0.001), ofloxacin (p = 0.005), levofloxacin (p < 0.001), benzalkonium chloride (p = 0.003), and ceftazidime (p = 0.001). A similar finding was found for ciprofloxacin, occupying 90.8% of S. pneumoniae isolates, 28.8% of S. oralis, and 23.3% of S. mitis. On the other hand, S. pneumoniae showed relatively low rates of resistance to ofloxacin (5.8%), levofloxacin (2.1%), and benzalkonium chloride (9.1%), whereas in the other two species, the rates were higher: resistance rates to ofloxacin, levofloxacin, and benzalkonium chloride were 28.8%, 21.2%, and 17.2%, respectively, for S. oralis, and 21.9%, 19.2%, and 22.7%, respectively, for S. mitis. The resistance rate of ceftazidime of S. oralis (68.2%) was markedly higher than S. pneumoniae (42.5%) and S. mitis (47.9%).
2.5. Analysis of Prognostic Correlations
In this study, follow-up records of 74 patients were analyzed, categorizing outcomes as good (clinical remission, Figure 3A) or poor (worsening, surgery, Figure 3B). S. pneumoniae keratitis had the worst prognosis, with 65% resulting in poor outcomes, whereas S. mitis keratitis showed a more favorable prognosis, with only 26% leading to poor outcomes.
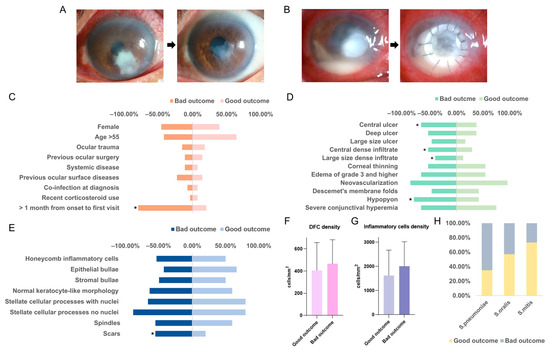
Figure 3.
Risk factors may affect outcome and prognostic differences among Streptococcus pneumoniae Streptococcus oralis and Streptococcus mitis keratitis. (A) The clinical manifestation of a good outcome of patients with Streptococcal keratitis. (B) The clinical manifestation of a good outcome of patients with Streptococcal keratitis. The correlation between demogsraphic and predisposing data (C), initial clinical characteristics (D), IVCM cellular features (E), dendritiform cell density in IVCM photographs (F), inflammatory cells density in IVCM photographs (G), and the outcome. (H) A comparison of outcomes of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis keratitis. *: significant p-value < 0.05.
Univariate and multivariate analyses (Figure 3C) revealed significant correlations between demographic and predisposing factors and patient outcomes. Notably, a longer interval from symptom onset to the first visit was associated with an increased likelihood of poor outcomes (p < 0.001). Other factors, such as age and sex, did not show significant correlations.
Furthermore, an analysis of slit-lamp photography features of Streptococcal keratitis indicated that central corneal ulcers (p = 0.042), dense central infiltrations (p = 0.043), large-sized infiltrations (p = 0.047), and hypopyon (p = 0.019) were all linked to worse outcomes (Figure 3D). In addition, the appearance of scar tissue on in vivo confocal microscopy (IVCM) was correlated with poor outcomes (Figure 3E, p = 0.037). Inflammatory cell and dendritic cell densities were slightly increased in patients with bad outcomes (Figure 3F,G).
3. Discussion
Streptococcal keratitis is a rapidly progressing corneal disease characterized by poor prognosis, often leading to corneal scarring, perforation, and permanent vision loss [10,11]. This study examines predisposing factors, clinical findings, antibiotic susceptibility patterns, and treatment outcomes of patients with Streptococcal keratitis treated at Beijing Tongren Hospital over an 18-year period, aligning with findings from previous studies in Shanghai [12], South India [13], and Australia [14]. The analysis identified S. pneumoniae as the most prevalent species, accounting for 29.8% of total Streptococcal isolates, consistent with a prior 5-year study that reported S. pneumoniae responsible for 38% of bacterial keratitis cases [15].
Streptococcal keratitis is typically an opportunistic infection, often arising from immune defects and various risk factors. While contact lens wearing is a common risk factor for bacterial keratitis in developed countries [16,17], none of the patients in this study reported contact lens use. The most prevalent risk factor identified was previous ocular disease, accounting for 24.6% of cases, including conditions such as glaucoma, uveitis, and blepharitis. This prevalence is comparable to a report from northern California (17.7%), but lower than findings from Iran (44.4%) [18]. These pre-existing ocular conditions can compromise the normal structure and defense mechanisms of the eye, increasing susceptibility to bacterial infections. Additionally, 21.4% of cases had undergone prior eye surgery, and 16.3% had experienced ocular trauma, consistent with results from a study in Singapore [19].
We evaluated slit-lamp images to detail Streptococcal keratitis manifestations. Nearly all patients (99.0%) developed corneal ulcers, indicating high epithelial barrier breakdown facilitating Streptococcal invasion [20]. Corneal thinning was another key feature, potentially linked to virulence factors [16]. Notably, we compared clinical signs among S. pneumoniae, S. oralis, and S. mitis keratitis. S. pneumoniae cases showed higher rates of central dense infiltrates and hypopyon, while S. oralis/S. mitis cases had more paracentral infiltrates and less hypopyon. S. mitis cases also tended to have smaller infiltrates. These differences reflected the species-specificity of disease progression, with S. pneumoniae being more virulent, which led to a higher risk of rapid disease progression and thus bad outcomes [17]. These findings provide new perspectives on distinguishing these Streptococcal keratitis types, informing future treatment.
IVCM serves as a noninvasive tool for the early diagnosis of fungal and amoebic keratitis by detecting fungal filaments and amoebic capsules in real time. However, the IVCM features of bacterial keratitis, particularly Streptococcal keratitis, have not been extensively documented [18,19]. Our study enhances the understanding of IVCM characteristics in Streptococcal keratitis, revealing that dendritiform cells were present in 73.9% of cases, with inflammatory cells arranged nonspecifically in almost all cases. Epithelial and stromal bullae were noted in half of the cases, occurring more frequently than in amoebic or fungal keratitis [21]. The high density of dendritiform and inflammatory cells suggests a severe immune response. Initially, inflammatory cells are confined to the epithelial layer, but as the disease progresses, they infiltrate the anterior and then the posterior stromal layers, indicating an increasing depth of inflammation. Additionally, the observation of cellular structure destruction allows for assessment of the damage inflicted by Streptococcus on epithelial cells and keratocytes.
Treatment of Streptococcal keratitis typically involves topical antimicrobial therapy with systemic antibiotics being commonly used in severe cases, and surgical interventions reserved for cases of resistance, progression despite treatment, or corneal perforation [22]. However, due to the widespread use of commonly used topical antibiotics in ophthalmic treatment, bacterial resistance is increasing in some cases, thus limiting the potential efficacy of such drugs [23,24]. A 20-year follow-up study in Taiwan showed increasing antibiotic resistance in Gram-positive bacteria, including rising azithromycin resistance in S. pneumoniae [25,26,27]. However, it is important to recognize that the established breakpoints for topical antibiotics are not well defined, making the interpretation of drug sensitivity data challenging. Topical antibiotics have several advantages over systemic medications, including the ability to deliver high concentrations of antimicrobial agents at the desired site of action and reduced systemic toxicity [23]. Even when pathogens exhibit resbuistance in vitro, the concentration of topical antibiotics at the site of infection often exceeds the MIC; therefore, while in vitro resistance patterns are valuable, the local drug concentration at the site of infection should also be considered when evaluating the efficacy of topical antibiotics, because that concentration may still be sufficient to effectively treat the infection. As illustrated in our study, amikacin and tobramycin had higher resistance rates suggesting that they are not suitable for the treatment of Streptococcal keratitis, whereas vancomycin and rifampicin had lower resistance rates. We also found that resistance rates to aminoglycosides, β-lactam antibiotics, and fluoroquinolone antibiotics have increased over the past few years. Among them, the rise in antibiotic resistance rates of fluoroquinolones from 12.0% to 41.6% is the most important to note. Fluoroquinolones are often used as the first-line treatment for eye infections, and the medications are generally effective [28,29]. This change highlights new challenges in managing Streptococcal keratitis and underscores the need to explore alternative antibiotic options to combat the growing resistance. The varying resistance rates among the three species of streptococci highlight the need for tailored antibiotic therapy in treating Streptococcal keratitis. Notably, ciprofloxacin showed significantly higher resistance in S. pneumoniae compared to the other species, while S. oralis exhibited a notably higher resistance to ceftazidime. Consequently, the use of both ciprofloxacin and ceftazidime would be contraindicated in these cases. However, it is crucial to consider that breakpoints for topical antibiotics are not well defined, and local drug concentrations may exceed the MIC, even in cases where resistance is detected. This discrepancy highlights the need for further research on the pharmacokinetics and efficacy of topical antibiotics in treating ocular infections.
We evaluated clinical signs associated with predicting the clinical outcome of Streptococcal keratitis: central corneal ulcer, central dense infiltrate, large-size dense infiltrate, and hypopyon are linked with bad outcomes. A central bacterial corneal infection is very serious because it can pose a severe threat to vision [30]. In infectious keratitis, the presence of a large infiltrate at the onset of the patient’s disease may be related to the late onset of the disease, the inadequate treatment received, the resistance of the pathogen to treatment, or the immune status of the patient, which ultimately leads to a poor prognosis [31]. Hypopyon is formatted by the lesion of leukocytes from blood vessels and gravitates at the bottom of the anterior chamber [32]. The higher proportion of hypopyon in S. pneumoniae keratitis than in the other two kinds could explain the correlation of these clinical features with worse outcomes in S. pneumoniae keratitis. The presence of inflammation in the anterior chamber indicates deeper lesions of streptococcal infection. This is supported by the results of our analysis of IVCM photographs. Patients with hypopyon tend to have inflammatory cells in the epithelial and stromal layer, suggesting a more severe progression of the infection and therefore a worse prognosis. The presence of scar tissue in the IVCM photographs also suggests severe progression of the infection and a poor prognosis.
This study has several shortcomings; due to its retrospective nature, only part of the participants can be included in the analysis of clinical features and antibiotic susceptibility patterns. The lack of data from follow-up examinations also limits the results of this study. Despite the shortness, this study is the largest study describing Streptococcal keratitis, elucidating the clinical features and risk factors for this disease, as well as its antibiotic susceptibility patterns.
In summary, this study provides a comprehensive analysis of Streptococcal keratitis, revealing the main risk factors, such as prior ocular surface disease, previous ocular surgery, and ocular trauma. We also found notable differences in clinical features and antibiotic susceptibility among various Streptococcal species. Furthermore, in vivo confocal microscopy (IVCM) proves to be an invaluable tool for monitoring the progression of infection, enhancing our understanding and management of this condition.
4. Materials and Methods
4.1. Participants
This retrospective study examined all cases of Streptococcal keratitis at Beijing Tongren Hospital from 1 January 2006 to 30 December 2023. The study adhered to the Declaration of Helsinki and received approval from the Medical Ethics Committee of Beijing Tongren Hospital (TRECKY2015-KY09). Cases were defined by clinical presentation and laboratory confirmation of Streptococcal etiology. Patients with incomplete initial or follow-up records were excluded.
4.2. Clinical Diagnosis Procedures
Patients’ information, including demographics, predisposing factors, clinical features, initial diagnosis, treatments, and follow-up records, was extracted from the electronic health record system using a standardized Excel proforma. Predisposing factors for Streptococcal keratitis were categorized as trauma, previous ocular surgery, systemic disease, previous ocular surface diseases, co-infection at diagnosis, and recent corticosteroid use.
Upon presentation, all patients underwent visual acuity measurement and slit-lamp biomicroscopy examination. Two experienced ophthalmologists carefully evaluated the clinical signs, including the location and size of the corneal ulcer and infiltrate, corneal thinning, neovascularization, Descemet’s membrane folding, and hypopyon. The size of the corneal ulcer and stromal infiltration was measured using corneal photographs, calculated by multiplying the longest diameter and the longest perpendicular width.
The Rostock Cornea Module of the Heidelberg Retina Tomograph III (HRT III/RCM, Heidelberg Engineering, Heidelberg, Germany) was utilized to obtain in vivo confocal microscopy (IVCM) images covering an area of 400 × 400 µm. IVCM photographs of Streptococcal keratitis at various stages of progression were evaluated across different corneal layers. The stages of Streptococcal keratitis were classified according to the different degrees of ulcer size [33]. Stage I: Early signs of infection with limited corneal lesions (≤3 mm). Stage II: Moderate infection with visible corneal inflammation, hypopyon and potential thinning with medium-sized lesions. Stage III: Advanced infection with significant corneal involvement, large-sized lesions (≥6 mm), hypopyon, neovascularization, possible perforation, or risk of endophthalmitis. The maximum density of inflammatory and dendritiform cells, from the epithelial to the endothelial layers, was quantified using the Cell Count function of ImageJ software v1.4.3.x [34].
Microbiological investigation was conducted for patients with clinically suspected corneal infections. This involved microscopic examination of corneal scrapings, including Gram staining, followed by microbiological culture and antimicrobial susceptibility testing. Corneal scrapes were inoculated on appropriate media, including chocolate agar, blood agar, and Sabouraud dextrose agar, to culture fastidious organisms, bacteria, and fungi, respectively. Isolate identification, including the detection of any mixed infections, was performed using the MALDI-TOF-MS system (Bruker, Bremen, Germany). All diagnostic procedures were carried out by experienced ophthalmologists and laboratory staff.
4.3. Antibiotic Susceptibility Testing
Antimicrobial susceptibility testing was interpreted following the Clinical and Laboratory Standards Institute (CLSI) guidelines. Antibiotic susceptibility, including vancomycin, ceftazidime, ofloxacin, levofloxacin, rifampin, moxifloxacin, ciprofloxacin, benzalkonium chloride, amikacin, gentamicin, and tobramycin, was assessed using the Kirby–Bauer disk diffusion method. The detailed CLSI breakpoints for each antibiotic are presented in Table S1.
Isolates were prepared to match the 0.5 McFarland standard for turbidity, ensuring a consistent bacterial concentration for testing of approximately 1.5 × 108 CFU/mL. Using a sterile swab, the standardized bacterial suspension was evenly spread across the surface of Mueller–Hinton agar plates to create a uniform lawn using a sterile swab. After allowing the inoculum to settle briefly, antibiotic-impregnated disks (vancomycin, ceftazidime, ofloxacin, levofloxacin, rifampin, moxifloxacin, ciprofloxacin, benzalkonium chloride, amikacin, gentamicin, and tobramycin) were carefully placed onto the agar surface using sterile forceps after allowing the inoculum to settle briefly. The plates were then incubated in an aerobic environment at 35 °C (21% O2) for 18–24 h.
Following incubation, the diameter of each inhibition zone around the antibiotic disks was measured to the nearest millimeter. These measurements were interpreted using CLSI breakpoints to classify each isolate’s response as resistant (R), intermediate (I), or sensitive (S) based on established zone diameter breakpoints for each antibiotic. Additionally, control strains (e.g., E. coli ATCC 25922) were used to validate the accuracy of the antimicrobial susceptibility testing and were tested at regular intervals to ensure the consistency of the results. All data on cases with mixed infections are presented in Table S3.
4.4. Treatment and Outcome
The treatment of Streptococcal keratitis involves both antibiotic therapy and surgical intervention. Adequate treatment is crucial to prevent serious complications, including empiric subconjunctival antibiotic therapy or systemic antibiotic therapy targeting any scleral or intraocular extension of the infection. Initial recommendations included monotherapy with fluoroquinolone, while current guidelines suggest empiric treatment with fluoroquinolone or intensive combination therapy with cefazolin and gentamicin [35]. The antibiotic regimen is subsequently modified based on clinical response and antibiotic susceptibility testing. Therapeutic penetrating keratoplasty (PK) remains the primary surgical intervention for rapidly progressing infections. The indications for surgical procedures are determined by experienced experts [36].
Prognosis was evaluated by analyzing the patients’ follow-up medical records for at least one month. During follow-up, the clinical progression of the disease was carefully monitored through regular corneal assessments and slit-lamp examinations to evaluate the size and depth of ulcers or infiltrates. The presence of complications such as corneal thinning, perforation, or the need for surgical intervention was noted. Patients were classified as having a good outcome if they experienced relief from the ulcers or infiltrates using only topical antibiotics. This was defined as complete or near-complete resolution of corneal lesions without the need for further surgical intervention, and with no signs of infection recurrence. In contrast, bad outcomes were defined as an inadequate response to antibiotic treatment, characterized by the enlargement of ulcers or infiltrates, progressive corneal thinning, or the need for surgical intervention. If surgical intervention, such as penetrating keratoplasty, was required due to the severity or progression of the infection, it was classified as a poor outcome.
4.5. Statistical Analysis
All statistical analyses were performed using SPSS 26.0 (SPSS, Chicago, IL, USA). Descriptive statistics were calculated, and the data were presented in figures and tables. Continuous variables were described using appropriate measures of central tendency and dispersion, while categorical variables were reported as percentages. The Chi-square test or Fisher’s exact test was used to compare the clinical characteristics of patients with Streptococcal keratitis and cases with good and bad outcomes. Bonferroni correction was applied to account for multiple comparisons. A p-value of 0.05 or less was considered statistically significant.
5. Conclusions
This study offers a detailed analysis of Streptococcal keratitis, highlighting key risk factors, differences in clinical features and antibiotic susceptibility among species, and the valuable role of in vivo confocal microscopy in monitoring infection progression and improving management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13121190/s1. Figure S1: Slit-lamp photographs of different stages of Streptococcal keratitis. (A): Stage I; (B): Stage II; (C): Stage III. Figure S2: In vivo confocal microscopy photographs showing cellular features in the corneal epithelial layer of different stages of Streptococcal keratitis. (A): Stage I; (B): Stage II; (C): Stage III. Figure S3: In vivo confocal microscopy photographs showing cellular features in the corneal epithelial basement layer of different stages of Streptococcal keratitis. (A): Stage I; (B): Stage II; (C): Stage III. Figure S4: In vivo confocal microscopy photographs showing cellular features in the corneal anterior stroma layer of different stages of Streptococcal keratitis. (A): Stage I; (B): Stage II; (C): Stage III. Figure S5: In vivo confocal microscopy photographs showing cellular features in the corneal posterior stroma layer of different stages of Streptococcal keratitis. (A): Stage I; (B): Stage II; (C): Stage III. Figure S6: In vivo confocal microscopy photographs showing cellular features in the corneal endothelial layer and post-corneal region of different stages of Streptococcal keratitis. (A): Stage I; (B): Stage II; (C): Stage III. Figure S7: Inflammatory cell density and dendritiform cell density changes during the progression of Streptococcal keratitis. (A) Inflammatory cell density; (B) dendritiform cell density. Table S1. Clinical and laboratory standards institute (CLSI) breakpoint standards for antimicrobial susceptibility testing of antibiotics. Table S2. Antimicrobial Susceptibility Results of isolated Streptococcal Strains. Table S3. Microorganisms involved in mixed Infections in patients with Streptococcal keratitis.
Author Contributions
Conceptualization, Q.L.; data curation, Z.C.; formal analysis, Z.C., Q.S.; investigation, B.P., Z.W. (Zhenyu Wei), Z.W. (Zhiqun Wang), Y.Z., K.C., X.X., and X.L.; methodology, Q.L.; software, Z.Z.; writing—original draft, Z.C.; writing—review and editing, Z.C., Q.S., and Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program, grant number 2021YFC2301000; National Natural Science Foundation of China, grant number 82171017; Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes (PWD&RPP-MRI, JYY2023-6).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Beijing Tongren Hospital (TRECKY2015-KY09).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The information used in our study is available upon request from the corresponding author. The dataset is not available to the public due to the need to protect patient privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Russello, G.; Moramarco, A.; Vizzini, L.; Farina, C.; Fontana, L.; Carretto, E. Diagnostic approach and epidemiology of Microbial Keratitis: Findings from an Italian Tertiary Care center. Diagn. Microbiol. Infect. Dis. 2021, 101, 115470. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, R.L.; Andreo, E.G.; Finotti, I.G.; Arcieri, E.S.; Ferreira, M.A.; Rocha, F.J. Epidemiology and etiologic diagnosis of infectious keratitis in Uberlandia, Brazil. Eur. J. Ophthalmol. 2010, 20, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Oydanich, M.; Dingle, T.C.; Hamula, C.L.; Ghisa, C.; Asbell, P. Retrospective report of antimicrobial susceptibility observed in bacterial pathogens isolated from ocular samples at Mount Sinai Hospital, 2010 to 2015. Antimicrob. Resist. Infect. Control 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Sirikul, T.; Prabriputaloong, T.; Smathivat, A.; Chuck, R.S.; Vongthongsri, A. Predisposing factors and etiologic diagnosis of ulcerative keratitis. Cornea 2008, 27, 283–287. [Google Scholar] [CrossRef]
- Srinivasan, M.; Mascarenhas, J.; Prashanth, C.N. Distinguishing infective versus noninfective keratitis. Indian J. Ophthalmol. 2008, 56, 203–207. [Google Scholar] [CrossRef]
- Jin, H.; Parker, W.T.; Law, N.W.; Clarke, C.L.; Gisseman, J.D.; Pflugfelder, S.C.; Wang, L.; Al-Mohtaseb, Z.N. Evolving risk factors and antibiotic sensitivity patterns for microbial keratitis at a large county hospital. Br. J. Ophthalmol. 2017, 101, 1483–1487. [Google Scholar] [CrossRef]
- Hardie, J.M.; Whiley, R.A. The genus Streptococcus. In The Genera of Lactic Acid Bacteria; Wood, B.J.B., Holzapfel, W.H., Eds.; Springer: Boston, MA, USA, 1995; pp. 55–124. [Google Scholar]
- Nanayakkara, U.; Khan, M.A.; Hargun, D.K.; Sivagnanam, S.; Samarawickrama, C. Ocular streptococcal infections: A clinical and microbiological review. Surv. Ophthalmol. 2023, 68, 678–696. [Google Scholar] [CrossRef]
- Liesegang, T.J. Bacterial keratitis. Infect. Dis. Clin. N. Am. 1992, 6, 815–829. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Khoo, P.; Watson, S.L. Infectious keratitis: A review. Clin. Exp. Ophthalmol. 2022, 50, 543–562. [Google Scholar] [CrossRef]
- Hong, J.; Chen, J.; Sun, X.; Deng, S.X.; Chen, L.; Gong, L.; Cao, W.; Yu, X.; Xu, J. Paediatric bacterial keratitis cases in Shanghai: Microbiological profile, antibiotic susceptibility and visual outcomes. Eye 2012, 26, 1571–1578. [Google Scholar] [CrossRef]
- Lalitha, P.; Manoharan, G.; Karpagam, R.; Prajna, N.V.; Srinivasan, M.; Mascarenhas, J.; Das, M.; Porco, T.C.; Lietman, T.M.; Cevallos, V.; et al. Trends in antibiotic resistance in bacterial keratitis isolates from South India. Br. J. Ophthalmol. 2017, 101, 108–113. [Google Scholar] [CrossRef]
- Watson, S.L.; Gatus, B.J.; Cabrera-Aguas, M.; Armstrong, B.H.; George, C.R.; Khoo, P.; Lahra, M.M. Bacterial Ocular Surveillance System (BOSS) Sydney, Australia 2017–2018. Commun. Dis. Intell. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Lalitha, P.; Prajna, N.V.; Srinivasan, M.; Das, M.; D’Silva, S.S.; Oldenburg, C.E.; Borkar, D.S.; Esterberg, E.J.; Lietman, T.M.; et al. Acanthamoeba, fungal, and bacterial keratitis: A comparison of risk factors and clinical features. Am. J. Ophthalmol. 2014, 157, 56–62. [Google Scholar] [CrossRef]
- Marquart, M.E.; Benton, A.H.; Galloway, R.C.; Stempak, L.M. Antibiotic susceptibility, cytotoxicity, and protease activity of viridans group streptococci causing endophthalmitis. PLoS ONE 2018, 13, e0209849. [Google Scholar] [CrossRef]
- Whatmore, A.M.; Efstratiou, A.; Pickerill, A.P.; Broughton, K.; Woodard, G.; Sturgeon, D.; George, R.; Dowson, C.G. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: Characterization of “Atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 2000, 68, 1374–1382. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Dart, J.K.G.; De, S.K.; Carnt, N.; Cleary, G.; Hau, S. Comparison of culture, confocal microscopy and PCR in routine hospital use for microbial keratitis diagnosis. Eye 2022, 36, 2172–2178. [Google Scholar] [CrossRef]
- Hau, S.C.; Dart, J.K.G.; Vesaluoma, M.; Parmar, D.N.; Claerhout, I.; Bibi, K.; Larkin, D.F.P. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br. J. Ophthalmol. 2010, 94, 982–987. [Google Scholar] [CrossRef]
- Termote, K.; Joe, A.W.; Butler, A.L.; McCarthy, M.; Blondeau, J.M.; Iovieno, A.; Holland, S.P.; Yeung, S.N. Epidemiology of bacterial corneal ulcers at tertiary centres in Vancouver, B.C. Can. J. Ophthalmol. 2018, 53, 330–336. [Google Scholar] [CrossRef]
- Chidambaram, J.D.; Prajna, N.V.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Macleod, D.; Lalitha, P.; Burton, M.J. In Vivo Confocal Microscopy Cellular Features of Host and Organism in Bacterial, Fungal, and Acanthamoeba Keratitis. Am. J. Ophthalmol. 2018, 190, 24–33. [Google Scholar] [CrossRef]
- Tabatabaei, S.A.; Soleimani, M.; Behrouz, M.J.; Torkashvand, A.; Anvari, P.; Yaseri, M. A randomized clinical trial to evaluate the usefulness of amniotic membrane transplantation in bacterial keratitis healing. Ocul. Surf. 2017, 15, 218–226. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Pawlak, J.; Saravolatz, S.N.; Johnson, L.B. In Vitro Activity of Retapamulin against Staphylococcus aureus Resistant to Various Antimicrobial Agents. Antimicrob. Agents Chemother. 2013, 57, 4547–4550. [Google Scholar] [CrossRef]
- Peng, M.Y.; Cevallos, V.; McLeod, S.D.; Lietman, T.M.; Rose-Nussbaumer, J. Bacterial Keratitis: Isolated Organisms and Antibiotic Resistance Patterns in San Francisco. Cornea 2018, 37, 84–87. [Google Scholar] [CrossRef]
- Asbell, P.A.; Sanfilippo, C.M.; Sahm, D.F.; DeCory, H.H. Trends in Antibiotic Resistance Among Ocular Microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol. 2020, 138, 439–450. [Google Scholar] [CrossRef]
- Egrilmez, S.; Yildirim-Theveny, Ş. Treatment-Resistant Bacterial Keratitis: Challenges and Solutions. Clin. Ophthalmol. 2020, 14, 287–297. [Google Scholar] [CrossRef]
- Sand, D.; She, R.; Shulman, I.A.; Chen, D.S.; Schur, M.; Hsu, H.Y. Microbial keratitis in los angeles: The doheny eye institute and the los angeles county hospital experience. Ophthalmology 2015, 122, 918–924. [Google Scholar] [CrossRef]
- Mantadakis, E.; Maraki, S.; Michailidis, L.; Gitti, Z.; Pallikaris, I.G.; Samonis, G. Antimicrobial susceptibility of Gram-positive cocci isolated from patients with conjunctivitis and keratitis in Crete, Greece. J. Microbiol. Immunol. Infect. 2013, 46, 41–47. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. Bacterial Keratitis; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fernandes, M.; Vira, D.; Dey, M.; Tanzin, T.; Kumar, N.; Sharma, S. Comparison Between Polymicrobial and Fungal Keratitis: Clinical Features, Risk Factors, and Outcome. Am. J. Ophthalmol. 2015, 160, 873–881.e872. [Google Scholar] [CrossRef]
- Rohatgi, J.N. Bacteriology of corneal ulcer with special reference to hypopyon corneal ulcer. Indian J. Ophthalmol. 1967, 15, 54–57. [Google Scholar]
- Acharya, M.; Farooqui, J.H.; Jain, S.; Mathur, U. Pearls and paradigms in Infective Keratitis. Rom. J. Ophthalmol. 2019, 63, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Blautain, B.; Rabut, G.; Dupas, B.; Riancho, L.; Liang, H.; Luzu, J.; Labbé, A.; Garrigue, J.S.; Brignole-Baudouin, F.; Baudouin, C.; et al. Multimodal Approach in Dry Eye Disease Combining In Vivo Confocal Microscopy and HLA-DR Expression. Transl. Vis. Sci. Technol. 2024, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Rhee, M.K.; Akpek, E.K.; Amescua, G.; Farid, M.; Garcia-Ferrer, F.J.; Varu, D.M.; Musch, D.C.; Dunn, S.P.; Mah, F.S. Bacterial Keratitis Preferred Practice Pattern®. Ophthalmology 2019, 126, P1–P55. [Google Scholar] [CrossRef] [PubMed]
- Ung, L.; Chodosh, J. Foundational concepts in the biology of bacterial keratitis. Exp. Eye Res. 2021, 209, 108647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).