Antimicrobial Resistance in Papua New Guinea: A Narrative Scoping Review
Abstract
:1. Introduction
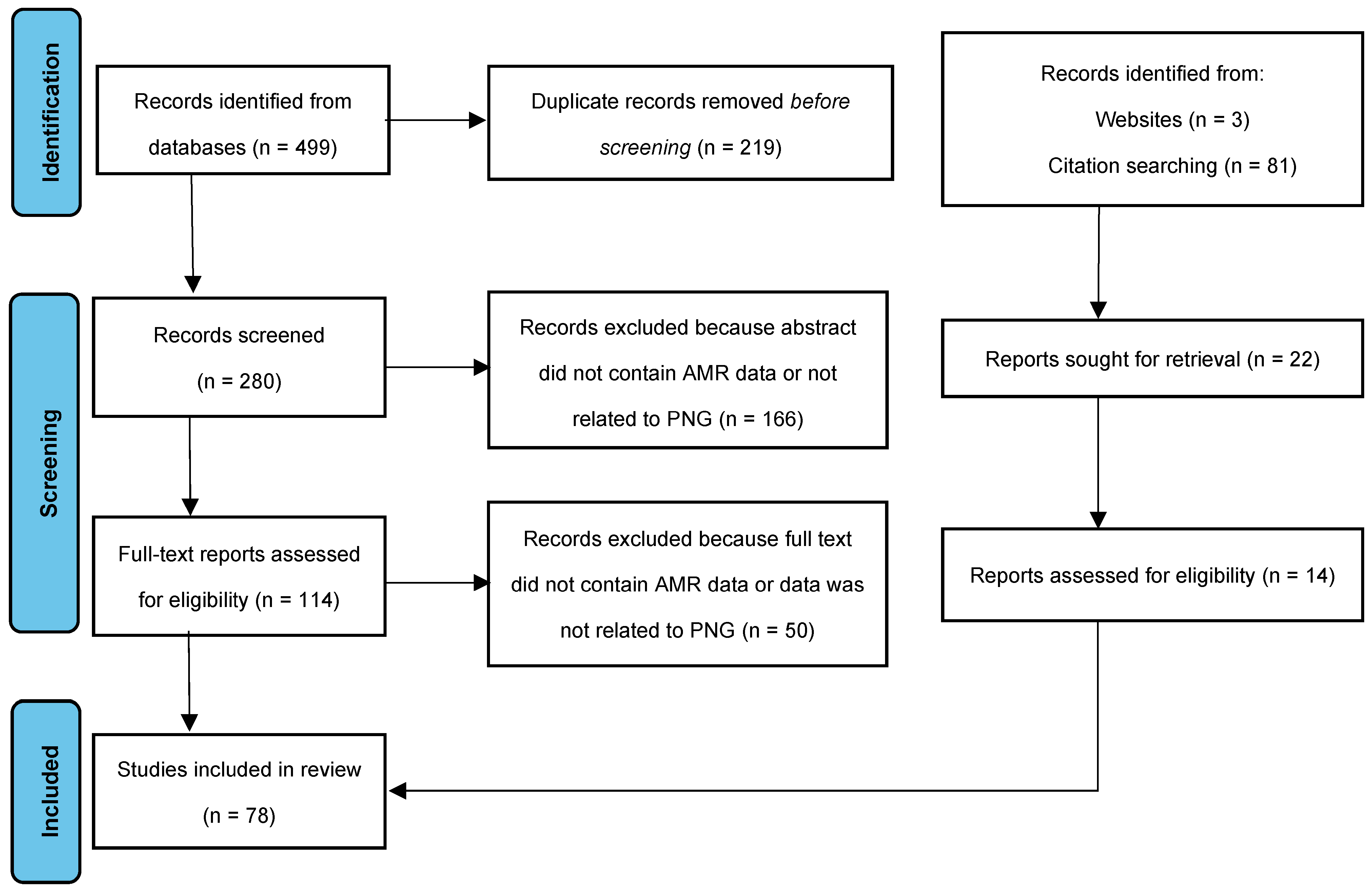
2. Methods
2.1. Antimicrobial Resistance among Gram-Positive Organisms
2.1.1. Staphylococcus aureus
2.1.2. Streptococcus pneumoniae
2.1.3. Streptococcus pyogenes (Group A Streptococcus)
2.1.4. Streptococcus agalactiae (Group B Streptococcus)
2.1.5. Other β-Hemolytic Streptococcus spp.
2.1.6. Corynebacterium spp.
2.2. Antimicrobial Resistance among Gram-Negative Organisms
2.2.1. Haemophilus influenzae
2.2.2. Escherichia coli
2.2.3. Klebsiella spp.
2.2.4. Enterobacter spp.
2.2.5. Proteus spp.
2.2.6. Providencia spp.
2.2.7. Morganella morganii
2.2.8. Pseudomonas spp.
2.2.9. Acinetobacter spp.
2.2.10. Burkholderia spp.
2.2.11. Aeromonas spp.
2.2.12. Citrobacter freundii
2.2.13. Alcaligenes spp.
2.2.14. Shigella spp.
2.2.15. Salmonella spp.
2.2.16. Campylobacter spp.
2.2.17. Vibrio cholerae
2.2.18. Neisseria meningitidis
2.2.19. Neisseria gonorrhoeae
2.3. Drivers of Antimicrobial Resistance in Papua New Guinea
3. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitur, U.; Adair, T.; Riley, I.; Lopez, A.D. Estimating the pattern of causes of death in Papua New Guinea. BMC Public. Health 2019, 19, 1322. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.; Mpirimbanyi, C.; Nziyomaze, E.; Niyomugabo, J.-P.; Niyonsenga, Z.; Muvunyi, C.M.; Mueller, A.; Bebell, L.M.; Nkubana, T.; Musoni, E.; et al. Widespread antimicrobial resistance among bacterial infections in a Rwandan referral hospital. PLoS ONE 2019, 14, e0221121. [Google Scholar] [CrossRef] [PubMed]
- Central Intelligence Agency. Papua New Guinea. In The World Factbook 2020; Central Intelligence Agency: Langley, VA, USA, 2020. [Google Scholar]
- World Bank. Papua New Guinea. Available online: https://data.worldbank.org/country/papua-new-guinea (accessed on 19 May 2020).
- United Nations Development Programme. Human Development Index (HDI). Available online: http://hdr.undp.org/en/content/human-development-index-hdi (accessed on 19 May 2020).
- Ventola, C.L. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Spellberg, B.; Gilbert, D.N. The Future of Antibiotics and Resistance: A Tribute to a Career of Leadership by John Bartlett. Clin. Infect. Dis. 2014, 59 (Suppl. S2), 71–75. [Google Scholar] [CrossRef] [PubMed]
- Aia, P.; Kal, M.; Lavu, E.; Lucy, N.; Johnson, K. The Burden of Drug-Resistant Tuberculosis in Papua New Guinea: Results of a Large Population-Based Survey. PLoS ONE 2016, 11, e0149806. [Google Scholar] [CrossRef] [PubMed]
- Cleary, E.; Hetzel, M.W.; Clements, A.C.A. A review of malaria epidemiology and control in Papua New Guinea 1900 to 2021: Progress made and future directions. Front. Epidemiol. 2022, 2, 980795. [Google Scholar] [CrossRef]
- Gare, J.; Toto, B.; Pokeya, P.; Le, L.-V.; Dala, N.; Lote, N.; John, B.; Yamba, A.; Soli, K.; DeVos, J.; et al. High prevalence of pre-treatment HIV drug resistance in Papua New Guinea: Findings from the first nationally representative pre-treatment HIV drug resistance study. BMC Infect. Dis. 2022, 22, 266. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 2001, 344, 11–16. [Google Scholar] [CrossRef]
- Scrimgeour, E.; Igo, J. Penicillin resistant Staphylococcus aureus in Port Moresby. PNG Med. J. 1981, 24, 261–263. [Google Scholar]
- Duke, T.; Michael, A.; Mgone, J.; Frank, D.; Wal, T.; Sehuko, R. Etiology of child mortality in Goroka, Papua New Guinea: A prospective two-year study. Bull. World Health Organ. 2002, 80, 16–25. [Google Scholar]
- Montgomery, J. The Aerobic Bacteriology of Infected Skin Lesions in Children of the Eastern Highlands Province. PNG Med. J. 1985, 28, 93–103. [Google Scholar]
- Montgomery, J.; West, B.; Michael, A.; Kadivaion, B. Bacterial Resistance in the Eastern Highlands Province. PNG Med. J. 1987, 30, 11–19. [Google Scholar]
- Laman, M.; Greenhill, A.; Coombs, G.W.; Robinson, O.; Pearson, J.; Davis, T.M.E.; Manning, L. Methicillin-resistant Staphylococcus aureus in Papua New Guinea: A community nasal colonization prevalence study. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 360–362. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Aglua, I.; Jaworski, J.; Drekore, J. Methicillin-Resistant Staphylococcus aureus in Melanesian Children with Haematogenous Osteomyelitis from the Central Highlands of Papua New Guinea. Int. J. Pediatr. 2018, 6, 8361–8370. [Google Scholar]
- Hansman, D.; Bullen, M. A resistant pneumococcus. Lancet 1967, 290, 264–265. [Google Scholar] [CrossRef]
- Gratten, M.; Naraqi, S.; Hansman, D. High prevalence of penicillin-insensitive pneumococci in Port Moresby, Papua New Guinea. Lancet 1980, 316, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Duke, T.; Michael, A.; Mokela, D.; Wal, T.; Reeder, J. Chloramphenicol or ceftriaxone, or both, as treatment for meningitis in developing countries? Arch. Dis. Child. 2003, 88, 536–539. [Google Scholar] [CrossRef]
- Manning, L.; Laman, M.; Greenhill, A.R.; Michael, A.; Siba, P.; Mueller, I.; Davis, T.M.E. Increasing chloramphenicol resistance in Streptococcus pneumoniae isolates from Papua New Guinean Children with acute bacterial meningitis. Antimicrob. Agents Chemother. 2011, 55, 4454–4456. [Google Scholar] [CrossRef]
- Laman, M.; Manning, L. Acute bacterial meningitis in Papua New Guinea: New treatment guidelines in response to increasing antibiotic resistance. PNG Med. J. 2011, 54, 1–3. [Google Scholar]
- Scrimgeour, E.M.; Kaven, J. Severe staphylococcal pneumonia complicating pyomyositis. Am. J. Trop. Med. Hyg. 1982, 31, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Browness, P.; Bower, M.; Montgomery, J.; Lupiwa, T.; Gratten, M.; Shann, F. The bacteriology of skin sores in Goroka children. PNG Med. J. 1984, 27, 83–87. [Google Scholar]
- Brian, M.J.; Michael, A. Community-acquired infection with methicillin-resistant Staphylococcus aureus in Papua New Guinea. Pediatr. Infect. Dis. J. 1989, 8, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Duke, T. Antibiotic-resistant bacterial sepsis in Papua New Guinea. PNG Med. J. 2000, 43, 82–90. [Google Scholar]
- Asa, H.; Laman, M.; Greenhill, A.R.; Siba, P.M.; Davis, T.M.E.; Maihua, J.; Manning, L. Bloodstream infections caused by resistant bacteria in surgical patients admitted to Modilon Hospital, Madang. PNG Med. J. 2012, 55, 5–11. [Google Scholar]
- Poka, H.; Duke, T. Clinical management of diarrhoea in children. PNG Med. J. 2013, 56, 156–161. [Google Scholar]
- Henriques-Normark, B.; Tuomanen, E.I. The Pneumococcus: Epidemiology, Microbiology, and Pathogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a010215. [Google Scholar] [CrossRef]
- Hansman, D.; Glasgow, H.; Sturt, J.; Devitt, L.; Douglas, R. Increased resistance to penicillin of pneumococci isolated from man. N. Engl. J. Med. 1971, 284, 175–177. [Google Scholar] [CrossRef]
- Hansman, D. Pneumococci insensitive to Penicillin. Nature 1971, 230, 407–408. [Google Scholar] [CrossRef]
- Hansman, D.; Devitt, L.; Riley, I. Pneumococci with increased resistance to penicillin. Br. Med. J. (Clin. Res. Ed.) 1973, 3, 405. [Google Scholar] [CrossRef] [PubMed]
- Gratten, M.; Barker, J.; Shann, F.; Gerega, G.; Montgomery, J.; Kajoi, M.; Lupiwa, T. The Aetiology of Purulent Meningitis in Highland Children: A Bacteriological Study. PNG Med. J. 1985, 28, 233–240. [Google Scholar]
- Barker, J.; Gratten, M.; Riley, I.; Lehmann, D.; Montgomery, J.; Kajoi, M.; Gratten, H.; Smith, D.; Marshall, T.F.D.C.; Alpers, M.P. Pneumonia in Children in the Eastern Highlands of Papua New Guinea: A Bacteriologic Study of Patients Selected by Standard Clinical Criteria. J. Infect. Dis. 1989, 159, 348–352. [Google Scholar] [CrossRef]
- Gratten, M.; Montgomery, J. The bacteriology of acute pneumonia and meningitis in children in Papua New Guinea: Assumptions, facts and technical strategies. PNG Med. J. 1991, 34, 185–198. [Google Scholar]
- Gratten, M. Carriage and Invasion of Respiratory Bacterial Pathogens in Melanesian Children. MSc Thesis, University of Papua New Guinea, Port Moresby, Papua New Guinea, 1984. [Google Scholar]
- Montgomery, J. A Longitudinal Study of Upper Respiratory Tract Bacterial Carriage and Its Association with Acute Lower Respiratory Infections in Children in the Highlands of Papua New Guinea. MSc Thesis, University of Papua New Guinea, Port Moresby, Papua New Guinea, 1989. [Google Scholar]
- Lehmann, D.; Gratten, M.; Montgomery, J. Susceptibility of pneumococcal carriage isolates to penicillin provides a conservative estimate of susceptibility of invasive pneumococci. Pediatr. Infect. Dis. J. 1997, 16, 297–305. [Google Scholar] [CrossRef]
- Lehmann, D.; Yeka, W.; Rongap, T.; Javati, A.; Saleu, G.; Clegg, A.; Michael, A.; Lupiwa, T.; Omena, M.; Alpers, M.P. Aetiology and clinical signs of bacterial meningitis in children admitted to Goroka Base Hospital, Papua New Guinea, 1989–1992. Ann. Trop. Paediatr. 1999, 19, 21–32. [Google Scholar] [CrossRef]
- Greenhill, A.R.; Phuanukoonnon, S.; Michael, A.; Yoannes, M.; Orami, T.; Smith, H.; Murphy, D.; Blyth, C.; Reeder, J.; Siba, P.; et al. Streptococcus pneumoniae and Haemophilus influenzae in paediatric meningitis patients at Goroka General Hospital, Papua New Guinea: Serotype distribution and antimicrobial susceptibility in the pre-vaccine era. BMC Infect. Dis. 2015, 15, 485. [Google Scholar] [CrossRef]
- Daimen, M. Acute bacterial meningitis in adult patients at the Port Moresby General Hospital 1998–2008. In Public Private Partnership in Health Care, 45th Annual Medical Symposium, 30 August–4 September 2009; Medical Society of Papua New Guinea: Port Moresby, Papua New Guinea, 2009. [Google Scholar]
- Martin, J.; Green, M. Group A Streptococcus. Semin. Pediatr. Infect. Dis. 2006, 17, 140–148. [Google Scholar] [CrossRef]
- Raabe, V.N.; Shane, A.L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7, 1–21. [Google Scholar] [CrossRef]
- Shah, M.; Centor, R.M.; Jennings, M. Severe acute pharyngitis caused by group C Streptococcus. J. Gen. Intern. Med. 2007, 22, 272–274. [Google Scholar] [CrossRef]
- Kalt, F.; Schulthess, B.; Sidler, F.; Herren, S.; Fucentese, S.F.; Zingg, P.O.; Berli, M.; Zinkernagel, A.S.; Zbinden, R.; Achermann, Y. Corynebacterium Species Rarely Cause Orthopedic Infections. J. Clin. Microbiol. 2018, 56, 1–8. [Google Scholar] [CrossRef]
- Agrawal, A.; Murphy, T.F. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J. Clin. Microbiol. 2011, 49, 3728–3732. [Google Scholar] [CrossRef]
- Shann, F.; Germer, S.; Hazlett, D.; Gratten, M.; Linnemann, V.; Payne, R. Aetiology of pneumonia in children in Goroka Hospital, Papua New Guinea. Lancet 1984, 324, 537–541. [Google Scholar] [CrossRef]
- Shann, F.; Gratten, M.; Montgomery, J.; Lupiwa, T.; Polume, H. Haemophilus influenzae Resistant to Penicillin in Goroka. PNG Med. J. 1982, 25, 23–24. [Google Scholar]
- Duke, T.; Mokela, D.; Frank, D.; Michael, A.; Paulo, T.; Mgone, J.; Kurubi, J. Management of meningitis in children with oral fluid restriction or intravenous fluid at maintenance volumes: A randomised trial. Ann. Trop. Paediatr. 2002, 22, 145–157. [Google Scholar] [CrossRef]
- Paediatric Society of Papua New Guinea. Standard Treatment for Common Illnesses of Children in Papua New Guinea, 10th ed.; Paediatric Society of Papua New Guinea: Port Moresby, Papua New Guinea, 2016. [Google Scholar]
- Packham, D.; Sorrell, T. Pneumonia with bacteraemia due to Escherichia coli. Aust. N. Z. J. Med. 1981, 11, 669–672. [Google Scholar] [CrossRef]
- Duke, T.; Michael, A. Increase in sepsis due to multi-resistant enteric gram-negative bacilli in Papua New Guinea. PNG Med. J. 1999, 353, 2210–2211. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Trevett, A.; SenGupta, S. Gentamicin resistance in fatal Klebsiella septicaemia. PNG Med. J. 1992, 35, 202–204. [Google Scholar]
- Lithgow, A.E.; Kilalang, C. Outbreak of nosocomial sepsis in the Special Care Nursery at Port Moresby General Hospital due to multiresistant Klebsiella pneumoniae: High impact on mortality. PNG Med. J. 2009, 52, 28–34. [Google Scholar]
- Hilty, M.; Sendi, P.; Seiffert, S.N.; Droz, S.; Perreten, V.; Hujer, A.M.; Bonomo, R.A.; Mühlemann, K.; Endimiani, A. Characterisation and clinical features of Enterobacter cloacae bloodstream infections occurring at a tertiary care university hospital in Switzerland: Is cefepime adequate therapy? Int. J. Antimicrob. Agents. 2013, 41, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Endimiani, A.; Luzzaro, F.; Brigante, G.; Perilli, M.; Lombardi, G.; Amicosante, G.; Rossolini, G.M.; Toniolo, A. Proteus mirabilis bloodstream infections: Risk factors and treatment outcome related to the expression of extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 2005, 49, 2598–2605. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, Y.K.; Kim, H.Y.; Park, J.E.; Uh, Y. Clinical and microbiological features of Providencia bacteremia: Experience at a tertiary care hospital. Korean J. Intern. Med. 2015, 30, 219–225. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Hu, Q.; Rao, X. Morganella Morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 2016, 50, 10–17. [Google Scholar] [CrossRef]
- de Bentzmann, S.; Plésiat, P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ. Microbiol. 2011, 13, 1655–1665. [Google Scholar] [CrossRef]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Barnes, D.J.; Naraqi, S.; Igo, J.D. Community-Acquired Acinetobacter Pneumonia in Adults in Papua New Guinea. Rev. Infect. Dis. 1988, 10, 636–639. [Google Scholar] [CrossRef]
- Sfeir, M.M. Burkholderia cepacia Complex Infections: More Complex Than the Bacterium Name Suggest. J. Infect. 2018, 77, 166–170. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B. Melioidosis. Nat. Rev. Dis. Prim. 2018, 4, 17107. [Google Scholar] [CrossRef]
- Baker, A.; Pearson, T.; Price, E.P.; Dale, J.; Keim, P.; Hornstra, H.; Greenhill, A.; Padilla, G.; Warner, J. Molecular Phylogeny of Burkholderia pseudomallei from a Remote Region of Papua New Guinea. PLoS ONE 2011, 6, e18343. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, K.P.; Ranjan, N. Citrobacter: An emerging health care associated urinary pathogen. Urol. Ann. 2013, 5, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.J.; Nizhu, L.N.; Rabbani, R. Bloodstream infection with pandrug-resistant Alcaligenes faecalis treated with double-dose of tigecycline. IDCases 2019, 18, e00600. [Google Scholar] [CrossRef]
- Zaidi, M.B.; Estrada-García, T. Shigella: A Highly Virulent and Elusive Pathogen. Curr. Trop. Med. Rep. 2014, 1, 81–87. [Google Scholar] [CrossRef]
- Curtis, P. The isolation, incidence and sensitivity of Shigella organisms. PNG Med. J. 1964, 7, 23–26. [Google Scholar]
- Rosewell, A.; Ropa, B.; Posanai, E.; Dutta, S.R.; Mola, G.; Zwi, A.; MacIntyre, C.R. Shigella spp. Antimicrobial Drug Resistance, Papua New Guinea, 2000–2009. Emerg. Infect. Dis. 2010, 16, 8–10. [Google Scholar] [CrossRef]
- Greenhill, A.R.; Guwada, C.; Siba, V.; Michael, A.; Yoannes, M.; Wawarie, Y.; Ford, R.; Siba, P.M.; Horwood, P.F. Antibiotic resistant Shigella is a major cause of diarrhoea in the Highlands of Papua New Guinea. J. Infect. Dev. Ctries. 2014, 8, 1391–1397. [Google Scholar] [CrossRef]
- Malau, E.; Ford, R.; Valcanis, M.; Jennison, A.V.; Mosse, J.; Bean, D.; Yoannes, M.; Pomat, W.; Horwood, P.F.; Greenhill, A.R. Antimicrobial sensitivity trends and virulence genes in Shigella spp. from the Oceania region. Infect. Genet. Evol. 2018, 64, 52–56. [Google Scholar] [CrossRef]
- Kurtz, J.R.; Goggins, J.A.; McLachlan, J.B. Salmonella infection: Interplay between the bacteria and host immune system. Immunol. Lett. 2017, 190, 42–50. [Google Scholar] [CrossRef]
- Howard, P.; Alexander, N.D.; Atkinson, A.; Clegg, A.; Gerega, G.; Javati, A.; Kajoi, M.; Lupiwa, S.; Lupiwa, T.; Mens, M.; et al. Bacterial, Viral and Parasitic Aetiology of Paediatric Diarrhoea in the Highlands of Papua New Guinea. J. Trop. Pediatr. 2000, 46, 10–14. [Google Scholar] [CrossRef]
- Faruque, S.; Albert, M.; Mekalanos, J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 1998, 62, 1301–1314. [Google Scholar] [CrossRef]
- Murhekar, M.; Dutta, S.; Ropa, B.; Dagina, R.; Posanaic, E.; Rosewell, A. Vibrio cholerae antimicrobial drug resistance, Papua New Guinea, 2009–2011. West. Pac. Surveill. Response J. WSPAR 2013, 4, 2009–2011. [Google Scholar] [CrossRef]
- Rouphael, N.G.; Stephens, D.S. Neisseria meningitidis: Biology, microbiology, and epidemiology. Methods Mol. Biol. 2012, 799, 1–20. [Google Scholar]
- Hudson, B.; van der Meijden, W.; Lupiwa, T.; Howard, P.; Tabua, T.; Tapsall, J.W.; Phillips, E.A.; Lennox, V.A.; Backhouse, J.L.; Pyakalyia, T. A survey of sexually transmitted diseases in five STD clinics in Papua New Guinea. PNG Med. J. 1994, 37, 152–160. [Google Scholar]
- Piszczek, J.; St Jean, R.; Khaliq, Y. Gonorrhea: Treatment update for an increasingly resistant organism. Can. Pharm. J. 2015, 148, 82–89. [Google Scholar] [CrossRef]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef]
- Lahra, M.M.; Lo, Y.R.; Whiley, D.M. Gonococcal antimicrobial resistance in the Western Pacific Region. Sex. Transm. Infect. 2013, 89 (Suppl. S4), 19–23. [Google Scholar] [CrossRef]
- WHO Western Pacific Region Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic susceptibility of Neisseria gonorrhoeae in the WHO Western Pacific Region. Genitourin. Med. 1997, 73, 355–361. [Google Scholar]
- Pusahai-Riman, P.; Soepol, N. A retrospective assessment of antibiotic susceptibility pattern of Neisseria gonorrhoeae and prevalence rate of gonorrhoea in Port Moresby General Hospital from 2005 to 2006. Pac. J. Med. Sci. 2008, 5, 82–90. [Google Scholar]
- Toliman, P.J.; Lupiwa, T.; Law, G.J.; Reeder, J.C.; Siba, P.M. Neisseria gonorrhoeae isolates from four centres in Papua New Guinea remain susceptible to amoxycillin-clavulanate therapy. PNG Med. J. 2010, 53, 15–20. [Google Scholar]
- Riley, I. Pneumonia in Papua New Guinea. Bachelor’s Thesis, University of Sydney, Sydney, Australia, 1979. [Google Scholar]
- Peacock, S.; Newton, P. Public health impact of establishing the cause of bacterial infections in rural Asia. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Standard Management of Sexually Transmitted Infections Genital Conditions in Papua New Guinea: A Manual for Health Workers in PNG; Papua New Guinea Department of Health: Port Moresby, Papua New Guinea, 2006.
- Blyth, C.C.; Ford, R.; Sapura, J.; Kumani, T.; Masiria, G.; Kave, J.; Yuasi, L.; Greenhill, A.; Hwaihwanje, I. Childhood pneumonia and meningitis in the Eastern Highlands Province, Papua New Guinea in the era of conjugate vaccines: Study methods and challenges. Pneumonia 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Zamunu, A.; Pameh, W.; Ripa, P.; Vince, J.; Duke, T. Antibiotic use in the management of children with the common cold at a provincial hospital in Papua New Guinea: A point-prevalence study. Paediatr. Int. Child. Health. 2018, 38, 261–265. [Google Scholar] [CrossRef]
- Eells, S.J.; Nguyen, M.; Jung, J.; Macias-Gil, R.; May, L.; Miller, L.G. Relationship between adherence to oral antibiotics and postdischarge clinical outcomes among patients hospitalized with Staphylococcus aureus skin infections. Antimicrob. Agents Chemother. 2016, 60, 2941–2948. [Google Scholar] [CrossRef]
- Wandi, F.; Kiagi, G.; Duke, T. Long-term outcome for children with bacterial meningitis in rural Papua New Guinea. J. Trop. Pediatr. 2005, 51, 51–53. [Google Scholar] [CrossRef]
- Weinstein, Z.B.; Zaman, M.H. Evolution of rifampin resistance in Escherichia coli and Mycobacterium smegmatis due to substandard drugs. Antimicrob. Agents Chemother. 2018, 63, e01243-18. [Google Scholar] [CrossRef]
- Hetzel, M.W.; Page-Sharp, M.; Bala, N.; Pulford, J.; Betuela, I.; Davis, T.M.E.; Lavu, E.K. Quality of antimalarial drugs and antibiotics in Papua New Guinea: A survey of the health facility supply chain. PLoS ONE 2014, 9, e96810. [Google Scholar] [CrossRef]
- Papua New Guinea Department of Health, World Health Organization. Papua New Guinea Pharmaceutical Country Profile. 2012. Available online: https://www.who.int/countries/png/ (accessed on 19 May 2020).
- Berndtson, A.E. Increasing Globalization and the Movement of Antimicrobial Resistance between Countries. Surg. Infect. 2020, 21, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Banks, G. Globalization, poverty, and hyperdevelopment in Papua New Guinea’s mining sector. Focaal 2005, 2005, 128–144. [Google Scholar] [CrossRef]
- John, B.; Bowler, D.; Gooch, P.; Lawson, J. Purulent meningitis in Papuan children. PNG Med. J. 1968, 11, 23–29. [Google Scholar]
- World Health Organization, Gonococcal Antimicrobial Surveillance Programme. Gonococcal antimicrobial susceptibility. In Report on Global Sexually Transmitted Infection Surveillance, 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic Resistant Threats in the United States; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 10325. [Google Scholar]
- Foxlee, N.D.; Townell, N.; McIver, L.; Lau, C.L. Antibiotic Resistance in Pacific Island Countries and Territories: A Systematic Scoping Review. Antibiotics 2019, 8, 29. [Google Scholar] [CrossRef]
- Observatory of Economic Complexity. Papua New Guinea Country Profile. Available online: https://oec.world/en/profile/country/png/ (accessed on 19 May 2020).
- Bainomugisa, A.; Pandey, S.; Donnan, E.; Simpson, G.; Foster, J.; Lavu, E.; Hiasihri, S.; McBryde, E.S.; Moke, R.; Vincent, S.; et al. Cross-border movement of highly drug-resistant Mycobacterium tuberculosis from Papua New Guinea to Australia through Torres Strait Protected Zone, 2010–2015. Emerg. Infect. Dis. 2019, 25, 406–415. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, B.; Adiunegiya, S. Antimicrobial Resistance in Papua New Guinea: A Narrative Scoping Review. Antibiotics 2023, 12, 1679. https://doi.org/10.3390/antibiotics12121679
Page B, Adiunegiya S. Antimicrobial Resistance in Papua New Guinea: A Narrative Scoping Review. Antibiotics. 2023; 12(12):1679. https://doi.org/10.3390/antibiotics12121679
Chicago/Turabian StylePage, Brady, and Simeon Adiunegiya. 2023. "Antimicrobial Resistance in Papua New Guinea: A Narrative Scoping Review" Antibiotics 12, no. 12: 1679. https://doi.org/10.3390/antibiotics12121679
APA StylePage, B., & Adiunegiya, S. (2023). Antimicrobial Resistance in Papua New Guinea: A Narrative Scoping Review. Antibiotics, 12(12), 1679. https://doi.org/10.3390/antibiotics12121679






