Phage–Antibiotic Combination Therapy against Recurrent Pseudomonas Septicaemia in a Patient with an Arterial Stent
Abstract
1. Introduction
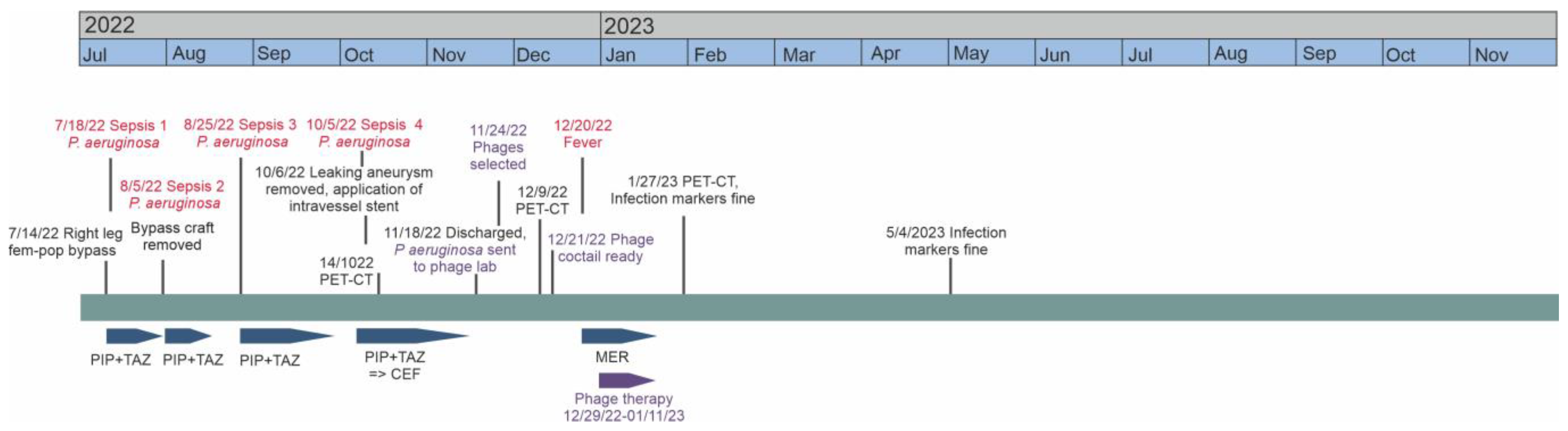
2. Case Report
2.1. The Patient
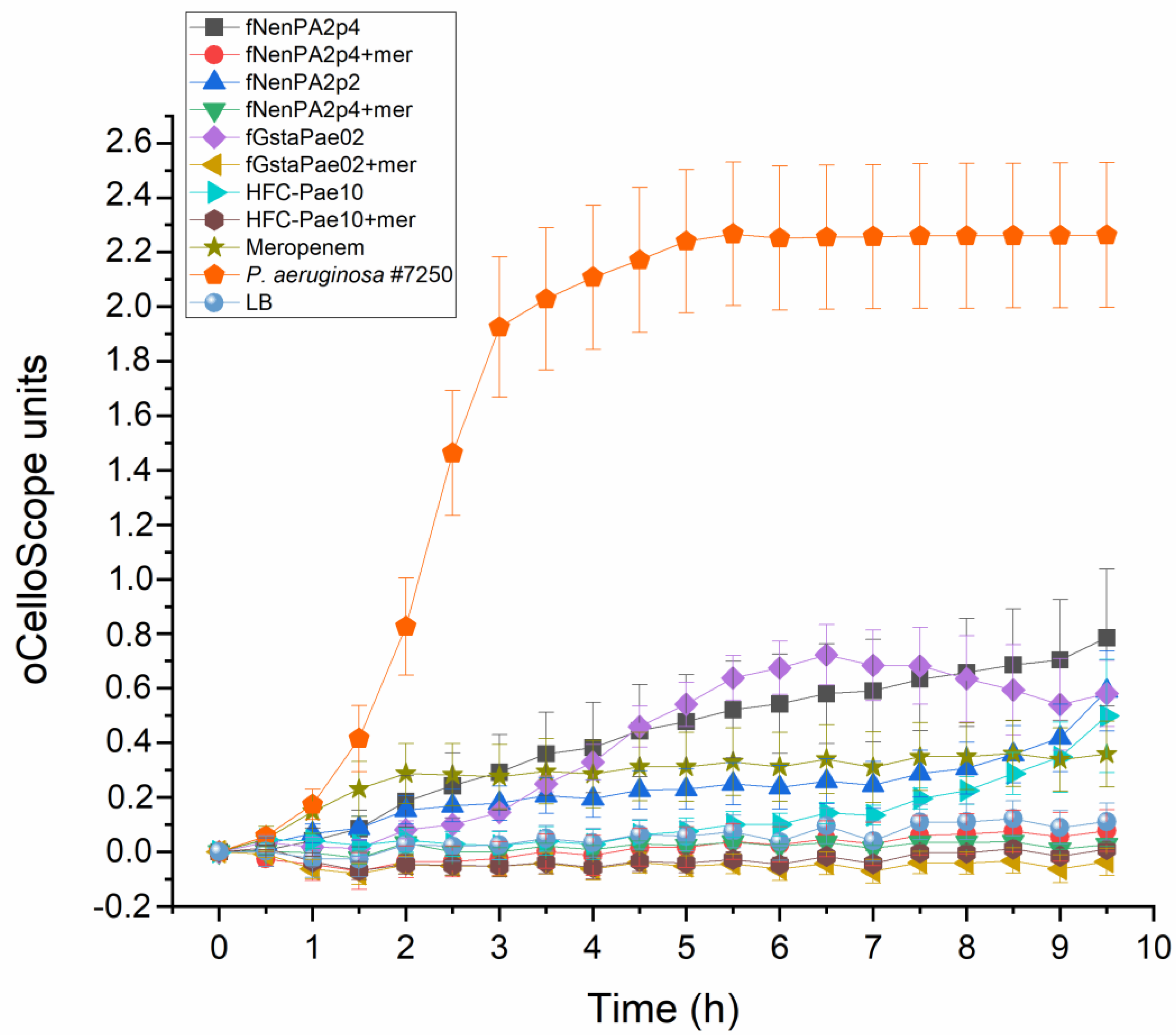
2.2. Phage Cocktail HFC-Pae10
2.3. Phage Treatment of the Patient
3. Discussion
4. Materials and Methods
4.1. Human Subject
4.2. Phage Sensitivity Testing of the Patient Isolate
4.3. Phage Cocktail Production
4.4. Phage-Neutralizing Antibodies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wouthuyzen-Bakker, M.; van Oosten, M.; Bierman, W.; Winter, R.; Glaudemans, A.; Slart, R.; Toren-Wielema, M.; Tielliu, I.; Zeebregts, C.J.; Prakken, N.H.J.; et al. Diagnosis and treatment of vascular graft and endograft infections: A structured clinical approach. Int. J. Infect. Dis. 2023, 126, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Bower, T.C.; Creager, M.A.; Amin-Hanjani, S.; O’Gara, P.T.; Lockhart, P.B.; Darouiche, R.O.; Ramlawi, B.; Derdeyn, C.P.; Bolger, A.F.; et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: A scientific statement from the american heart association. Circulation 2016, 134, e412–e460. [Google Scholar] [CrossRef] [PubMed]
- Elmassry, M.M.; Colmer-Hamood, J.A.; Kopel, J.; San Francisco, M.J.; Hamood, A.N. Anti-Pseudomonas aeruginosa vaccines and therapies: An assessment of clinical trials. Microorganisms 2023, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the eskape pathogens. Expert Rev. Anti Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Chan, B.K.; Turner, P.E.; Kim, S.; Mojibian, H.R.; Elefteriades, J.A.; Narayan, D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health 2018, 2018, 60–66. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; De Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S.; et al. Personalized bacteriophage therapy outcomes for 100 consecutive cases: A multicentre, multinational, retrospective observational study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Alves, D.R. Phage therapy in the 21st century: Is there modern, clinical evidence of phage-mediated efficacy? Pharmaceuticals 2021, 14, 1157. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef]
- Blasco, L.; Lopez-Hernandez, I.; Rodriguez-Fernandez, M.; Perez-Florido, J.; Casimiro-Soriguer, C.S.; Djebara, S.; Merabishvili, M.; Pirnay, J.P.; Rodriguez-Bano, J.; Tomas, M.; et al. Case report: Analysis of phage therapy failure in a patient with a Pseudomonas aeruginosa prosthetic vascular graft infection. Front. Med. 2023, 10, 1199657. [Google Scholar] [CrossRef]
- Chakfe, N.; Diener, H.; Lejay, A.; Assadian, O.; Berard, X.; Caillon, J.; Fourneau, I.; Glaudemans, A.; Koncar, I.; Lindholt, J.; et al. Editor’s choice—European society for vascular surgery (esvs) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 339–384. [Google Scholar] [CrossRef]
- Racenis, K.; Lacis, J.; Rezevska, D.; Mukane, L.; Vilde, A.; Putnins, I.; Djebara, S.; Merabishvili, M.; Pirnay, J.P.; Kalnina, M.; et al. Successful bacteriophage-antibiotic combination therapy against multidrug-resistant Pseudomonas aeruginosa left ventricular assist device driveline infection. Viruses 2023, 15, 1210. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.A.; MacLeod, C.S.; Stacey, H.J.; Nagy, J.; Jones, J.D. The safety and efficacy of phage therapy for infections in cardiac and peripheral vascular surgery: A systematic review. Antibiotics 2023, 12, 1684. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage therapy for critical infections related to cardiothoracic surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Grambow, E.; Junghans, S.; Kroger, J.C.; Reisinger, E.C.; Krause, B.J.; Gross, J. Treatment of an infected tevar with extra- and endovascular bacteriophage application. EJVES Vasc. Forum 2022, 56, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Berkson, J.D.; Wate, C.E.; Allen, G.B.; Schubert, A.M.; Dunbar, K.E.; Coryell, M.P.; Sava, R.L.; Gao, Y.; Hastie, J.L.; Smith, E.M.; et al. Phage-specific immunity impairs efficacy of bacteriophage targeting vancomycin resistant Enterococcus in a murine model. Nat. Commun. 2024, 15, 2993. [Google Scholar] [CrossRef]
- Bernabéu-Gimeno, M.; Pardo-Freire, M.; Chan, B.K.; Turner, P.E.; Gil-Brusola, A.; Pérez-Tarazona, S.; Carrasco-Hernández, L.; Quintana-Gallego, E.; Domingo-Calap, P. Neutralizing antibodies after nebulized phage therapy in cystic fibrosis patients. Med 2024, 5, 1096–1111.e6. [Google Scholar] [CrossRef]
- Kovacs, C.J.; Rapp, E.M.; Rankin, W.R.; McKenzie, S.M.; Brasko, B.K.; Hebert, K.E.; Bachert, B.A.; Kick, A.R.; Burpo, F.J.; Barnhill, J.C. Combinations of bacteriophage are efficacious against multidrug-resistant Pseudomonas aeruginosa and enhance sensitivity to carbapenem antibiotics. Viruses 2024, 16, 1000. [Google Scholar] [CrossRef]
- Manohar, P.; Loh, B.; Nachimuthu, R.; Leptihn, S. Phage-antibiotic combinations to control Pseudomonas aeruginosa-Candida two-species biofilms. Sci. Rep. 2024, 14, 9354. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, B.; Van der Linden, D.; Chatzis, O.; Lood, C.; Wagemans, J.; Lavigne, R.; Schroven, K.; Paeshuyse, J.; de Magnee, C.; Sokal, E.; et al. Bacteriophage-antibiotic combination therapy against extensively drug-resistant Pseudomonas aeruginosa infection to allow liver transplantation in a toddler. Nat. Commun. 2022, 13, 5725. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 9 April 2022).
- Khatami, A.; Foley, D.A.; Warner, M.S.; Barnes, E.H.; Peleg, A.Y.; Li, J.; Stick, S.; Burke, N.; Lin, R.C.Y.; Warning, J.; et al. Standardised treatment and monitoring protocol to assess safety and tolerability of bacteriophage therapy for adult and paediatric patients (stamp study): Protocol for an open-label, single-arm trial. BMJ Open 2022, 12, e065401. [Google Scholar] [CrossRef]
- Sambrook, J.; Russel, D.W. Molecular Cloning, a Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Patpatia, S.; Schaedig, E.; Dirks, A.; Paasonen, L.; Skurnik, M.; Kiljunen, S. Rapid hydrogel-based phage susceptibility test for pathogenic bacteria. Front. Cell Infect. Microbiol. 2022, 12, 1032052. [Google Scholar] [CrossRef] [PubMed]
- Hietala, V.; Horsma-Heikkinen, J.; Carron, A.; Skurnik, M.; Kiljunen, S. The removal of endo- and enterotoxins from bacteriophage preparations. Front. Microbiol. 2019, 10, 1674. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otava, U.E.; Tervo, L.; Havela, R.; Vuotari, L.; Ylänne, M.; Asplund, A.; Patpatia, S.; Kiljunen, S. Phage–Antibiotic Combination Therapy against Recurrent Pseudomonas Septicaemia in a Patient with an Arterial Stent. Antibiotics 2024, 13, 916. https://doi.org/10.3390/antibiotics13100916
Otava UE, Tervo L, Havela R, Vuotari L, Ylänne M, Asplund A, Patpatia S, Kiljunen S. Phage–Antibiotic Combination Therapy against Recurrent Pseudomonas Septicaemia in a Patient with an Arterial Stent. Antibiotics. 2024; 13(10):916. https://doi.org/10.3390/antibiotics13100916
Chicago/Turabian StyleOtava, Ulla Elina, Laura Tervo, Riikka Havela, Liisa Vuotari, Matti Ylänne, Annette Asplund, Sheetal Patpatia, and Saija Kiljunen. 2024. "Phage–Antibiotic Combination Therapy against Recurrent Pseudomonas Septicaemia in a Patient with an Arterial Stent" Antibiotics 13, no. 10: 916. https://doi.org/10.3390/antibiotics13100916
APA StyleOtava, U. E., Tervo, L., Havela, R., Vuotari, L., Ylänne, M., Asplund, A., Patpatia, S., & Kiljunen, S. (2024). Phage–Antibiotic Combination Therapy against Recurrent Pseudomonas Septicaemia in a Patient with an Arterial Stent. Antibiotics, 13(10), 916. https://doi.org/10.3390/antibiotics13100916






