Abstract
The global emergence of extended-spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae presents a significant public health threat and complicates antibiotic treatment for infections. This study aimed to determine the prevalence of ESBL-producing K. pneumoniae in a clinical setting, analyze their antimicrobial susceptibility profiles, and characterize both phenotypic and genetic determinants. A total of 507 non-duplicate clinical isolates of Enterobacterales were collected between 2019 and 2020, and third-generation cephalosporin resistance was screened by disk diffusion. Identification of K. pneumoniae was confirmed using biochemical tests and PCR with species-specific primers. Antimicrobial susceptibility testing was conducted using disk diffusion, and phenotypic ESBL production was confirmed using the combined disk method. Multiplex PCR detected ESBL genes (blaTEM, blaSHV, and blaCTX-M) and identified blaCTX-M groups. The genetic relatedness of ESBL-producing strains was assessed using the ERIC-PCR approach. Fitty-four isolates were confirmed as ESBL producers, all classified as multidrug-resistant (MDR). All ESBL-producing K. pneumoniae isolates exhibited resistance to ampicillin and cefotaxime, with high resistance rates for ciprofloxacin (98.2%), azithromycin (94.4%), piperacillin–tazobactam (88.9%), and trimethoprim (83.3%). Genotypic analysis revealed blaCTX-M was present in 94.4% of isolates, blaSHV in 87%, and blaTEM in 55.5%. The blaCTX-M-1 group was the most prevalent, accounting for 96.1% of isolates. Co-harboring of blaCTX-M, blaSHV, and blaTEM occurred in 42.6% of isolates, with co-carrying of blaCTX-M, and blaSHV was observed in 23/54 isolates. The ERIC-PCR analysis revealed 15 distinct types, indicating high genetic diversity. These findings highlight the urgent need for ongoing monitoring to control the spread of ESBL among K. pneumoniae and emphasize the importance of early detection and appropriate antibiotic selection for effectively treating infection caused by these pathogens.
1. Introduction
The global emergence of multidrug-resistant (MDR) bacteria poses a significant threat to public health, particularly in low-and middle-income countries (LMICs). Decades of inappropriate antibiotic use in human and animal health have exerted selective pressure, compromising the efficacy of beta-lactam antibiotics. In LMICs, which are a resource-limited setting, an inadequate antimicrobial stewardship program and unrestricted access to antibiotics have exacerbated this issue [1]. The indiscriminate use of third-generation cephalosporins has precipitated the rise of antimicrobial-resistant (AMR) organisms, notably resistant E. coli and K. pneumoniae, in both community and nosocomial settings. Consequently, AMR-associated hospital-acquired infections in LMICs’ ICUs exceed prevalence rates observed in high-income countries, complicating treatment strategies and threatening global health initiatives [2].
Klebsiella pneumoniae is a significant opportunistic pathogen in healthcare settings [3,4]. It is responsible for a wide range of infectious diseases, including pneumonia, bacteremia, sepsis, burn and wound infections, and urinary tract infections (UTIs) [5]. K. pneumoniae is primarily associated with hospital-acquired infections (HAIs), particularly among immunocompromised patients and those with underlying conditions such as diabetes, chronic obstructive pulmonary disease (COPD), or a history of antibiotic use [6]. The pathogen’s high prevalence and the ability of many strains to exhibit resistance to commonly used antibiotics, including carbapenems, have contributed to a notable increase in mortality and morbidity over the years [6]. K. pneumoniae’s capacity to acquire resistance genes across various antibiotic classes, such as the production of carbapenemases like NDM-1 and OXA-48, further enhances its pathogenic potential and leads to severe disease manifestations [7]. Consequently, the World Health Organization (WHO) has classified antibiotic-resistant K. pneumoniae as a Priority 1 (critical group) pathogen, indicating the urgent need for research and development of new antibiotics to combat this significant threat to global health [8].
K. pneumoniae exhibits resistance to beta-lactam antibiotics primarily through the production of beta-lactamases, particularly extended-spectrum beta-lactamases (ESBLs). These enzymes can hydrolyze first-, second-, and third-generation cephalosporins, as well as aztreonam, although they are inhibited by clavulanic acid. The coexistence of various modifying enzymes on the same plasmid can confer resistance to additional antibiotic classes, including fluoroquinolones, aminoglycosides, tetracyclines, and trimethoprim-sulfamethoxazole. ESBL-encoding genes are categorized into several families, with blaTEM variants, blaSHV variants, and blaCTX-M being the most prevalent [9]. Among these, CTX-M enzymes, classified as class A ESBLs, are the most widely distributed worldwide, including in developing countries. These ESBL genes are typically located on plasmids but can occasionally be found on chromosomes. More than 50 allelic variants of CTX-M have been identified, which are grouped into six clusters: CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, CTX-M-25, and KLUC based on a ≥10% variance in amino acid sequence identity, with several minor variants within the groups. CTX-M-type ESBLs are predominantly detected in plasmid incompatibility groups, although chromosomal integration has also been reported [10]. In humans, CTX-M-15 (CTX-M-1 group) and CTX-M-14 (CTX-M-9 group) are more prevalent, whereas CTX-M-1 (CTX-M-1 group) is more predominant in animals. Other CTX-M groups have been reported in specific regions, such as the CTX-M-2 and CTX-M-8 groups in South America and the CTX-M-2 group in Japan [11]. The most identified CTX-M groups in Gram-negative pathogens are CTX-M-1, CTX-M-2, CTX-M-8, and CTX-M-9 [12].
In Thailand, the prevalence of ESBL-producing K. pneumoniae ranges from 12.7% to 30.2% [13,14,15]. The blaCTX-M is believed to be the dominant type in the Asia–Pacific region, contributing to dissemination and outbreak in several countries. Therefore, ongoing surveillance of ESBL-producing K. pneumoniae is crucial to monitor and develop effective countermeasures against potential nosocomial infections. This study aimed to characterize ESBL-producing K. pneumoniae isolates collected between 2019 and 2020 from two tertiary hospitals in northeastern Thailand. The objectives included determining the prevalence of ESBL-producing K. pneumoniae, assessing their antimicrobial susceptibility profiles, and characterizing their phenotypic and genetic features.
2. Results
2.1. Characteristics of the Recovered K. pneumoniae Isolates
Among 507 non-duplicate Enterobacterales clinical isolates, K. pneumoniae was identified in 70 isolates (13.8%) recovered from various clinical samples, including feces (n = 14), urine (n = 16), blood (n = 5), pus (n = 7), and sputum (n = 28). Phenotypically, K. pneumoniae is characterized as a Gram-negative, rod-shaped, non-motile bacterium that grows on MacConkey agar, producing mucoid colonies that are pink to red due to lactose fermentation. Additional phenotypic characteristics include indole positivity, urease negativity, citrate negativity, gas production, hydrogen sulfide negativity, and lysine decarboxylase positivity.
2.2. Phenotypic Determination for ESBL-Producing K. pneumoniae Isolates
All 70 K. pneumoniae isolates exhibited resistance, as determined by disk diffusion assays using cefotaxime (30 μg) and ceftazidime (30 μg). Among these, all K. pneumoniae isolates were resistant to cefotaxime, while 56 isolates (80%, 56/70) demonstrated resistance to both cefotaxime and ceftazidime. Further analysis using the combination disk method in Figure 1 revealed that 54 out of the 70 isolates (77.1%) produced ESBLs. The ESBL-producing K. pneumoniae isolates were recovered from various clinical specimens, including sputum (37.0%, n = 20/54), feces (25.9%, n = 14/54), urine (16.7%, n = 13/54), pus (7.4%, n = 4/54), and blood (5.5%, n = 3/54).
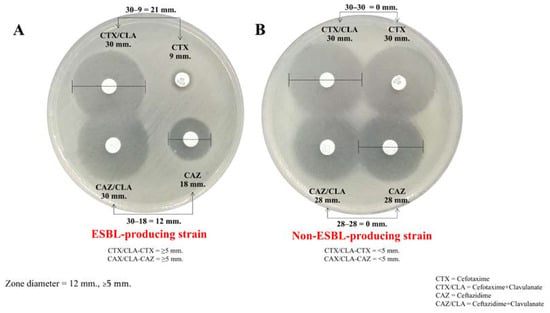
Figure 1.
The combination disk method for ESBL detection. (A) Zone diameter: ≥5 mm difference between the cefotaxime (CTX) and cefotaxime/clavulanic acid (CTX/CLA) disks and the ceftazidime (CAZ) and ceftazidime/clavulanic acid (CAZ/CLA) disks, which suggests an ESBL-producing strain (top). (B) Zone diameter: <5 mm difference between the CTX and CTX/CLA disks and the CAZ and CAZ/CLA disks, which does not suggest an ESBL-producing strain.
2.3. Antimicrobial Susceptibility Pattern of ESBL-Producing K. pneumoniae Isolates
The antimicrobial resistance profile of the ESBL-producing K. pneumoniae isolates is illustrated in Figure 2. All isolates exhibited complete resistance to ampicillin and cefotaxime, with relatively high resistance rates observed for ciprofloxacin (98.6%, 69/70), azithromycin (95.7%, 67/70), piperacillin-tazobactam (91.4%, 64/70), trimethoprim (87.1%, 61/70), ceftazidime (80%, 56/70), nitrofurantoin (65.7%, 46/70), and tetracycline (62.9%, 44/70). All ESBL-producing K. pneumoniae isolates were classified as multidrug-resistant (MDR), defined as resistance to at least one agent in three or more antimicrobial categories. Additionally, 14.8% (8/54) of the ESBL-producing K. pneumoniae isolates were identified as extensively drug-resistant (XDR), isolates resistant to at least one drug in all but two or fewer antimicrobial categories. The antimicrobial resistance patterns are presented in Table 1.
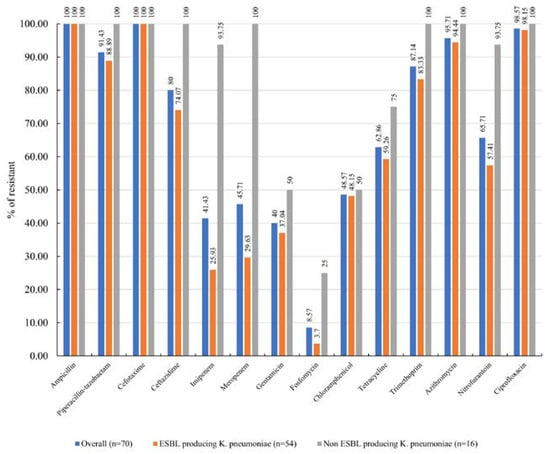
Figure 2.
Antimicrobial resistance of ESBL-producing K. pneumoniae isolates.

Table 1.
Antimicrobial resistance patterns of ESBL-producing K. pneumoniae isolates (n = 54).
2.4. Molecular Characterization of β-Lactamase Genes
The presence of beta-lactamase genes in all 54 ESBL-producing K. pneumoniae strains was determined using multiplex PCR, as shown in Figure 3. The predominant genes identified in our study were blaCTX-M (94.4%, 51/54), followed by blaSHV (87.0%, 47/54) and blaTEM (55.5%, 30/54). The most prevalent combination patterns were blaTEM + blaCTX-M-1 + blaSHV and blaCTX-M + blaSHV (42.6%, 23/54). Among the 51 ESBL-producing K. pneumoniae isolates carrying blaCTX-M, the majority belonged to the blaCTX-M-1 group (96.0%, 49/54 isolates), while the blaCTX-M-9 group was found in only one isolate. One isolate carried an unidentified group of blaCTX-M (Table 2).
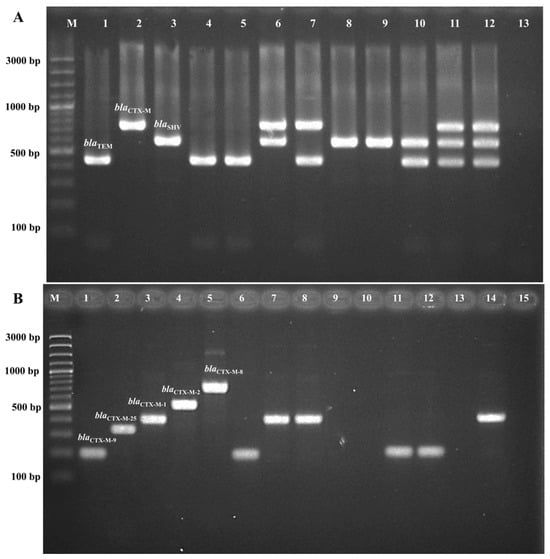
Figure 3.
Multiplex PCR for ESBL gene detection. (A). Multiplex PCR for blaTEM, blaSHV, and blaCTX-M. Lane M is a 100 bp molecular weight marker, lane 1 is a blaTEM positive control, lane 2 is a blaSHV positive control, lane 3 is a blaCTX-M positive control, lanes 4–12 are samples, and lane 13 is a negative control. (B). Multiplex PCR for blaCTX-M groups, including blaCTX-M-1, blaCTX-M-2, blaCTX-M-8, blaCTX-M-9, and blaCTX-M-25. Lane M is a 100 bp molecular weight marker, lane 1 is a blaCTX-M-1 positive control, lane 2 is a blaCTX-M-2 positive control, lane 3 is a blaCTX-M-8 positive control, lane 4 is a blaCTX-M-9 positive control, lane 5 is a blaCTX-M-25 positive control, lanes 6–14 are samples, and lane 15 is a negative control.

Table 2.
Distribution of β-lactamase genes in ESBL-producing K. pneumoniae isolates in clinical specimens.
2.5. ERIC-PCR Analysis
The genetic relatedness among ESBL-producing K. pneumoniae isolates was analyzed using ERIC-PCR; the analysis identified 15 different ERIC types, including seven common types and eight unique types (Figure 4(A1,A2)). A total of 15 different ERIC profiles (E-types) were observed, designated as P1 to P15. Out of 54 isolates, 24 belonged to P2, 9 were P1, and 3 each were P5, P6, and P15. Additionally, two isolates were categorized as P11 and P12, while eight isolates exhibited unique patterns. At a 95% similarity level, these isolates were classified into two clusters: A and B. The predominant cluster B contained 51 isolates (94%), while cluster A contained 3 isolates (5.5%) (Figure 4B). Notably, the strains of AMR0207 from hospital A and 5-197 and 367 from hospital B displayed the same ERIC-PCR pattern (P6). Furthermore, strains of AMR2442, AMR2435, AMR2433, AMR0210, AMR0206, AMR0203, AMR0198, and P37.6 from hospital A and AMR0428, AMR0423, AMR0146, AMR0139, AMR0137, AMR0134, AMR0131, AMR0130, AMR0129, 6-100, 2-245.2, 2-117, 339, 198, P37.6, 6-227, and 2-708 from hospital B revealed the same pattern of ERIC-PCR (P2). These results suggest the possibility of clonal distribution between hospital A and hospital B.
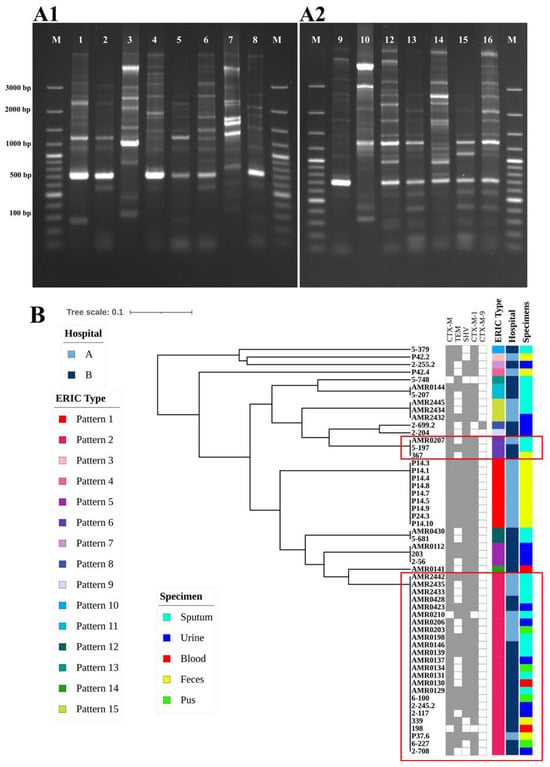
Figure 4.
(A) (A1,A2). Gel electrophoresis analysis of ERIC-PCR profiles of ESBL-producing K. pneumoniae isolated in our study. (B) Dendrogram generated from ERIC-PCR fingerprinting for ESBL-producing K. pneumoniae isolated in our study. Fifty-four isolates were designated to 15 patterns.
3. Discussion
The widespread emergence of ESBLs in pathogenic bacteria presents a significant public health concern. These enzymes confer resistance to a broad spectrum of beta-lactam antibiotics, compromising their efficacy and creating therapeutic challenges. This situation necessitates the development of reliable detection methods in clinical microbiology laboratories to guide appropriate antimicrobial therapy and infection control measures. Notably, K. pneumoniae, a major nosocomial pathogen, exhibits a high prevalence of ESBL production, further underscoring the need for effective detection strategies.
The present study revealed a prevalence of 10.7% (54/507) for ESBL-producing K. pneumoniae among Enterobacterales clinical isolates. This finding is similar to the 10.2% prevalence reported by Siriphap et al. in northern Thailand [13] and the 7.5% prevalence found by Ko et al. in the Asia–Pacific region, including Thailand [16]. However, it is notably lower than the 27.4% prevalence described by Sawatwong et al. in a different region of Thailand [15], suggesting potential variability within the country. Additionally, the prevalence of ESBL-producing Gram-negative bacteria or Enterobacterales was 24% in Egypt [17], 27.6% in Nigeria [18], 29.6% in Cameroon [19],15.3% in Ethiopia [20], 14.3% in India [21], and 16.8% in Nepal [22]. Upon recalculating the prevalence rates based on data from those studies, the prevalence of ESBL-producing K. pneumoniae is comparable to our current study finding, with rates of 11.4% (12/105) in Egypt [17], 12% (26/217) in Nigeria [18], 13.5% (19/141) in Ethiopia [20], 14.3% (72/504) in India [21], and 15% (322/2153) in Nepal [22]. Conversely, our findings indicate a higher prevalence compared to studies conducted in Cameroon, at 5.3% (8/152) [19], and Japan, at 4.8% (144/2987) [23]. These variations in prevalence across different studies and geographical regions may be attributed to multiple factors, including differences in study methodologies, such as sample size, sampling technique, participant demographics, antibiotic usage patterns, and regional infection control practices. Further investigation into these contributing factors could provide valuable insights into the global epidemiology of ESBL-producing K. pneumoniae.
Among the fifty-four ESBL-producing K. pneumoniae isolates, sputum samples (37.0%) constituted the predominant source, followed by feces, urine, pus, and blood. This result is comparable to studies conducted in northern Thailand (44.2%) [13]. However, several studies have identified urine as the major reservoir of ESBL producers, including investigations conducted in southern Thailand [24], northern Portugal [25], Nigeria [26], Uganda [27], and Ghana [28]. Additionally, some studies have reported blood [29] and feces [30] as the main sources of ESBL-producing K. pneumoniae. These variations likely reflect differences in sampling techniques, specimen collection, patient demographics, and geographic distribution.
The high resistance profile observed for penicillin and cephalosporin in our studies is consistent with findings from other studies [24,31,32,33,34,35]. This resistance can be attributed to the excessive use of amoxicillin and cefotaxime, ceftriaxone, and ceftazidime in hospitals, where hospitalization itself has been identified as a risk factor for infection with ESBL-producing Enterobacterales. Plasmids carrying genes that encode ESBLs can be easily transmitted horizontally between different bacteria in the hospital environment. Our study revealed an association between ESBL production and resistance to multiple antibiotic classes.
According to our finding, the overall prevalence of MDR among ESBL-producing K. pneumoniae isolates was 100%. This finding aligns with studies conducted in Ethiopia, which reported an MDR rate of 100% and 93.6% [36,37] and a 96.4% MDR rate in Ghana [34]. Moreover, our study shows that 14.8% of ESBL-producing K. pneumoniae isolates were XDR, which is lower than the 41% reported by Romyasamit C et al. in Southern Thailand [24].
Among the ESBL-producing K. pneumoniae isolates, the blaCTX-M gene was the most frequently detected, which is consistent with other studies, such as 83.1% in Nigeria [38] and 61.5% in Japan [39]. In our study, the prevalence of the blaCTX-M gene was 94.4% (Table 2). This result aligns with data obtained from the central part of Thailand, where the prevalence of blaCTX-M among clinical ESBL-K. pneumoniae was 99.2% (126/127) [14], and from Nigeria at 93.3% (98/105) [38], Japan at 97% (131/135) [39], Malaysia at 93.5% (87/93) [31], Brazil at 67.8% (120/177) [40], and China at 90.8% (167/184) [41]. The importance of genetic studies of ESBL genes lies in their capacity to spread horizontally to other bacterial species. This horizontal transfer leads to widespread ESBL activity among different pathogens in hospitals. The ongoing surveillance of genotypes in ESBL-producing K. pneumoniae is essential to monitor the emergence of new resistant clones in various regions. In our study, the blaCTX-M-1 group was the primary ESBL genotype identified among ESBL-producing K. pneumoniae. Our results are consistent with the predominance of the blaCTX-M-1 group report in Japan [23,39], China [41], the United States [42], Canada [29], and Ghana [43].
Additionally, we present evidence suggesting the potential of the same ESBL-producing K. pneumoniae clones (P2 and P6) between hospitals. This may indicate that these clones are commonly distributed and disseminated among human and non-human subjects in the community [41] or through a patient referral system between the hospitals [30,44]. Our study identified 15 distinct ERIC types, highlighting the genetic diversity of K. pneumoniae isolated from two hospitals. The prevalence of type P2 in both hospitals suggests that this clone may represent a major strain disseminating across multiple healthcare facilities. However, it is important to note that ERIC-PCR has limitations for K. pneumoniae typing. This method exhibits low discriminatory power. Therefore, complementary techniques such as pulsed-field gel electrophoresis (PFGE) or whole-genome sequencing could offer further insights into the genetic relationship among these isolates. However, we could tentatively cluster our isolates in the current study to guide clonal relationships.
The limitations of our study are noteworthy. First, this research was conducted in only two tertiary hospitals in northeastern Thailand, which may not be representative of the prevalence of ESBL-producing Klebsiella pneumoniae isolates in the region. Sample size, types of samples collected, and the restricted time frame further constrain the generalizability of our findings. It would be beneficial to extend this study to encompass a broader geographic area to obtain more relevant results. In addition, we identified 70 K. pneumoniae strains among 507 Enterobacterales isolates, and 54 out of 70 were classified as ESBL producers. The low prevalence of K. pneumoniae in our study may be influenced by the types and quantities of samples collected. Furthermore, the CTX-M variants were not sequenced in this study, which restricts our understanding of the genetic diversity of these CTX-M. These limitations highlight the necessity for large-scale, multicenter prospective studies employing standardized methodologies and comprehensive molecular analyses to better elucidate the epidemiology, resistance mechanisms, and clinical implications of ESBL-producing K. pneumoniae.
4. Materials and Methods
4.1. Bacterial Isolation and Identification
Among the 507 non-duplicate clinical isolates of Enterobacterales obtained from various clinical specimens, including urine (n = 229), feces (n = 142), blood (39), pus (n = 52), and sputum (n = 45), between January 2019 and December 2020, the isolates were collected from two tertiary care hospitals located in a rural region of northeastern Thailand. The identification of K. pneumoniae was performed using a conventional method. The isolates were cultured on MacConkey agar plates (HIMEDIA, Mumbai, India) and incubated at 37 °C for 24 h before undergoing Gram staining. Presumptive identification of K. pneumoniae was conducted using biochemical tests, including catalase, oxidase, IMViC (indole, methyl red, Voges–Proskauer, citrate utilization), triple sugar iron, urease, motility, and oxidative fermentation tests [45]. Subsequently, species confirmation was performed by a polymerase chain reaction (PCR) assay using specific primers [46]. The primer sequence and conditions are listed in Table 3. The reaction mixture contained PCRBIO HS Taq DNA Polymerase & Mixes (London, UK), 0.3 µM of each primer, sterile deionized water, and 50 ng of bacterial DNA template. PCR was performed using a Thermal Cycler (Bio-Rad, Hercules, CA, USA) with the following program: initial denaturation at 95 °C for 3 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 45 s, and a final extension of amplification at 72 °C for 5 min. The PCR products were analyzed using gel electrophoresis on 1.5% (w/v) agarose gel in 0.5× TBE buffer at a constant voltage of 100 V for 30 min (Mupid exU system; Takara; Tokyo, Japan). The gels were stained with ethidium bromide and visualized under ultraviolet light (GeneGenius Bioimaging System; SynGene; Cambridge, UK). The sizes of the PCR products were determined by comparison with molecular size standards (GeneRuler™ 100 bp Plus DNA ladder; Thermo Fisher Scientific; Vilnius, Lithuania). K. pneumoniae was cultured and inoculated into 20% glycerol–tryptic soy broth and stored in a −80 °C freezer.

Table 3.
Primer sequences for PCR detection in this study.
4.2. Phenotypic Detection of ESBL-Producing K. pneumoniae
K. pneumoniae strains were initially screened for third-generation cephalosporin resistance using the disk diffusion assay with ceftazidime (30 μg) and cefotaxime (30 μg). The cephalosporin-resistant isolates were non-susceptible (intermediate or resistant) to cefotaxime or ceftazidime, or both. The ESBL production was determined using the combination disk method according to the guidelines of the Clinical and Laboratory Standards Institute [50]. Briefly, bacterial cultures adjusted to a 0.5 McFarland standard were inoculated onto Mueller–Hinton agar plates (Merck, Frankfurter, Germany). Ceftazidime (30 µg), ceftazidime/clavulanate (30/10 µg), cefotaxime (30 µg), and cefotaxime/clavulanate (30/10 µg) disks (MASTDISCS®AST, Mereyside, UK) were placed on the inoculated plates, which were incubated at 37 °C for 18 h. The isolates that exhibited ≥5 mm inhibition zones with combination disks compared to individual antibiotics (without clavulanate) were designated as ESBL producers.
4.3. Antimicrobial Susceptibility Testing of K. pneumoniae
The antimicrobial susceptibility of the isolates was assessed following the CLSI 2023 guidelines. The following antibiotic disks (MASTDISCS®AST, Mereyside, UK) were used: piperacillin–tazobactam (PTZ 110 µg), ampicillin (AP 10 µg), ceftazidime (CAZ 30 µg), cefotaxime (CTX 30 µg), ciprofloxacin (CIP 5 µg), gentamicin (GM 10 µg), imipenem (IMP; 10 µg), meropenem (MEM; 10 µg), tetracycline (T 30 µg), chloramphenicol (C 30 µg), trimethoprim (TM 15 µg), and nitrofurantoin (NI 300 µg). The plates were incubated at 37 °C for 18–24 h. After incubation, the zones of inhibition were measured and interpreted as susceptible, intermediate, or resistant based on CLSI recommendations [50]. Isolates resistant to two or more classes of antimicrobial agents were classified as multidrug-resistant (MDR) according to the guidelines described by Magiorakos et al. [51].
4.4. DNA Extraction and Quantification
Genomic DNA from ESBL-producing K. pneumoniae strains was extracted using a ZymoBIOMICsTM DNA Miniprep Kit (Zymo Research; Irvine, CA, USA) following the manufacturer’s instructions. The extracted DNA was stored at −20 °C until further analysis. To quantify the DNA concentration and assess its purity, absorbance readings were taken at 260 and 280 nm using a NanoDropTM 2000 Spectrophotometer (Thermo Fisher Scientific; Waltham, MA, USA).
4.5. Molecular Detection of Beta-Lactamase Genes by PCR
The presence of blaTEM, blaSHV, blaCTX-M, and the blaCTX-M subgroup (blaCTX-M groups 1, 2, 8, 9, and 25) in ESBL-producing isolates was determined using multiplex PCR, following previously described protocols [47,48]. All primers were synthesized by Integrated DNA Technologies (Singapore). The target genes, primers sequence, cycle conditions, and expected amplicon size are described in Table 3. PCR was conducted using a T100 thermal cycler (BioRad, Hercules, CA, USA) with a total volume of 25 µL, which included PCRBIO HS Taq DNA Polymerase & Mixes (London, UK), 0.3 µM of each primer, sterile deionized water, and 50 ng of DNA template. The PCR amplification program consisted of an initial denaturation at 95 °C for 3 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 1 min, and a final amplification at 72 °C for 7 min. For the blaCTX-M subgroup, the PCR conditions were modified to include an initial denaturation at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 25 s, annealing at 52 °C for 40 s, extension at 72 °C for 50 s, and a final amplification at 72 °C for 6 min.
The PCR products from each reaction were subjected to electrophoresis on 1.5% agarose gel alongside a DNA marker (GeneRuler™ 100 bp Plus DNA ladder; Thermo Fisher Scientific; Vilnius, Lithuania). The gels were visualized and photographed under UV transillumination (SYNGENE, Cambridge, UK).
4.6. Quality Control
For the standardization of the drug susceptibility test, K. pneumoniae (ATCC 2524) and E. coli (ATCC 25922) were used as the control strains. For the PCR controls, sterile water served as the negative control, while known positive DNA and negative controls from the previous extraction were processed to ensure the accuracy of the PCR procedure.
4.7. Enterobacterial Repetitive Intergenic Consensus–Polymerase Chain Reaction (ERIC-PCR)
Genetic relatedness among the fifty-four ESBL-producing K. pneumoniae strains was assessed using ERIC-PCR with ERIC-1 and ERIC-2 primers [49]. The sequence primers are shown in Table 3. The reaction mixture contained PCRBIO HS Taq DNA Polymerase & Mixes (London, UK), 0.5 µM of each primer, sterile deionized water, and 50 ng of bacterial DNA template. The cycling conditions were as follows: an initial denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 92 °C for 30 s, annealing at 52 °C for 1 min, extension at 72 °C for 8 min, and a final extension at 72 °C for 16 min. The ERIC-PCR fragments were visualized by 1% agarose gel electrophoresis stained with GelRed (Biotium, Inc., Hayward, CA, USA).
The pattern of DNA fingerprints was visually compared, and patterns differing by at least one amplification band were classified as different. For constructing a computerized dendrogram, the presence and absence of bands were recorded as 1 and 0, respectively. ERIC profiles were compared using the Dice similarity matrix coefficient and clustered by the neighbor-joining method to prepare the phylogenetic tree. Isolates exhibiting two or more different bands in the ERIC banding pattern were considered different ERIC types. The dendrogram was constructed using the FreeTree program, employing an unweighted paired group method with arithmetic mean (UPGMA) according to published guidelines by [52]. The UPGMA tree was visualized by iToLv5 [53].
5. Conclusions
The findings of this study confirm a high prevalence rate of 94.4% for ESBL-producing K. pneumoniae, with a significant proportion of isolates exhibiting MDR. The highest levels of resistance were observed against ampicillin and cefotaxime, with the blaCTX-M-1 group identified as the most prevalent ESBL gene. These results align with broader concerns regarding antibiotic-resistant ESKAPE pathogens, which are associated with increased mortality risk and economic costs, particularly in developing countries. Furthermore, there is a pressing need for broader studies and ongoing surveillance to monitor resistance patterns beyond the geographic and temporal limitations of this research. Routine screening for ESBL-producing pathogens is crucial for early detection and appropriate antibiotic selection. On a larger scale, developing rapid diagnostics, new antibiotics, and vaccines, along with creating an international platform for real-time surveillance of antimicrobial resistance, are vital steps in containing this global threat and ensuring high-quality, effective therapeutic options.
Author Contributions
Conceptualization, A.K. and P.C.; Formal analysis, S.C.; Funding acquisition, P.C.; Investigation, S.C., N.P. (Nattamol Phetburom) and P.B.; Methodology, S.C., N.P. (Nattamol Phetburom) and P.C.; Resources, P.K., N.P. (Nuntiput Putthanachote) and N.W.; Supervision, A.K.; Validation, S.C. and P.C.; Writing—original draft, S.C. and P.C.; Writing—review and editing, A.K. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Graduate Program Scholarship from The Graduate School, Kasetsart University, Bangkok, Thailand.
Institutional Review Board Statement
Ethical approval was not required for this study. The research utilized only pre-identified isolates from microbiology laboratories, precluding patient contact or direct handling of original samples. Sample sources were anonymously extracted from clinical records, and all data were analyzed and managed anonymously, strictly for research purposes.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Takahashi, E.; Hongsuwan, M.; Wuthiekanun, V.; Thamlikitkul, V.; Hinjoy, S.; Day, N.P.J.; Peacock, S.J.; Limmathurotsakul, D. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife 2016, 5, e18082. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Anes, J.; Devineau, S.; Fanning, S. Klebsiella pneumoniae: Prevalence, Reservoirs, Antimicrobial Resistance, Pathogenicity, and Infection: A Hitherto Unrecognized Zoonotic Bacterium. Foodborne Pathog. Dis. 2021, 18, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, Q.; Ma, Y.; Deng, Y.; Li, S.; Shi, N.; Niu, H.; Liu, X.Y.; Cai, J. Causality of Opportunistic Pathogen Klebsiella pneumoniae to Hypertension Development. Hypertension 2022, 79, 2743–2754. [Google Scholar] [CrossRef]
- Odewale, G.; Jibola-Shittu, M.Y.; Ojurongbe, O.; Olowe, R.A.; Olowe, O.A. Genotypic Determination of Extended Spectrum β-Lactamases and Carbapenemase Production in Clinical Isolates of Klebsiella pneumoniae in Southwest Nigeria. Infect. Dis. Rep. 2023, 15, 339–353. [Google Scholar] [CrossRef]
- Yazgan, B.; Türkel, İ.; Güçkan, R.; Kılınç, Ç.; Yıldırım, T. Comparison of biofilm formation and efflux pumps in ESBL and carbapenemase producing Klebsiella pneumoniae. J. Infect. Dev. Ctries. 2018, 12, 156–163. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Núñez-Samudio, V.; Pimentel-Peralta, G.; Herrera, M.; Pecchio, M.; Quintero, J.; Landires, I. Molecular Genetic Epidemiology of an Emerging Antimicrobial-Resistant Klebsiella pneumoniae Clone (ST307) Obtained from Clinical Isolates in Central Panama. Antibiotics 2022, 11, 1817. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- D’Andrea, M.M.; Arena, F.; Pallecchi, L.; Rossolini, G.M. CTX-M-type β-lactamases: A successful story of antibiotic resistance. Int. J. Med. Microbiol. 2013, 303, 305–317. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, R.; Abdullah, A.; Ahmed, D.; Hussain, A. High Prevalence of blaCTX-M-15 Gene among Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolates Causing Extraintestinal Infections in Bangladesh. Antibiotics 2020, 9, 796. [Google Scholar] [CrossRef] [PubMed]
- Siriphap, A.; Kitti, T.; Khuekankaew, A.; Boonlao, C.; Thephinlap, C.; Thepmalee, C.; Suwannasom, N.; Khoothiam, K. High prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates: A 5-year retrospective study at a Tertiary Hospital in Northern Thailand. Front. Cell. Infect. Microbiol. 2022, 12, 955774. [Google Scholar] [CrossRef] [PubMed]
- Kiratisin, P.; Apisarnthanarak, A.; Laesripa, C.; Saifon, P. Molecular characterization and epidemiology of extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-M family is endemic. Antimicrob. Agents Chemother. 2008, 52, 2818–2824. [Google Scholar] [CrossRef]
- Sawatwong, P.; Sapchookul, P.; Whistler, T.; Gregory, C.J.; Sangwichian, O.; Makprasert, S.; Jorakate, P.; Srisaengchai, P.; Thamthitiwat, S.; Promkong, C.; et al. High Burden of Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae Bacteremia in Older Adults: A Seven-Year Study in Two Rural Thai Provinces. Am. J. Trop. Med. Hyg. 2019, 100, 943–951. [Google Scholar] [CrossRef]
- Ko, W.C.; Stone, G.G. In vitro activity of ceftazidime-avibactam and comparators against Gram-negative bacterial isolates collected in the Asia-Pacific region as part of the INFORM program (2015–2017). Ann. Clin. Microbiol. Antimicrob. 2020, 19, 14. [Google Scholar] [CrossRef]
- Elsayed, A.G.A.; Badr, D.F.; El Kheir, N.Y.A.; Zaki, M.E.S.; Mossad, A.E.M.; Mahmoud, E.M.F. Prevalence of extended-spectrum beta-lactamase and molecular detection of blaTEM, blaSHV, and blaCTX-M genotypes among gram-negative Bacilli isolates from hospital acquired infections in pediatrics, one institutional study. Ital. J. Pediatr. 2024, 50, 31. [Google Scholar] [CrossRef]
- Jesumirhewe, C.; Springer, B.; Allerberger, F.; Ruppitsch, W. Whole genome sequencing of extended-spectrum β-lactamase genes in Enterobacteriaceae isolates from Nigeria. PLoS ONE 2020, 15, e0231146. [Google Scholar] [CrossRef]
- Nkengkana, O.A.; Founou, R.C.; Founou, L.L.; Dimani, B.D.; Koudoum, P.L.; Zemtsa, J.R.; Mbossi, A.; Mawout, C.S.; Tegang, L.T.; Noubom, M. Phenotypic and genotypic characterization of multidrug resistant and extended-spectrum β-lactamase-producing Enterobacterales isolated from clinical samples in the western region in Cameroon. BMC Infect. Dis. 2023, 23, 819. [Google Scholar] [CrossRef]
- Moges, F.; Gizachew, M.; Dagnew, M.; Amare, A.; Sharew, B.; Eshetie, S.; Abebe, W.; Million, Y.; Feleke, T.; Tiruneh, M. Multidrug resistance and extended-spectrum beta-lactamase producing Gram-negative bacteria from three Referral Hospitals of Amhara region, Ethiopia. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 16. [Google Scholar] [CrossRef]
- Bobbadi, S.; Bobby, M.N.; Chinnam, B.K.; Reddy, P.N.; Kandhan, S. Phenotypic and genetic screening of Klebsiella pneumoniae isolates from human UTI patients for beta-lactamases and their genetic diversity analysis by ERIC and REP PCRs. Braz. J. Microbiol. [Publ. Braz. Soc. Microbiol.] 2023, 54, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Zellweger, R.M.; Maharjan, N.; Dongol, S.; Prajapati, K.G.; Thwaites, G.; Basnyat, B.; Dixit, S.M.; Baker, S.; Karkey, A. A high prevalence of multi-drug resistant Gram-negative bacilli in a Nepali tertiary care hospital and associated widespread distribution of Extended-Spectrum Beta-Lactamase (ESBL) and carbapenemase-encoding genes. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Tetsuka, N.; Morioka, H.; Iguchi, M.; Kawamura, K.; Hayashi, K.; Yanagiya, T.; Morokuma, Y.; Watari, T.; Kiyosuke, M.; et al. Genetic and epidemiological analysis of ESBL-producing Klebsiella pneumoniae in three Japanese university hospitals. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2022, 28, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Romyasamit, C.; Sornsenee, P.; Kawila, S.; Saengsuwan, P. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: Insights from a tertiary hospital in Southern Thailand. Microbiol. Spectr. 2024, 12, e00213-24. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Carvalho, J.A.; Martínez-Álvarez, S.; Sadi, M.; Capita, R.; Alonso-Calleja, C.; Rabbi, F.; Dapkevicius, M.d.L.N.E.; Igrejas, G.; Torres, C.; et al. Characterization of ESBL-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Clinical Samples in a Northern Portuguese Hospital: Predominance of CTX-M-15 and High Genetic Diversity. Microorganisms 2021, 9, 1914. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Sani, Y.; Saleh, Q.; Saleh, A.; Hakeem, G. Phenotypic Detection of Extended Spectrum Beta lactamase and Carbapenemase Co-producing Clinical Isolates from Two Tertiary Hospitals in Kano, North West Nigeria. Ethiop. J. Health Sci. 2017, 27, 3–10. [Google Scholar] [CrossRef]
- Kateregga, J.N.; Kantume, R.; Atuhaire, C.; Lubowa, M.N.; Ndukui, J.G. Phenotypic expression and prevalence of ESBL-producing Enterobacteriaceae in samples collected from patients in various wards of Mulago Hospital, Uganda. BMC Pharmacol. Toxicol. 2015, 16, 14. [Google Scholar] [CrossRef]
- Obeng-Nkrumah, N.; Twum-Danso, K.; Krogfelt, K.A.; Newman, M.J. High levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: The need for regular monitoring and evaluation of antibiotic resistance. Am. J. Trop. Med. Hyg. 2013, 89, 960–964. [Google Scholar] [CrossRef]
- Denisuik, A.J.; Karlowsky, J.A.; Adam, H.J.; Baxter, M.R.; Lagacé-Wiens, P.R.S.; Mulvey, M.R.; Hoban, D.J.; Zhanel, G.G. Dramatic rise in the proportion of ESBL-producing Escherichia coli and Klebsiella pneumoniae among clinical isolates identified in Canadian hospital laboratories from 2007 to 2016. J. Antimicrob. Chemother. 2019, 74, IV64–IV71. [Google Scholar] [CrossRef]
- Fils, P.E.L.; Cholley, P.; Gbaguidi-Haore, H.; Hocquet, D.; Sauget, M.; Bertrand, X. ESBL-producing Klebsiella pneumoniae in a University hospital: Molecular features, diffusion of epidemic clones and evaluation of cross-transmission. PLoS ONE 2021, 16, e0247875. [Google Scholar] [CrossRef]
- Al-Marzooq, F.; Mohd Yusof, M.Y.; Tay, S.T. Molecular Analysis of Antibiotic Resistance Determinants and Plasmids in Malaysian Isolates of Multidrug Resistant Klebsiella pneumoniae. PLoS ONE 2015, 10, e0133654. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.B.; Anwar, K.A. Phenotypic and genotypic detection of extended spectrum beta lactamase enzyme in Klebsiella pneumoniae. PLoS ONE 2022, 17, e0267221. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schulte, E.; Tuo, M.N.; Akoua-Koffi, C.; Schaumburg, F.; Becker, S.L. High prevalence of ESBL-producing Klebsiella pneumoniae in clinical samples from central Côte d’Ivoire. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 91, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S.; Muhsen, K.; Johnson, S.A.M.; Kotey, F.C.N.; Dayie, N.T.K.D.; Tetteh-Quarcoo, P.B.; Tette, E.M.A.; Osei, M.M.; Egyir, B.; Nii-Trebi, N.I.; et al. Multicenter Surveillance of Antimicrobial Resistance among Gram-Negative Bacteria Isolated from Bloodstream Infections in Ghana. Antibiotics 2023, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Ghenea, A.E.; Zlatian, O.M.; Cristea, O.M.; Ungureanu, A.; Mititelu, R.R.; Balasoiu, A.T.; Vasile, C.M.; Salan, A.I.; Iliuta, D.; Popescu, M.; et al. TEM,CTX-M,SHV Genes in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Clinical Samples in a County Clinical Emergency Hospital Romania-Predominance of CTX-M-15. Antibiotics 2022, 11, 503. [Google Scholar] [CrossRef]
- Desta, K.; Woldeamanuel, Y.; Azazh, A.; Mohammod, H.; Desalegn, D.; Shimelis, D.; Gulilat, D.; Lamisso, B.; Makonnen, E.; Worku, A.; et al. High Gastrointestinal Colonization Rate with Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Patients: Emergence of Carbapenemase-Producing K. pneumoniae in Ethiopia. PLoS ONE 2016, 11, e0161685. [Google Scholar] [CrossRef]
- Tola, M.A.; Abera, N.A.; Gebeyehu, Y.M.; Dinku, S.F.; Tullu, K.D. High prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae fecal carriage among children under five years in Addis Ababa, Ethiopia. PLoS ONE 2021, 16, e0258117. [Google Scholar] [CrossRef]
- Duru, C.; Olanipekun, G.; Odili, V.; Kocmich, N.; Rezac, A.; Ajose, T.O.; Medugu, N.; Umoru, D.; Onuchukwu, C.; Munir, H.; et al. Molecular characterization of invasive Enterobacteriaceae from pediatric patients in Central and Northwestern Nigeria. PLoS ONE 2020, 15, e0230037. [Google Scholar] [CrossRef]
- Watanabe, N.; Watari, T.; Otsuka, Y.; Ito, M.; Yamagata, K.; Fujioka, M. Antimicrobial resistance and AmpC production in ESBL-producing Klebsiella pneumoniae and Klebsiella quasipneumoniae: A retrospective study in Japanese clinical isolates. PLoS ONE 2024, 19, e0303353. [Google Scholar] [CrossRef]
- Da Nogueira, K.S.; Conte, D.; Maia, F.V.; Dalla-Costa, L.M. Distribution of extended-spectrum β-lactamase types in a Brazilian tertiary hospital. Rev. Soc. Bras. Med. Trop. 2015, 48, 162–169. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, K.; Zheng, B.; Zhao, L.; Shen, P.; Ji, J.; Wei, Z.; Li, L.; Zhou, J.; Xiao, Y. High Prevalence of ESBL-Producing Klebsiella pneumoniae Causing Community-Onset Infections in China. Front. Microbiol. 2016, 7, 1830. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Kimbrough, J.H.; Devries, S.; Mendes, R.E.; Sader, H.S. Trends of β-Lactamase Occurrence among Escherichia coli and Klebsiella pneumoniae in United States Hospitals during a 5-Year Period and Activity of Antimicrobial Agents against Isolates Stratified by β-Lactamase Type. Open Forum Infect. Dis. 2023, 10, ofad038. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, F.K.M.; Ablordey, A.; Obeng-Nkrumah, N.; Opintan, J.A. Extended-spectrum beta-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae recovered from patients at the Tamale Teaching Hospital, Ghana. PLoS ONE 2024, 19, e0300596. [Google Scholar] [CrossRef] [PubMed]
- Anudit, C.; Saraisuwan, P.; Kimterng, C.; Puangmanee, C.; Bamphensin, N.; Kerdsin, A. Dissemination of Urinary Escherichia coli Phylogroup B2 in Provincial and Community Hospitals in Uthai Thani, Central Thailand. Jpn. J. Infect. Dis. 2024, 77, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S. Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and Other Enterobacteriaceae, 10th ed.; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2011. [Google Scholar]
- Hatrongjit, R.; Chopjitt, P.; Boueroy, P.; Kerdsin, A. Multiplex PCR Detection of Common Carbapenemase Genes and Identification of Clinically Relevant Escherichia coli and Klebsiella pneumoniae Complex. Antibiotics 2022, 12, 76. [Google Scholar] [CrossRef]
- Monstein, H.J.; Östholm-Balkhed, Å.; Nilsson, M.V.; Nilsson, M.; Dornbusch, K.; Nilsson, L.E. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2007, 115, 1400–1408. [Google Scholar] [CrossRef]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J. Antimicrob. Chemother. 2006, 57, 154–155. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, J.R.; Plaza, O.B. 1nstitute for Molecular Genetics and department of Pediatrics, Baylor College of Medicine. Cell 1991, 19, 6823–6831. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, M100, 32nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Hampl, V.; Pavlíček, A.; Flegr, J. Construction and bootstrap analysis of DNA fingerprinting-based phylogenetic trees with the freeware program FreeTree: Application to trichomonad parasites. Int. J. Syst. Evol. Microbiol. 2001, 51, 731–735. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).