Effects of a Primary Care Antimicrobial Stewardship Program on Meticillin-Resistant Staphylococcus aureus Strains across a Region of Catalunya (Spain) over 5 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting, and Study Periods
2.2. Sources of Information
2.3. Measurement of Consumption and Microbiological Impact Outcomes
2.4. Evaluation Methods
2.5. Statistical Analysis
3. Results
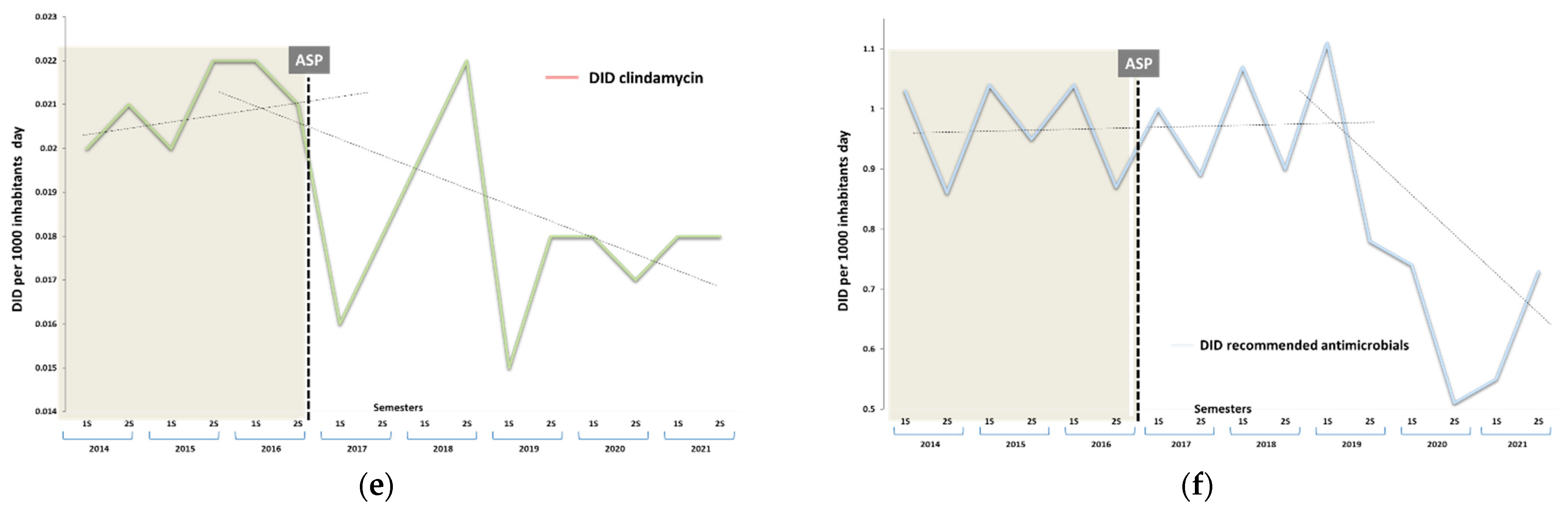
3.1. Impact on Antibiotic Consumption
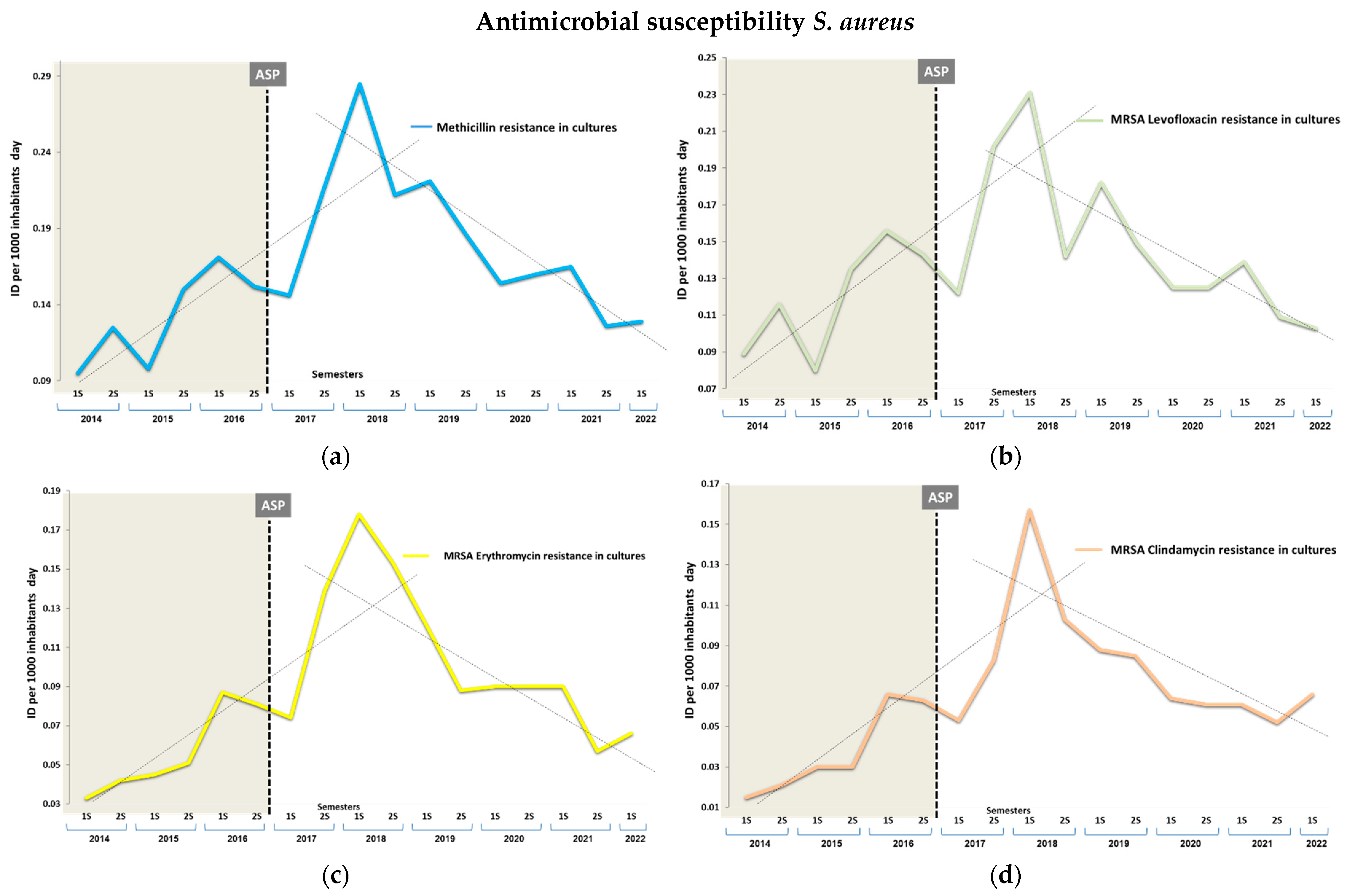
3.2. Impact on Antimicrobial Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; MacKenzie, F.M. Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.; Fatima, M.; Zaheer, C.-N.F.; Muneer, A.; Murtaza, M.; et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 2023, 13, 1067284. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Pfaller, M.A.; Shortridge, D.; Zervos, M.; Jones, R.N. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY antimicrobial surveillance program. Open Forum Infect. Dis. 2019, 6 (Suppl. S1), S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Garcia Reeves, A.B.; Trogdon, J.G.; Stearns, S.C.; Lewis, J.W.; Weber, D.J.; Weinberger, M. Are rates of methicillin-resistant Staphylococcus aureus and Clostridioides difficile associated with quality and clinical outcomes in US acute care hospitals? Am. J. Med. Qual. 2021, 36, 90–98. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Otto, M.; Kreiswirth, B.N.; Chambers, H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010, 375, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Quero, S.; Serras-Pujol, M.; Párraga-Niño, N.; Torres, C.; Navarro, M.; Vilamala, A.; Puigoriol, E.; Ríos, J.D.d.L.; Arqué, E.; Serra-Pladevall, J.; et al. Methicillin-resistant and methicillin-sensitive Staphylococcus aureus in pork industry workers, Catalonia, Spain. One Health 2023, 16, 100538. [Google Scholar] [CrossRef]
- Aldeyab, M.A.; Bond, S.E.; Conway, B.R.; Lee-Milner, J.; Sarma, J.B.; Lattyak, W.J. A threshold logistic modelling approach for identifying thresholds between antibiotic use and methicillin-resistant Staphylococcus aureus incidence rates in hospitals. Antibiotics 2022, 11, 1250. [Google Scholar] [CrossRef]
- Tacconelli, E.; De Angelis, G.; Cataldo, M.A.; Pozzi, E.; Cauda, R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J. Antimicrob. Chemother. 2008, 61, 26–38. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.A.; Grillo, S.; Carrera-Salinas, A.; González-Díaz, A.; Cuervo, G.; Grau, I.; Camoez, M.; Martí, S.; Berbel, D.; Tubau, F.; et al. Molecular epidemiology, antimicrobial susceptibility, and clinical features of methicillin-resistant Staphylococcus aureus bloodstream infections over 30 Years in Barcelona, Spain (1990–2019). Microorganisms 2022, 10, 2401. [Google Scholar] [CrossRef]
- Bruyndonckx, R.; Hoxha, A.; Quinten, C.; Ayele, G.M.; Coenen, S.; Versporten, A.; ESAC-Net Study Group. Change-points in antibiotic consumption in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. S2), ii68–ii78. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Lawes, T.; Lopez-Lozano, J.M.; Nebot, C.A.; Macartney, G.; Subbarao-Sharma, R.; Dare, C.R.; Wares, K.D.; Gould, I.M. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and communityassociated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: A non-linear time-series study. Lancet Infect. Dis. 2015, 15, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Kurotschka, P.K.; Fulgenzio, C.; Da Cas, R.; Traversa, G.; Ferrante, G.; Massidda, O.; Gágyor, I.; Aschbacher, R.; Moser, V.; Pagani, E.; et al. Effect of fluoroquinolone use in primary care on the development and gradual decay of Escherichia coli resistance to fluoroquinolones: A matched case-control study. Antibiotics 2022, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Hammond, A.; Stuijfzand, B.; Avison, M.B.; Hay, A.D. Antimicrobial resistance associations with national primary care antibiotic stewardship policy: Primary care-based, multilevel analytic study. PLoS ONE 2020, 15, e0232903. [Google Scholar] [CrossRef]
- Gágyor, I.; Hay, A.D. Outcome selection in primary care antimicrobial stewardship research. J. Antimicrob. Chemother. 2021, 77, 7–12. [Google Scholar] [CrossRef]
- Avent, M.L.; Cosgrove, S.E.; Price-Haywood, E.G.; van Driel, M.L. Antimicrobial stewardship in the primary care setting: From dream to reality? BMC Fam. Pract. 2020, 21, 134. [Google Scholar] [CrossRef]
- Jover-Sáenz, A.; Ramírez-Hidalgo, M.; Bellés Bellés, A.; Ribes Murillo, E.; Batlle Bosch, M.; Cayado Cabanillas, J.; Garrido-Calvo, S.; Vilas, M.I.G.; Navés, L.G.; Caudevilla, M.J.J.; et al. Impact of a Primary Care Antimicrobial Stewardship Program on Bacterial Resistance Control and Ecological Imprint in Urinary Tract Infections. Antibiotics 2022, 11, 1776. [Google Scholar] [CrossRef]
- Jover-Sáenz, A.; Grup d’estudi i equip PROAP. P-ILERDA -Programa integrat local extrahospitalari de racionalització, millora i desprescripció antibiòtica a Lleida-. Primers resultats d’un programa d’optimització d’ús d’antimicrobians (PROA) en atenció primària. Annals Medicina 2018, 4, 160–166. (In Catalan) [Google Scholar]
- Rodríguez-Baño, J.; Paño-Pardo, J.R.; Alvarez-Rocha, L.; Asensio, A.; Calbo, E.; Cercenado, E.; Cisneros, J.M.; Cobo, J.; Delgado, O.; Garnacho-Montero, J.; et al. Programs for optimizing the use of antibiotics (PROA) in Spanish hospitals: GEIH-SEIMC, SEFH and SEMPSPH consensus document. Enferm. Infecc. Microbiol. Clin. 2012, 30, 22.e1–22.e23. [Google Scholar] [CrossRef]
- Bakhit, M.; Hoffmann, T.; Scott, A.M.; Beller, E.; Rathbone, J.; Del Mar, C. Resistance decay in individuals after antibiotic exposure in primary care: A systematic review and meta-analysis. BMC Med. 2018, 16, 126. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 8.0. 2018. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 5 December 2023).
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. Guideline Committee of the European Study Group on Clostridioides difficile. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. S2), S1–S21. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Consejo de la Unión Europea. Recomendación del Consejo sobre la intensificación de las medidas de la UE para luchar contra la resistencia a los antimicrobianos de acuerdo con el concepto «Una sola salud». D. Of. De La Unión Eur. 2023, 220, 1–20. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net) AER for 2021 (europa.eu). Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2021 (accessed on 4 October 2023).
- Lee, P.C.; Turnidge, J.; McDonald, P.J. Fine-needle aspiration biopsy in diagnosis of soft tissue infections. J. Clin. Microbiol. 1985, 22, 80–83. [Google Scholar] [CrossRef]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R.; et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 2018, 67, e1–e94. [Google Scholar] [CrossRef] [PubMed]
- Ezemma, O.; Korman, A.M.; Wang, H.E.; Kaffenberger, B. Diagnostic methods for the confirmation of non-purulent cellulitis: A review. Arch. Dermatol. Res. 2023, 315, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.; López-Vázquez, P.; Gonzalez-Gonzalez, C.; Vázquez-Lago, J.M.; Piñeiro-Lamas, M.; López-Durán, A.; Sánchez, C.; Herdeiro, M.T.; GREPHEPI Group. Impact of a multifaceted intervention to improve antibiotic prescribing: A pragmatic cluster-randomised controlled trial. Antimicrob. Resist. Infect. Control. 2020, 9, 195. [Google Scholar] [CrossRef]
- Rocha, V.; Estrela, M.; Neto, V.; Roque, F.; Figueiras, A.; Herdeiro, M.T. Educational interventions to reduce prescription and dispensing of antibiotics in primary care: A systematic review of economic impact. Antibiotics 2022, 11, 1186. [Google Scholar] [CrossRef]
- Arnold, S.R.; Straus, S.E. Interventions to improve antibiotic prescribing practices in ambulatory care. Evid. Based Child. Health 2006, 1, 623–690. [Google Scholar] [CrossRef]
- Drekonja, D.M.; Filice, G.A.; Greer, N.; Olson, A.; MacDonald, R.; Rutks, I.; Wilt, T.J. Antimicrobial stewardship in outpatient settings: A systematic review. Infect. Control Hosp. Epidemiol. 2015, 36, 142–152. [Google Scholar] [CrossRef]
- Dyar, O.J.; Beović, B.; Vlahović-Palčevski, V.; Verheij, T.; Pulcini, C. How can we improve antibiotic prescribing in primary care? Expert. Rev. Anti-Infect. Ther. 2016, 14, 403–413. [Google Scholar] [CrossRef] [PubMed]
- McNulty, C.; Hawking, M.; Lecky, D.; Jones, L.; Owens, R.; Charlett, A.; Butler, C.; Moore, P.; Francis, N. Effects of primary care antimicrobial stewardship outreach on antibiotic use by general practice staff: Pragmatic randomized controlled trial of the TARGET antibiotics work-shop. J. Antimicrob. Chemother. 2018, 73, 1423–1432. [Google Scholar] [CrossRef]
- Rice, L.B. Antimicrobial stewardship and antimicrobial resistance. Med. Clin. North. Am. 2018, 102, 805–818. [Google Scholar] [CrossRef]
- Chin, J.; Green, S.B.; McKamey, L.J.; Gooch, M.D.; Chapin, R.W.; Gould, A.P.; Milliken, S.F.; Blanchette, L.M. Restriction-free antimicrobial stewardship initiative targeting fluoroquinolone reduction across a regional health-system. Infect. Prev. Pract. 2019, 1, 100019. [Google Scholar] [CrossRef]
- López-Lozano, J.M.; Lawes, T.; Nebot, C.; Beyaert, A.; Bertrand, X.; Hocquet, D.; Aldeyab, M.; Scott, M.; Conlon-Bingham, G.; Farren, D.; et al. A nonlinear time-series analysis approach to identify thresholds in associations between population antibiotic use and rates of resistance. Nat. Microbiol. 2019, 4, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Lindner, V.; Quach, C.; Hanley, J.A.; Suissa, S. Antibacterial drugs and the risk of community-associated methicillin-resistant Staphylococcus aureus in children. Arch. Pediatr. Adolesc. Med. 2011, 165, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Lawes, T.; López-Lozano, J.M.; Nebot, C.; Macartney, G.; Subbarao-Sharma, R.; Dare, C.R.; Edwards, G.F.S.; Gould, I.M. Turning the tide or riding the waves? Impacts of antibiotic stewardship and infection control on MRSA strain dynamics in a Scottish region over 16 years: Non-linear time series analysis. BMJ Open 2015, 5, e006596. [Google Scholar] [CrossRef] [PubMed]
- El Mammery, A.; Ramírez de Arellano, E.; Cañada-García, J.E.; Cercenado, E.; Villar-Gómara, L.; Casquero-García, V.; García-Cobos, S.; Lepe, J.A.; Bordes, E.R.d.G.; Calvo-Montes, J.; et al. An increase in erythromycin resistance in methicillin-susceptible Staphylococcus aureus from blood correlates with the use of macrolide/lincosamide/streptogramin antibiotics. EARS-Net Spain (2004–2020). Front. Microbiol. 2023, 14, 1220286. [Google Scholar] [CrossRef]
- Oteo, J.; Aracil, M.B. Caracterización de mecanismos de resistencia por biología molecular: Staphylococcus aureus resistente a meticilina, β-lactamasas de espectro extendido y carbapenemasas. Enferm. Infecc. Microbiol. Clin. 2015, 33 (Suppl. S2), 27–33. [Google Scholar] [CrossRef] [PubMed]
- Poku, E.; Cooper, K.; Cantrell, A.; Harnan, S.; Sin, M.A.; Zanuzdana, A.; Hoffmann, A. Systematic review of time lag between antibiotic use and rise of resistant pathogens among hospitalized adults in Europe. JAC Antimicrob. Resist. 2023, 5, dlad001. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef] [PubMed]
- Bakhit, M.; Del Mar, C.; Scott, A.M.; Hoffmann, T. An analysis of reporting quality of prospective studies examining community antibiotic use and resistance. Trials 2018, 19, 656. [Google Scholar] [CrossRef] [PubMed]
- Aldeyab, M.A.; Monnet, D.L.; Lopez-Lozano, J.M.; Hughes, C.M.; Scott, M.G.; Kearney, M.P.; Magee, F.A.; McElnay, J.C. Modelling the impact of antibiotic use and infection control practices on the incidence of hospital-acquired methicillin-resistant Staphylococcus aureus: A time-series analysis. J. Antimicrob. Chemother. 2008, 62, 593–600. [Google Scholar] [CrossRef]
- Kaier, K.; Hagist, C.; Frank, U.; Conrad, A.; Meyer, E. Two time-series analyses of the impact of antibiotic consumption and alcohol-based hand disinfection on the incidences of nosocomial methicillin-resistant Staphylococcus aureus infection and Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 2009, 30, 346–353. [Google Scholar] [CrossRef]
- Mahamat, A.; MacKenzie, F.M.; Brooker, K.; Monnet, D.L.; Daures, J.P.; Gould, I.M. Impact of infection control interventions and antibiotic use on hospital MRSA: A multivariate interrupted time-series analysis. Int. J. Antimicrob. Agents. 2007, 30, 169–176. [Google Scholar] [CrossRef]
- Berger, P.; Pascal, L.; Sartor, C.; Delorme, J.; Monge, P.; Ragon, C.P.; Charbit, M.; Sambuc, R.; Drancourt, M. Generalized additive model demonstrates fluoroquinolone use/resistance relationships for Staphylococcus aureus. Eur. J. Epidemiol. 2004, 19, 453–460. [Google Scholar] [CrossRef]
- Vernaz, N.; Sax, H.; Pittet, D.; Bonnabry, P.; Schrenzel, J.; Harbarth, S. Temporal effects of antibiotic use and hand rub consumption on the incidence of MRSA and Clostridium difficile. J. Antimicrob. Chemother. 2008, 62, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Aldeyab, M.A.; Scott, M.G.; Kearney, M.P.; Alahmadi, Y.M.; Magee, F.A.; Conlon, G.; McELNAY, J.C. Impact of an enhanced antibiotic stewardship on reducing methicillin-resistant Staphylococcus aureus in primary and secondary healthcare settings. Epidemiol. Infect. 2014, 142, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Parienti, J.J.; Cattoir, V.; Thibon, P.; Lebouvier, G.; Verdon, R.; Daubin, C.; du Cheyron, D.; Leclercq, R.; Charbonneau, P. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: A 10-year interrupted time-series analysis. J. Hosp. Infect. 2011, 78, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Dantes, R.; Mu, Y.; Hicks, L.A.; Cohen, J.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Farley, M.M.; Holzbauer, S.; Meek, J.; et al. Association between outpatient antibiotic prescribing practices and community-associated Clostridium difficile infection. Open Forum Infect. Dis. 2015, 2, ofv113. [Google Scholar] [CrossRef] [PubMed]
- Rhea, S.; Jones, K.; Endres-Dighe, S.; Munoz, B.; Weber, D.J.; Hilscher, R.; MacFarquhar, J.; Sickbert-Bennett, E.; DiBiase, L.; Marx, A.; et al. Modeling inpatient and outpatient antibiotic stewardship interventions to reduce the burden of Clostridioides difficile infection in a regional healthcare network. PLoS ONE 2020, 15, e0234031. [Google Scholar] [CrossRef]
- Miller, A.C.; Arakkal, A.T.; Sewell, D.K.; Segre, A.M.; Tholany, J.; Polgreen, P.M.; CDC MInD-Healthcare Group. Comparison of different antibiotics and the risk for community-associated Clostridioides difficile infection: A Case-Control Study. Open Forum Infect. Dis. 2023, 10, ofad413. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.; Sferra, T.J.; Hernandez, A.V.; Donskey, C.J. Community-associated Clostridium difficile infection and antibiotics: A meta-analysis. J. Antimicrob. Chemother. 2013, 68, 1951–1961. [Google Scholar] [CrossRef]
- Alcalá, L.; Martín, A.; Marín, M.; Sánchez-Somolinos, M.; Catalán, P.; Peláez, T.; Bouza, E.; Spanish Clostridium difficile Study Group. The undiagnosed cases of Clostridium difficile infection in a whole nation: Where is the problem? Clin. Microbiol. Infect. 2012, 18, E204–E213. [Google Scholar] [CrossRef] [PubMed]
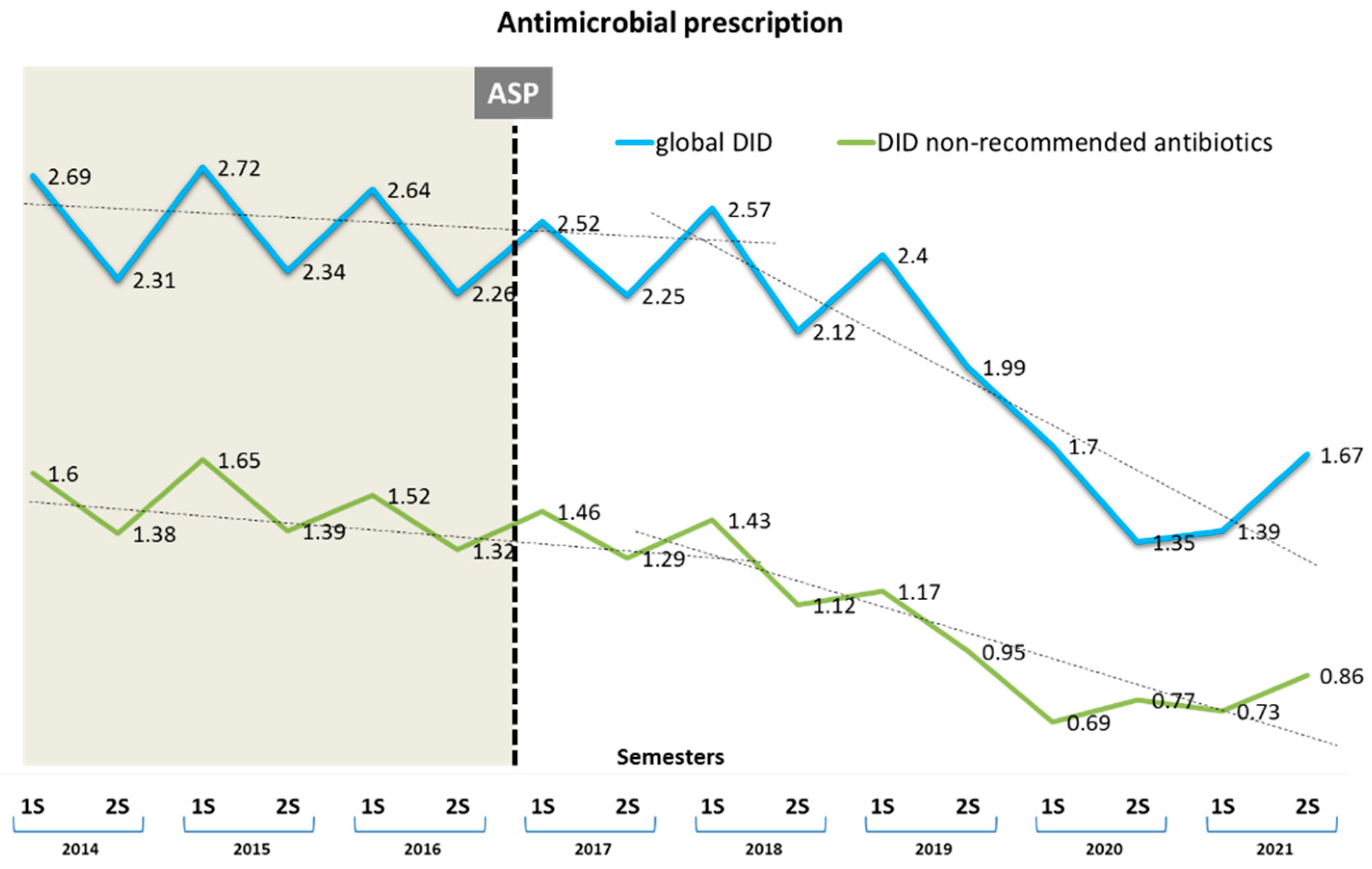


| Prescribed Antibiotic | DID Pre-Intervention Period | Relative Change First Semester 2017 (95% CI) | Relative Change First Semester 2019 (95% CI) | Relative Change Second Semester 2021 (95% CI) | Absolute Effect Post-Intervention Period | Relative Effect (%) |
|---|---|---|---|---|---|---|
| Total antibiotics (J01) | 2.496 | 0.892 (0.890 to 0.894) | 0.790 (0.787 to 0.781) | 0.670 (0.668 to 0.672) | −0.688 (−0.691 to −0.685) | 27.57 (27.65 to 27.49) |
| Total antibiotics not recommended (ANR) | 1.476 | 0.989 (0.987 to 0.992) | 0.796 (0.793 to 0.797) | 0.581 (0.579 to 0.583) | −0.079 (−0.079 to −0.079) | 37.57 (37.48 to 37.66) |
| Co-amoxclav (J01CR02) | 0.704 | 0.940 (0.938 to 0.943) | 0.821 (0.819 to 0.824) | 0.659 (0.657 to 0.662) | −0.250 (−0.251 to −0.249) | 35.59 (35.50 to 35.68) |
| Quinolones (J01M) | 0.311 | 0.903 (0.897 to 0.908) | 0.588 (0.584 to 0.593) | 0.328 (0.325 to 0.331) | −0.294 (−0.295 to −0.294) | 94.74 (94.72 to 94.75) |
| Ciprofloxacin (J01MA02) | 0.114 | 0.779 (0.770 to 0.788) | 0.556 (0.549 to 0.564) | 0.439 (0.433 to 0.446) | −0.052 (−0.052 to −0.051) | 45.39 (45.07 to 45.70) |
| Levofloxacin (J01MA12) | 0.132 | 1.055 (1.046 to 1.065) | 0.730 (0.722 to 0.738) | 0.338 (0.332 to 0.344) | −0.065 (−0.065 to 0.064) | 49.18 (48.93 to 49.44) |
| Cephalosporins (J01D) | 0.111 | 1.115 (1.104 to 1.126) | 0.785 (0.776 to 0.794) | 0.807 (0.798 to 0.816) | −0.025 (−0.026 to −0.025) | 22.99 (22.61 to 23.38) |
| Cefuroxime (J01DC02) | 0.061 | 0.739 (0.726 to 0.751) | 0.614 (0.603 to 0.625) | 0.433 (0.424 to 0.442) | −0.025 (−0.025 to −0.025) | 40.96 (40.50 to 41.41) |
| Third-generation cephalosporins (J01DD) | 0.046 | 1.223 (1.204 to 1.242) | 0.967 (0.971 to 1.004) | 1.275 (1.256 to 1.294) | 0.001 (0.001 to 0.001) | 2.32 (1.57 to 3.07) |
| Azithromycin (J01FA10) | 0.152 | 1.204 (1.194 to 1.213) | 1.119 (1.110 to 1.128) | 0.533 (0.527 to 0.539) | −0.042 (−0.043 to −0.041) | 27.67 (27.36 to 27.98) |
| Clindamycin (J01FF01) | 0.021 | 0.771 (0.750 to 0.793) | 0.720 (0.699 to 0.741) | 0.846 (0.824 to 0.869) | −0.004 (−0.005 to −0.004) | 21.57 (20.61 to 22.51) |
| Total recommended antibiotics (RA) | 0.969 | 1.032 (1.028 to 1.035) | 1.146 (1.143 to 1.150) | 0.748 (0.746 to 0.751) | −0.052 (−0.052 to −0.051) | 21.29 (21.17 to 22.42) |
| Amoxicillin (J01CA04) | 0.925 | 1.028 (1.027 to 1.029) | 1.081 (1.081 to 1.082) | 0.711 (0.709 to 0.712) | −0.218 (−0.218 to −0.217) | 23.53 (23.48 to 23.59) |
| Cloxacillin (J01CF02) | 0.018 | 1.018 (0.991 to 1.046) | 1.032 (1.005 to 1.060) | 0.722 (0.700 to 0.745) | −0.004 (−0.005 to −0.004) | 25.17 (24.18 to 26.15) |
| Cefadroxil (J01DB05) | 0.001 | 1.652 (1.418 to 1.924) | 2.702 (2.380 to 3.066) | 8.835 (8.066 to 9.678) | 0.002 (0.002 to 0.002) | 84.02 (82.80 to 85.14) |
| Cotrimoxazole (J01EE01) | 0.027 | 1.158 (1.134 to 1.182) | 1.416 (1.390 to 1.443) | 1.956 (1.924 to 1.988) | 0.014 (0.013 to 0.014) | 33.89 (33.26 to 34.52) |
| Antimicrobial Resistance | Comparisons by Semesters (S) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second S 2016 vs. Second S 2017 | Second S 2016 vs. Second S 2019 | Second S 2016 vs. First S 2022 | |||||||||||||
| % (n/N) Pre-Intervention Resistance | % (n/N) Post-Intervention Resistance | OR CI 95% | p | % (n/N) Pre-Intervention Resistance | % (n/N) Post-Intervention Resistance | OR CI 95% | p | Prevention Rate (%) | % (n/N) Pre-Intervention Resistance | % (n/N) Post-Intervention Resistance | OR CI 95% | p | Prevention Rate (%) | ||
| MSSA | Clindamycin | 6.54 (48/734) | 16.08 (23/143) | 2.73 (1.60–4.67) | <0.001 | 6.53 (48/734) | 17.89 (34/190) | 3.11 (1.94–4.99) | <0.001 | NA | 6.53 (48/734) | 19.10 (34/178) | 3.37 (2.09–5.42) | <0.001 | NA |
| Levofloxacin | 17.71 (130/734) | 13.22 (19/143) | 0.71 (0.42–1.19) | NS | 17.71 (130/734) | 6.84 (13/190) | 0.34 (0.18–0.61) | <0.001 | 61.4 (33.2–77.6) | 17.71 (130/734) | 10.11 (18/178) | 0.52 (0.30–0.88) | 0.014 | 42.9 (9.10–64.1) | |
| Erythromycin | 19.20 (141/734) | 22.07 (33/143) | 1.26 (0.82–1.93) | NS | 19.20 (141/734) | 21.57 (41/190) | 1.15 (0.78–1.71) | NS | NA | 19.20 (141/734) | 22.47 (40/178) | 1.21 (0.81–1.81) | NS | NA | |
| MRSA | Clindamycin | 28.30 (75/265) | 38.35 (28/73) | 1.57 (0.91–2.71) | NS | 28.30 (75/265) | 45.31 (29/64) | 2.09 (1.19–3.67) | 0.009 | NA | 28.30 (75/265) | 51.11 (23/45) | 2.64 (1.39–5.03) | 0.002 | NA |
| Levofloxacin | 90.94 (241/265) | 93.15 (68/73) | 1.35 (0.49–3.68) | NS | 90.94 (241/265) | 79.63 (50/64) | 0.39 (0.18–0.81) | 0.010 | 12.37 (0.27–23.01) | 90.94 (241/265) | 81.25 (39/45) | 0.43 (0.18–0.99) | 0.044 | 10.6 (−2.83–22.4) | |
| Erythromycin | 42.64 (113/265) | 56.62 (47/73) | 1.75 (1.06–2.88) | 0.026 | 42.64 (113/265) | 46.87 (30/64) | 1.18 (0.68–2.05) | NS | NA | 42.64 (113/265) | 51.11 (23/45) | 1.40 (0.74–2.64) | NS | NA | |
| Antimicrobial Resistance | ID Pre-Intervention Period (95% CI) | Relative Change Second Semester 2017 (95% CI) | Relative Change Second Semester 2019 (95% CI) | Relative Change First Semester 2022 (95% CI) | Absolute Effect Post-Intervention Period | Relative Preventable Effect (%) |
|---|---|---|---|---|---|---|
| MSSA | ||||||
| Clindamycin | 0.024 (0.024 to 0.024) | 2.860 (1.740 to 4.703) | 4.170 (2.687 to 6.471) | 4.071 (2.623 to 6.371) | 0.065 (0.054 to 0.076) | 73.11 (63.67 to 80.10) |
| Levofloxacin | 0.065 (0.064 to 0.065) | 0.872 (0.539 to 1.412) | 0.588 (0.332 to 1.041) | 0.795 (0.486 to 1.302) | −0.008 (−0.021 to 0.005) | 12.33 (−8.65 to 29.25) |
| Erithromycin | 0.071 (0.070 to 0.071) | 1.397 (0.956 to 2.040) | 1.712 (1.209 to 2.424) | 1.630 (1.147 to 2.316) | 0.041 (0.026 to 0.056) | 39.92 (23.81 to 47.77) |
| MRSA | ||||||
| Clindamycin | 0.037 (0.037 to 0.038) | 2.229 (1.444 to 3.440) | 2.276 (1.483 to 3.494) | 1.762 (1.104 to 2.812) | 0.040 (0.028 to 0.052) | 51.93 (38.18 to 62.62) |
| Levofloxacin | 0.120 (0.119 to 0.120) | 1.684 (1.287 to 2.204) | 1.246 (0.921 to 1.685) | 0.858 (0.604 to 1.218) | 0.022 (0.003 to 0.041) | 15.48 (1.80 to 27.26) |
| Erithromycin | 0.056 (0.056 to 0.057) | 2.483 (1.767 to 3.489) | 1.563 (1.045 to 2.337) | 1.169 (0.747 to 1.831) | 0.045 (0.031 to 0.060) | 44.73 (31.96 to 55.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jover-Sáenz, A.; Ramírez-Hidalgo, M.; Bellés Bellés, A.; Ribes Murillo, E.; Batlle Bosch, M.; Ribé Miró, A.; Mari López, A.; Cayado Cabanillas, J.; Piqué Palacín, N.; Garrido-Calvo, S.; et al. Effects of a Primary Care Antimicrobial Stewardship Program on Meticillin-Resistant Staphylococcus aureus Strains across a Region of Catalunya (Spain) over 5 Years. Antibiotics 2024, 13, 92. https://doi.org/10.3390/antibiotics13010092
Jover-Sáenz A, Ramírez-Hidalgo M, Bellés Bellés A, Ribes Murillo E, Batlle Bosch M, Ribé Miró A, Mari López A, Cayado Cabanillas J, Piqué Palacín N, Garrido-Calvo S, et al. Effects of a Primary Care Antimicrobial Stewardship Program on Meticillin-Resistant Staphylococcus aureus Strains across a Region of Catalunya (Spain) over 5 Years. Antibiotics. 2024; 13(1):92. https://doi.org/10.3390/antibiotics13010092
Chicago/Turabian StyleJover-Sáenz, Alfredo, María Ramírez-Hidalgo, Alba Bellés Bellés, Esther Ribes Murillo, Meritxell Batlle Bosch, Anna Ribé Miró, Alba Mari López, José Cayado Cabanillas, Neus Piqué Palacín, Sònia Garrido-Calvo, and et al. 2024. "Effects of a Primary Care Antimicrobial Stewardship Program on Meticillin-Resistant Staphylococcus aureus Strains across a Region of Catalunya (Spain) over 5 Years" Antibiotics 13, no. 1: 92. https://doi.org/10.3390/antibiotics13010092
APA StyleJover-Sáenz, A., Ramírez-Hidalgo, M., Bellés Bellés, A., Ribes Murillo, E., Batlle Bosch, M., Ribé Miró, A., Mari López, A., Cayado Cabanillas, J., Piqué Palacín, N., Garrido-Calvo, S., Ortiz Valls, M., Gracia Vilas, M. I., Gros Navés, L., Javierre Caudevilla, M. J., Montull Navarro, L., Bañeres Argiles, C., Vaqué Castilla, P., Ichart Tomás, J. J., Saura Codina, M., ... on behalf of Clinical Microbiology and Antibiotic Resistance Group -IRBLleida-. (2024). Effects of a Primary Care Antimicrobial Stewardship Program on Meticillin-Resistant Staphylococcus aureus Strains across a Region of Catalunya (Spain) over 5 Years. Antibiotics, 13(1), 92. https://doi.org/10.3390/antibiotics13010092






