Antimicrobial Resistance in Streptococcus pneumoniae before and after the Introduction of Pneumococcal Conjugate Vaccines in Brazil: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. Assessment of the Methodological Quality of the Articles
2.2. Data Extraction
2.3. Statistical Analysis
3. Discussion
4. Materials and Methods
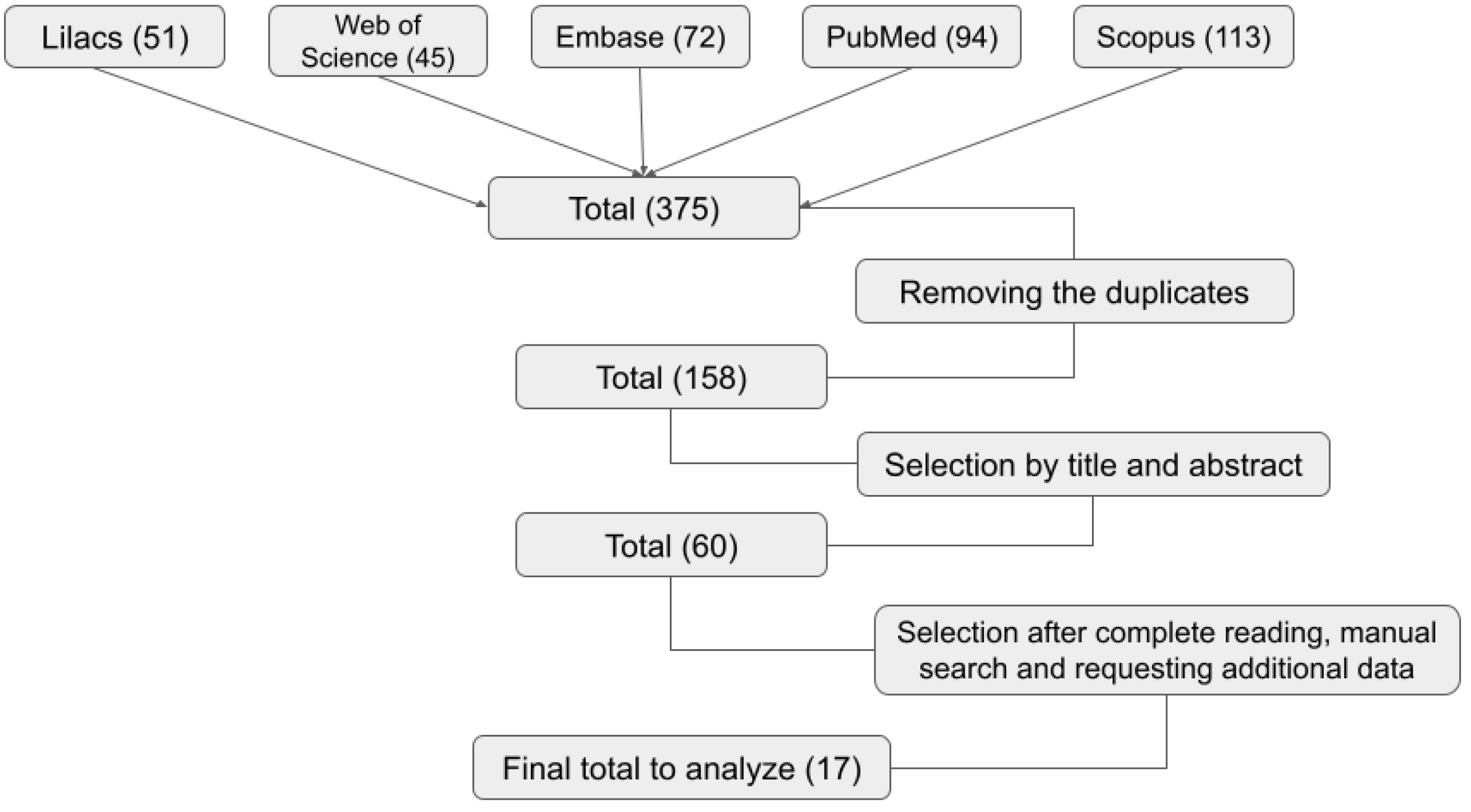
4.1. Search Strategy
4.2. Article Selection and Data Extraction
4.3. Quality Assessment
4.4. Data Compilation
4.5. Statistical Analysis
4.6. Ethical Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, A.; Weiser, J.N.; Paton, J.C.; Andrew, P.W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008, 6, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Drijkoningen, J.J.C.; Rohde, G.G.U. Pneumococcal infection in adults: Burden of disease. Clin. Microbiol. Infect. 2014, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, E.; Bonanni, P.; Rohde, G.; Sayiner, A.; Torres, A. The remaining challenges of pneumococcal disease in adults. Eur. Respir. Rev. 2012, 21, 57–65. [Google Scholar] [CrossRef]
- Briles, D.E.; Paton, J.C.; Mukerji, R.; Swiatlo, E.; Crain, M.J. Pneumococcal vaccines. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Rappuoli, R.; De Gregorio, E.; Costantino, P. On the mechanisms of conjugate vaccines. Proc. Natl. Acad. Sci. USA 2019, 116, 14–16. [Google Scholar] [CrossRef]
- Pneumococcal 7-Valent Conjugate Vaccine (Diphtheria CRM197 Protein) Prevnar®. European Medicines Agency: London, UK. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar (accessed on 17 July 2023).
- Synflorix: Pneumococcal Polysaccharide Conjugate Vaccine (Adsorbed). European Medicines Agency: London, UK. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000973/WC500054346.Pdf (accessed on 17 July 2023).
- Approved Products: Prevnar 13: Pneumococcal 13-Valent Conjugate Vaccine (Diphtheria CRM 197 Protein). Food and Drug Administration: Washington, DC, USA. Available online: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM574852.pdf (accessed on 17 July 2023).
- Food and Drug Administration. Summary Basis for Regulatory Action—PREVNAR20. US Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA. Available online: https://www.fda.gov/media/150388/download (accessed on 17 July 2023).
- Food and Drug Administration. Summary Basis for Regulatory Action—VAXNEUVANCE. US Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA. Available online: https://www.fda.gov/media/151201/download (accessed on 17 July 2023).
- Huang, L.; Wasserman, M.; Grant, L.; Farkouh, R.; Snow, V.; Arguedas, A.; Chilson, E.; Sato, R.; Perdrizet, J. Burden of pneumococcal disease due to serotypes covered by the 13-valent and new higher-valent pneumococcal conjugate vaccines in the United States. Vaccine 2022, 40, 4700–4708. [Google Scholar] [CrossRef]
- Brasil Departamento de Vigilância Epidemiológica, Secretaria de Vigilância em Saúde, Ministério da Saúde. Informe técnico da vacina pneumocócica 10-valente (conjugada). Brasília: Ministério da Saúde (Série A. Normas e Manuais Técnicos). Available online: http://epidemiologia.alfenas.mg.gov.br/download/informe_t%C3%A9cnico_pneumo_.pdf (accessed on 17 July 2023).
- Brasil Ministério da Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Portaria nº 14, de 1º de março de 2019. Torna pública a decisão de incorporar a vacina pneumocócica conjugada 13-valente contra doenças pneumocócicas em pacientes de risco, no âmbito do Sistema Único de Saúde—SUS. Diário Oficial da União, Brasília, DF, 6 de março de 2019. p. 79. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sctie/2019/prt0014_06_03_2019.html (accessed on 29 October 2023).
- Brasil Ministério da Saúde. Programa Nacional de Imunizações: 30 anos. Brasília: Ministério da Saúde; 2003. 212p. Série C. Projetos e Programas e Relatórios. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/livro_30_anos_pni.pdf (accessed on 1 November 2023).
- Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. Programa Nacional de Imunizações (PNI): 40 anos. Brasília: Ministério da Saúde; 2013; 236p. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/programa_nacional_imunizacoes_pni40.pdf (accessed on 1 November 2023).
- Sociedade Brasileira de Imunizações—SBIM. Vacinas pneumocócicas conjugadas. 2023. Available online: https://familia.sbim.org.br/vacinas/vacinas-disponiveis/vacinas-pneumococicas-conjugadas (accessed on 29 October 2023).
- Jefferies, J.M.; Macdonald, E.; Faust, S.N.; Clarke, S.C. 13-valent pneumococcal conjugate vaccine (PCV13). Hum. Vaccines 2011, 7, 1012–1018. [Google Scholar] [CrossRef]
- Jarovsky, D.; Berezin, E.N. Impact of PCV10 on pediatric pneumococcal disease burden in Brazil: Time for new recommendations? J. Pediatr. 2023, 99, S46–S56. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. DATASUS. Brasília: Ministério da Saúde; 2023. Available online: http://tabnet.datasus.gov.br/cgi/dhdat.exe?bd_pni/cpnibr.def/ (accessed on 26 December 2023).
- Neves, F.P.G.; Cardoso, N.T.; Snyder, R.E.; Marlow, M.A.; Cardoso, C.A.A.; Teixeira, L.M.; Riley, L.W. Pneumococcal carriage among children after four years of routine 10-valent pneumococcal conjugate vaccine use in Brazil: The emergence of multidrug resistant serotype 6C. Vaccine 2017, 35, 2794–2800. [Google Scholar] [CrossRef]
- Fortuna, L.B.; Miranda, F.M.; Antunes, I.M.; Silva, A.B.; Cabral, A.S.; Dolores, I.M.; Cardoso-Marques, N.T.; Teixeira, L.M.; Neves, F.P. Prevalence, capsular types, antimicrobial resistance and risk factors associated with pneumococcal carriage among children after long-term 10-valent pneumococcal conjugate vaccine use in Brazil. Vaccine 2023, 41, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Kupek, E.; Vieira, I.L.V. O impacto da vacina pneumocócica PCV10 na redução da mortalidade por pneumonia em crianças menores de um ano em Santa Catarina, Brasil. Cad. Saúde Pública 2016, 32, e00131414. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.L.; Afonso, E.T.; Minamisava, R.; Bierrenbach, A.L.; Cristo, E.B.; Morais-Neto, O.L.; Policena, G.M.; Domingues, C.M.A.S.; Toscano, C.M. Direct and indirect impact of 10-valent pneumococcal conjugate vaccine introduction on pneumonia hospitalizations and economic burden in all age-groups in Brazil: A time-series analysis. PLoS ONE 2017, 12, e0184204. [Google Scholar] [CrossRef] [PubMed]
- Brasil Ministério da Saúde/SVS—Sistema de Informação de Agravos de Notificação—Sinan Net. Available online: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/meninbr.def (accessed on 13 October 2023).
- Von Specht, M.; García Gabarrot, G.; Mollerach, M.; Bonofiglio, L.; Gagetti, P.; Kaufman, S.; Vigliarolo, L.; Toresani, I.; Lopardo, H.A. Resistance to β-lactams in Streptococcus pneumoniae. Rev. Argent. Microbiol. 2021, 53, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Cherazard, R.; Epstein, M.; Doan, T.L.; Salim, T.; Bharti, S.; Smith, M.A. Antimicrobial resistant Streptococcus pneumoniae: Prevalence, mechanisms, and clinical implications. Am. J. Ther. 2017, 24, e361–e369. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Alegría, A.R.; Pintado, V.; Barbolla, I. Treatment and prevention of invasive pneumococcal disease. Rev. Clin. Esp. (Engl. Ed.) 2018, 218, 244–252. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Wright, M.T.; Nelson, T.N. Otitis media: Rapid evidence review. Am. Fam. Physician 2019, 100, 350–356. Available online: https://www.aafp.org/pubs/afp/issues/2019/0915/p350.pdf (accessed on 17 July 2023).
- Modi, A.R.; Kovacs, C.S. Community-acquired pneumonia: Strategies for triage and treatment. Cleve. Clin. J. Med. 2020, 87, 145–151. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 17 July 2023).
- Centers for Disease Control and Prevention (CDC). Antimicrobial Resistance: 2019 AR Threats Report. 2019. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 29 October 2023).
- Pinto, T.C.A.; Neves, F.P.G.; Souza, A.R.V.; Oliveira, L.M.A.; Costa, N.S.; Castro, L.F.S.; Mendonça-Souza, C.R.d.V.; Peralta, J.M.; Teixeira, L.M. Evolution of Penicillin Non-susceptibility Among Streptococcus pneumoniae Isolates Recovered from Asymptomatic Carriage and Invasive Disease over 25 years in Brazil, 1990–2014. Front. Microbiol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Velasquez, P.A.; Parussolo, L.; Cardoso, C.L.; Tognim, M.C.B.; Garcia, L.B. High prevalence of children colonized with penicillin-resistant Streptococcus pneumoniae in public day-care centers. J. Pediatr. (Rio J.) 2009, 85, 516–522. [Google Scholar] [CrossRef]
- Pereira, M.; Pereira, M.R.; Cantarelli, V.; Costa, S.S. Prevalence of bacteria in children with otitis media with effusion. J. Pediatr. (Rio J.) 2004, 80, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, D.M.; Nascimento-Carvalho, C.M.; Brandão, M.A.; Azevedo, G.M.S.; De Souza, F.R.; Silva, N.M.S.; Brandão, A.P.; Andrade, A.L.; Brandileone, M.C.C. Antimicrobial resistance and serotypes of nasopharyngeal strains of Streptococcus pneumoniae in Brazilian adolescents. Microb. Drug Resist. 2006, 12, 29–32. [Google Scholar] [CrossRef]
- Franco, C.M.; Andrade, A.L.S.; Andrade, J.G.; e Silva, S.A.; Oliveira, C.R.M.; Pimenta, F.C.; Lamaro-Cardoso, J.; Brandão, A.P.; Almeida, S.C.G.; Calix, J.J.; et al. Survey of nonsusceptible nasopharyngeal Streptococcus pneumoniae isolates in children attending day-care centers in Brazil. Pediatr. Infect. Dis. J. 2010, 29, 77. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.P.; Cardoso, N.T.; Cardoso, C.A.; Teixeira, L.M.; Riley, L.W. Direct effect of the 13-valent pneumococcal conjugate vaccine use on pneumococcal colonization among children in Brazil. Vaccine 2019, 37, 5265–5269. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.N.; Palma, T.; Ribeiro, G.S.; Pinheiro, R.M.; Ribeiro, C.T.; Cordeiro, S.M.; Da Silva Filho, H.P.; Moschioni, M.; Thompson, T.A.; Sprat, B.; et al. Transmission of Streptococcus pneumoniae in an urban slum community. J. Infect. 2008, 57, 204–213. [Google Scholar] [CrossRef]
- Neves, F.P.G.; Pinto, T.C.A.; Corrêa, M.A.; Barreto, R.A.; Moreira, L.S.G.; Rodrigues, H.G.; Cardoso, C.A.; Barros, R.R.; Teixeira, L.M. Nasopharyngeal carriage, serotype distribution and antimicrobial resistance of Streptococcus pneumoniae among children from Brazil before the introduction of the 10-valent conjugate vaccine. BMC Infect. Dis. 2013, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira, B.J. Resistência antimicrobiana, genotipagem capsular e detecção de genes de resistência de Streptococcus pneumoniae isolados de crianças não vacinadas usuárias de creches em Fortaleza. Ph.D. Thesis, Federal University of Ceara, Fortaleza, Brazil, 2014. Available online: https://repositorio.ufc.br/handle/riufc/15459 (accessed on 18 July 2023).
- Da Silva, A.B.; Cardoso-Marques, N.T.; Dolores, I.M.; Teixeira, L.M.; Neves, F.P.G. Carriage prevalence, serotype distribution, antimicrobial resistance, pspA typing and pilus islets of Streptococcus pneumoniae isolated from adults living in a Brazilian urban slum. Vaccine 2023, 41, 1431–1437. [Google Scholar] [CrossRef]
- Fonseca, P.B.B.; Braga, J.A.P.; Machado, A.M.D.O.; Brandileone, M.C.D.C.; Farhat, C.K. Colonização nasofaríngea pelo Streptococcus pneumoniae em crianças com doença falciforme usando penicilina profilática. J. Pediatr. (Rio J.) 2005, 81, 149–154. [Google Scholar] [CrossRef]
- Brandileone, M.C.D.C.; Zanella, R.C.; Almeida, S.C.; Cassiolato, A.P.; De Lemos, A.P.S.; Salgado, M.M.; Higa, F.T.; Minamisava, R.; Andrade, A.L. Long-term effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae in children in Brazil. Vaccine 2019, 37, 5357–5363. [Google Scholar] [CrossRef]
- Zanella, R.C.; Brandileone, M.C.D.C.; Almeida, S.C.G.; De Lemos, A.P.S.; Sacchi, C.T.; Gonçalves, C.R.; Gonçalves, M.G.; Fukasawa, L.O.; Saraiva, M.D.; Rangel, L.F.; et al. Nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in a Brazilian elderly cohort. PLoS ONE 2019, 14, e0221525. [Google Scholar] [CrossRef]
- Rezende, R.P.V.D.; Cardoso-Marques, N.T.; Rodrigues, L.A.S.; Almeida, J.P.C.L.D.; Pillegi, G.S.; Teixeira, L.M.; Klumb, E.M.; Neves, F.P.G. Carriage prevalence, serotype distribution, and antimicrobial susceptibility among pneumococcal isolates recovered from adults with systemic lupus erythematosus. Lupus 2021, 30, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Laval, C.B.; De Andrade, A.L.S.S.; Pimenta, F.C.; De Andrade, J.G.; De Oliveira, R.M.; Silva, S.A.; De Lima, E.C.; Di Fabio, J.L.; Casagrande, S.T.; Brandileone, M.C.C. Serotypes of carriage and invasive isolates of Streptococcus pneumoniae in Brazilian children in the era of pneumococcal vaccines. Clin. Microbiol. Infect. 2006, 12, 50–55. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization [Homepage on the Internet]. OPAS. Available online: https://www3.paho.org/hq/index.php?option=com_docman&view=list&slug=sireva-ii-8059&Itemid=270&lang=pt#gsc.tab=0 (accessed on 18 July 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores, 2000–2005; OPS: Washington, DC, USA, 2007; Available online: https://www.paho.org/es/node/72322 (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II, 2006: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores; OPS: Washington, DC, USA, 2008; Available online: https://www.paho.org/es/documentos/informe-regional-sireva-ii-2006 (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II, 2007: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores; OPS: Washington, DC, USA, 2008; Available online: https://www3.paho.org/hq/dmdocuments/2009/SirevaII2007.pdf (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II, 2008: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores; OPS: Washington, DC, USA, 2009; Available online: https://www3.paho.org/hq/dmdocuments/2012/Informe-Regional-SIREVAII-2008.pdf (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II, 2009: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores; OPS: Washington, DC, USA, 2010; Available online: https://www3.paho.org/hq/dmdocuments/2010/SIREVA%20II%202009.pdf (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II, 2010: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores; OPS: Washington, DC, USA, 2011; Available online: https://www3.paho.org/hq/dmdocuments/2011/SIREVA-II-2010.pdf (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II, 2011: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores; OPS: Washington, DC, USA, 2012; Available online: https://www3.paho.org/hq/dmdocuments/2012/SIREVA-II-2011-Sp.pdf (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II, 2012: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasores; OPS: Washington, DC, USA, 2013; Available online: https://www3.paho.org/hq/index.php?option=com_docman&view=download&alias=30766-informe-regional-sireva-ii-2012-766&category_slug=sireva-ii-8059&Itemid=270&lang=pt (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe Regional de SIREVA II, 2013: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis en procesos invasivos bacterianos; OPS: Washington, DC, USA, 2016; Available online: https://iris.paho.org/handle/10665.2/31147 (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe regional de SIREVA II, 2014: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, em procesos invasivos bacterianos; OPS: Washington, DC, USA, 2017; Available online: https://iris.paho.org/handle/10665.2/33875 (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe regional de SIREVA II, 2015: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, em procesos invasivos bacterianos; OPS: Washington, DC, USA, 2018; Available online: https://iris.paho.org/bitstream/handle/10665.2/49091/9789275320099_spa.pdf?ua=1 (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe regional de SIREVA II, 2016: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, em procesos invasivos bacterianos; OPS: Washington, DC, USA, 2019; Available online: https://iris.paho.org/handle/10665.2/51781 (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe regional de SIREVA II, 2017; OPS: Washington, DC, USA, 2020; Available online: https://iris.paho.org/handle/10665.2/53136 (accessed on 12 November 2023).
- Organización Panamericana de la Salud. Informe regional de SIREVA II, 2018; OPS: Washington, DC, USA, 2021; Available online: https://iris.paho.org/handle/10665.2/54567 (accessed on 12 November 2023).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 19 July 2023).
- Sugianli, A.K.; Ginting, F.; Parwati, I.; de Jong, M.D.; van Leth, F.; Schultsz, C. Antimicrobial resistance among uropathogens in the Asia-Pacific region: A systematic review. JAC Antimicrob. Resist. 2021, 3, dlab003. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Cornick, J.E.; Harris, S.R.; Parry, C.M.; Moore, M.J.; Jassi, C.; Kamng’ona, A.; Kulohoma, B.; Heyderman, R.S.; Bentley, S.D.; Everett, D.B. Genomic identification of a novel co-trimoxazole resistance genotype and its prevalence amongst Streptococcus pneumoniae in Malawi. J. Antimicrob. Chemother. 2014, 69, 368–374. [Google Scholar] [CrossRef][Green Version]
- Domingues, C.M.; Verani, J.R.; Montenegro Renoiner, E.I.; de Cunto Brandileone, M.C.; Flannery, B.; de Oliveira, L.H.; Santos, J.B.; de Moraes, J.C. Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: A matched case-control study. Lancet Respir. Med. 2014, 2, 464–471. [Google Scholar] [CrossRef]
- Gupta, V.; Yu, K.C.; Schranz, J.; Gelone, S.P. A multicenter evaluation of the US prevalence and regional variation in macrolide-resistant S. pneumoniae in ambulatory and hospitalized adult patients in the United States. Open Forum Infect. Dis. 2021, 8, ofab063. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing (CLSI) M100-S17. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
| Newcastle—Ottawa | Selection | Comparability | Outcome | Total | Classification | |||
|---|---|---|---|---|---|---|---|---|
| Article | 1 | 2 | 1 | 1 | 2 | 3 | Number of “Yes”/Total | |
| Pinto et al., 2019 [38] | No | No | No | Yes | Yes | No | 2/6 | Poor |
| Velasquez et al., 2009 [39] | Yes | Yes | Yes | Yes | Yes | Yes | 6/6 | Excellent |
| Pereira et al., 2004 [40] | Yes | Yes | Yes | Yes | Yes | Yes | 6/6 | Excellent |
| Cardozo et al., 2006 [41] | Yes | Yes | No | Yes | Yes | No | 4/6 | Good |
| Franco et al., 2010 [42] | Yes | Yes | Yes | Yes | Yes | No | 5/6 | Excellent |
| Neves et al., 2019 [43] | Yes | Yes | No | Yes | Yes | No | 4/6 | Good |
| Neves et al., 2017 [21] | Yes | Yes | No | Yes | Yes | No | 4/6 | Good |
| Reis et al., 2008 [44] | Yes | Yes | Yes | Yes | Yes | No | 5/6 | Excellent |
| Neves et al., 2013 [45] | Yes | Yes | No | Yes | Yes | No | 4/6 | Good |
| Laranjeira, 2014 [46] | Yes | Yes | No | Yes | Yes | Yes | 5/6 | Excellent |
| Da Silva et al., 2023 [47] | Yes | Yes | Yes | Yes | Yes | No | 5/6 | Excellent |
| Fortuna et al., 2023 [22] | Yes | Yes | No | Yes | Yes | No | 4/6 | Good |
| Fonseca et al., 2005 [48] | Yes | Yes | No | Yes | Yes | No | 4/6 | Good |
| Brandileone et al., 2019 [49] | Yes | Yes | Yes | Yes | Yes | No | 5/6 | Excellent |
| Zanella et al., 2019 [50] | Yes | Yes | Yes | Yes | Yes | No | 5/6 | Excellent |
| Rezende et al., 2021 [51] | Yes | Yes | No | Yes | Yes | No | 4/6 | Good |
| Laval et al., 2006 [52] | Yes | Yes | No | Yes | Yes | No | 4/6 | Good |
| Reference | Collection Date | City of Study | Clinical Source | Age Range | Study Scenario | Number of Isolates and Main Findings |
|---|---|---|---|---|---|---|
| (a) | ||||||
| [34] | April to October 2008 | Umuarama | NP swab Colonization | Children aged 3 months to 6 years | Nine daycare centers. | 92 isolates from 570 children Full susceptibility to LEV, LIN, ofloxacin, RIF, telithromycin, and VAN. Non-susceptibility frequencies: - CHL (1.1%), CLI (1.1%), ERY (8.7%), MDR (9.8%), PEN-I (34.8%), PEN-R (22.8%), SXT (72.8%), and TET (6.5%). |
| [35] | June 2001 to October 2002 | Porto Alegre | Children’s middle ear effusion (NIPD) | Children aged 11 months to 10 years | Pediatric otorhinolaryngology outpatient clinic. | S. pneumoniae was detected by PCR in 16 (12.5%) and by culture in 8 (6.3%) of 128 clinical specimens. Of 8 isolates: 3 (37.5%) PEN-S, 3 (37.5%) PEN-I, and 2 (25%) PEN-R. |
| [36] | November 2002 to July 2003 | Salvador | NP swab Colonization | Adolescents: 37.3% (aged 10–13 years), 49.4% (aged 14–16 years), and 13.3% (aged 17–19 years) | Public schools. | 83 isolates Full susceptibility to CHL, CLI, RIF, and VAN. No resistance to CTX in 18 isolates tested. Non-susceptibility frequencies: - ERY (4.8%), PEN-I (7.2%), SXT (37.3%), and TET (18.1%). |
| [37] | August to December 2005 | Goiânia | NP swab Colonization | Children aged 2 to 59 months | 62 of the 70 municipal daycare Centers. | 686 isolates Full susceptibility to LEV and VAN. - PNSP: 178 (25.9%) Results for 141 PNSP isolates tested with antimicrobial agents other than PEN (non susceptible): - CLI (2.1%), CHL (14.2%), ERY (6.4%), MDR (24.8%), SXT (82.3%), and TET (10.6%). |
| [39] | July 2000 to May 2001 | Salvador | NP swab Colonization | 33 children aged < 5 years; 43 children aged 5–17 years, and 19 adults aged > 17 years | Slum in Northeastern Amaralina. | 95 isolates Non-susceptibility frequencies: - CHL (3%), MDR (5%), PEN (9%), ERY (2%), SXT (39%), and TET (15%). |
| [40] | March to June 2010 | Niterói | NP swab Colonization | Children aged ≤ 6 years | Children at a daycare center (n = 102) and at the emergency room of a pediatric hospital (n = 140). | 121 isolates Full susceptibility to CLI, LEV, RIF, and VAN. Non-susceptibility frequencies: - CHL (3.3%), ERY (1.7%), SXT (51.2%), and TET (8.3%). - PNSP: 27.3%, with MICs of 0.12–4 μg/mL. |
| [43] | 9 April 2002 to 28 February 2003 | São Paulo | NP swab Colonization | From 4 months to 17 years (average and standard deviation of 6.8 ± 4.7 years) | Children with sickle cell disease being followed up at Hospital São Paulo. | 14 isolates Full susceptibility to CTX, ERY, and VAN. Non-susceptibility frequencies: - OXA disc screening: 35.7% non-susceptible to PEN; MIC = 0.25 µg/mL; LEV (42.9%), and SXT (64.3%). |
| [47] | Winters of 2000 and 2001 (May–August) | Goiânia | NP swab Colonization | Children aged < 5 years | 20 large pediatric hospitals with healthy children from local childcare program. | 227 isolates - PNSP: 19.8%. |
| CHL = chloramphenicol; CLI = clindamycin; CTX = ceftriaxone; ERY = erythromycin; IPD = invasive pneumococcal disease; LEV = levofloxacin; LIN = linezolid; MIC = minimum inhibitory concentration; MDR = multidrug-resistant (isolate resistant to three or more classes of antimicrobial agents); NIPD = non-invasive pneumococcal disease; NP = nasopharyngeal; OP = oropharyngeal; OXA = oxacillin; PCV10 = 10-valent pneumococcal conjugate vaccine; PEN = penicillin; PNSP = penicillin non-susceptible pneumococci; SXT = sulfamethoxazole-trimethoprim; TET = tetracycline; VAN = vancomycin. | ||||||
| (b) | ||||||
| [38] | 29 September to 5 December 2014 | Niterói | NP swab Colonization | Children aged ≥ 2 months and <6 years | Pediatric (2 private and 1 public) clinics. | 9 isolates - Full susceptibility to CHL, LEV, RIF, and VAN. Non-susceptibility frequencies: - ERY (22.2%) and MDR (33.3%). - PNSP: 44.4% (PEN and CTX MICs ranged from 0.12 to 4.0 μg/mL and 0.023–0.5 μg/mL, respectively). |
| [21] | 29 September to 5 December 2014 | Niterói | NP swab Colonization | Children aged ≤ 6 years | Pediatric (2 private and 1 public) clinics. | 118 isolates - Full susceptibility to LEV, RIF, and VAN; Non-susceptibility frequencies: - CHL (1.7%), CLI (20.3%), ERY (28%), MDR (22%), SXT (39.8%), and TET (29.7%). - PNSP: 38.9% (PEN and CTX MICs ranged from 0.12 to 8.0 μg/mL and 0.012–1.0 μg/mL, respectively). |
| [41] | January to December, 2011 | Fortaleza | NP swab Colonization | Children aged 20 to 65 months | 14 municipal kindergartens. | 162 isolates Full susceptibility to amoxicillin and CTX; Non-susceptibility frequencies: CLI (10.5%), ERY (13.6%), PNSP (27.7%), and SXT (100%). |
| [42] | October to December, 2016 | Niterói | NP swab Colonization | Adults aged 18 to 89 years | Patients assisted at a public health center that serves the population of an urban slum. | 35 isolates Full susceptibility to CHL, LEV, and VAN. Non-susceptibility frequencies: - CLI (5.7%), ERY (5.7%), MDR (11.4%), RIF (2.9%), SXT (31.4%), and TET (20%). - PNSP: 22.9% (PEN MICs of 0.38–1.5 μg/mL) |
| [22] | 2 September to 17 December 2019 | Niterói | NP swab Colonization | Children aged ≤ 6 years | Pediatric (2 private and 2 public) clinics | 75 isolates Full susceptibility to LIN, LEV, RIF, and VAN. Non-susceptibility frequencies: - CHL (1.7%), CLI (24%), ERY (25.3%), azithromycin (25.3%), MDR (22.7%), SXT (41.3%), and TET (25.3%) - PNSP: 37.3% (PEN and CTX MICs ranged from 0.12–4.0 μg/mL and 0.064–4.0 μg/mL, respectively.). |
| [44] | August 2017 | São Paulo | NP swab Colonization | Children aged 12 to <24 months | Recruitment during an immunization campaign in 20 public health units in 5 different regions. | 348 isolates - MIC to PEN ≥ 0.12 mg/L: 62%; - MIC to CTX ≥ 1.0 mg/L: 6.9%. - MIC90 to PEN and CTX: 1.0 mg/L and 0.5 mg/L, respectively; - MIC50 to PEN and CTX: 0.12 mg/L and 0.06 mg/L, respectively. |
| [45] | April to August 2017 (visit 1) and September to December 2017 (visit 2). | São Paulo | NP swab Colonization | Visit 1: mean age 81.5 years; range: 60–102 years). Visit 2: mean age 81.9 years | Outpatients treated at the Geriatrics Division of the Hospital das Clínicas of the Faculty of Medicine of the University of São Paulo. | 32 isolates - PEN-resistant: 9.4%, two with MIC = 0.125 mg/L and one with MIC = 2 mg/L; - CTX-resistant (MIC = 1 mg/L): 3.1%. |
| [46] | 19 June 2018 to 29 January 2019 | Niterói and Rio de Janeiro | NP and OP swabs Colonization | Adults aged ≥ 18 years | Patients with systemic lupus erythematosus at 2 teaching hospitals. | 11 isolates Full susceptibility to CHL, LEV, RIF, and VAN. Non-susceptibility frequencies: - CLI (18.2%), ERY (27.3%), MDR (27.3%), PEN (36.4%), TET (36.4%), and SXT (9.1%). |
| CHL = chloramphenicol; CLI = clindamycin; CTX = ceftriaxone; ERY = erythromycin; IPD = invasive pneumococcal disease; LEV = levofloxacin; LIN = linezolid; MIC = minimum inhibitory concentration; MDR = multidrug-resistant (isolate resistant to three or more classes of antimicrobial agents); NIPD = non-invasive pneumococcal disease; NP = nasopharyngeal; OP = oropharyngeal; OXA = oxacillin; PCV10 = 10-valent pneumococcal conjugate vaccine; PEN = penicillin; PNSP = penicillin non-susceptible pneumococci; SXT = sulfamethoxazole-trimethoprim; TET = tetracycline; VAN = vancomycin. | ||||||
| (c) | ||||||
| Reference | Collection Date | City of Study | Clinical Source | Age Range | Number of Isolates and Main Findings | |
| [33] | 2000–2010 | Angra dos Reis, Niterói, Porto Alegre, Ribeirão Preto, Rio de Janeiro, and São Paulo | NP and OP swabs | Pre-PCV10 period Colonization | 225 results for antimicrobial agents. | |
| [33] | 2011–2017 | Campos dos Goytacazes, Niterói, and Rio de Janeiro | NP and OP swabs | Post-PCV10 period Colonization | 229 results for antimicrobial agents. | |
| [33] | 2000–2007 | Niterói and Rio de Janeiro | Empyema aspirate, spinal aspirate, blood culture, CSF, pericardial fluid, peritoneal fluid, pleural fluid, peritoneal fluid, blood (catheter), blood/long-term central catheter, blood/PICC type, blood/CSF, blood/peritoneal fluid, pleural cavity secretion, thoracic cavity secretion, chest tube secretion, peritoneal secretion, meningeal specimen. | Pre-PCV10 period IPD | 39 results for antimicrobial agents. | |
| [33] | 2000–2009 | Niterói, Porto Alegre, and Rio de Janeiro | Ear abscess, cervical abscess, buttock abscess, nasal/eye abscess, bronchial aspirate, corneal aspirate, sinus aspirate, pulmonary aspirate, tracheal aspirate, sputum, aqueous humor, vitreous humor, bronchoalveolar lavage, auricular secretion, bronchial secretion, tear duct secretion, conjunctival secretion, wound secretion, ocular secretion, skin secretion, pulmonary secretion, postauricular secretion, tracheal secretion, rectal swab, corneal ulcer, urine. | Pre-PCV10 period NIPD | 82 results for antimicrobial agents. | |
| [33] | 2011–2015 | Niterói, Porto Alegre, and Rio de Janeiro | Ear abscess, cervical abscess, buttock abscess, nasal/eye abscess, bronchial aspirate, corneal aspirate, sinus aspirate, pulmonary aspirate, tracheal aspirate, sputum, aqueous humor, vitreous humor, bronchoalveolar lavage, secretion, auricular secretion, bronchial secretion, tear duct secretion, conjunctival secretion, wound secretion, ocular secretion, skin secretion, pulmonary secretion, postauricular secretion, tracheal secretion, rectal swab, corneal ulcer, urine. | Post-PCV10 Period NIPD | 27 results for antimicrobial agents. | |
| CSF = cerebrospinal fluid; IPD = invasive pneumococcal disease; NIPD = non-invasive pneumococcal disease; NP = nasopharyngeal; OP = oropharyngeal; PCV10 = 10-valent pneumococcal conjugate vaccine; PICC = peripherally inserted central catheter. | ||||||
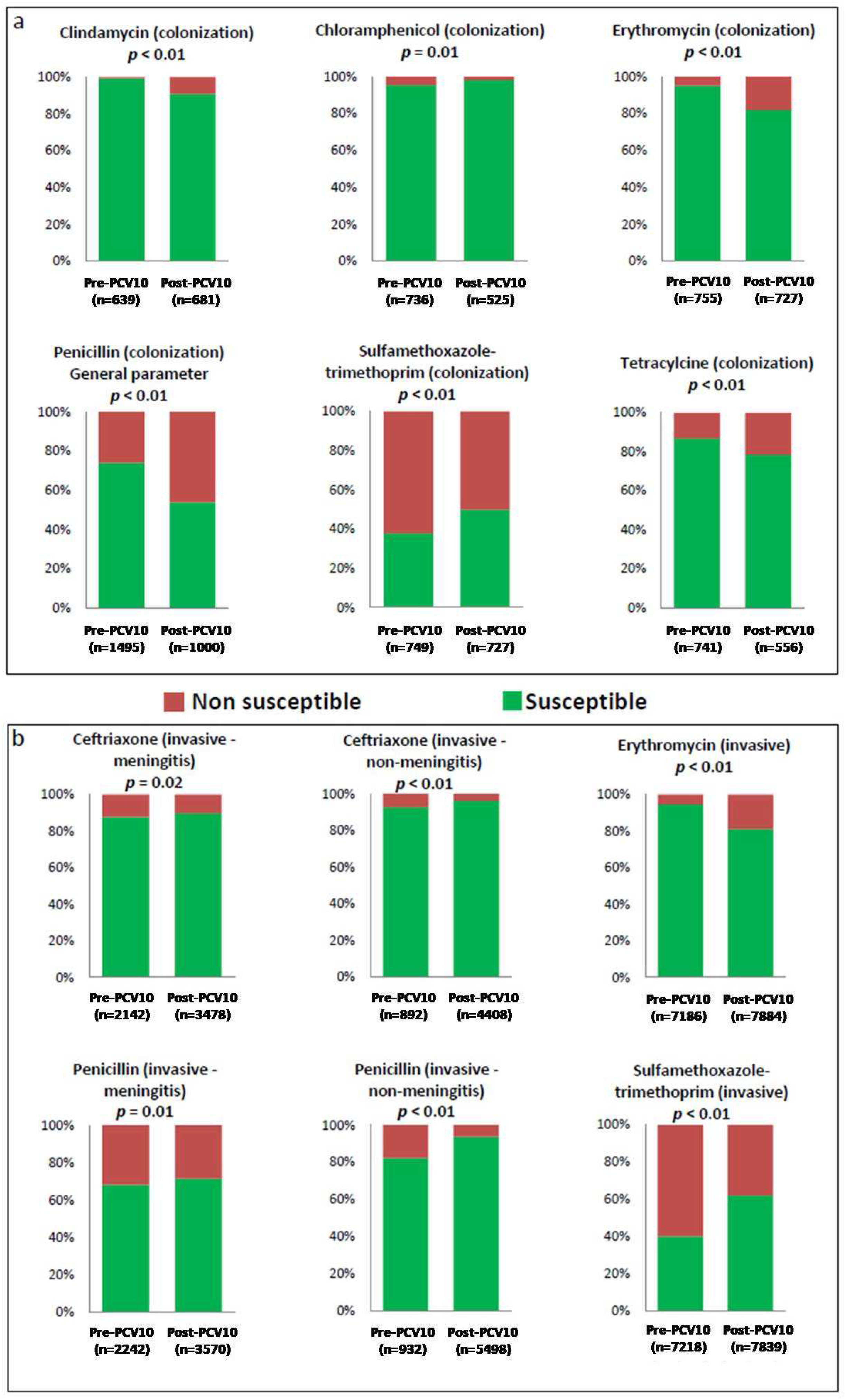
| Antimicrobial Agents | Colonization Isolates | Non-Invasive Isolates | Invasive Isolates | N | |||
|---|---|---|---|---|---|---|---|
| S (%) | NS (%) | S (%) | NS (%) | S (%) | NS (%) | ||
| Chloramphenicol | 703 (8.8%) | 33 (0.4%) | 43 (0.5%) | 4 (0.05%) | 7155 (89.5%) | 58 (0.7%) | 7996 |
| Erythromycin | 719 (8.9%) | 36 (0.4%) | 49 (0.6%) | 2 (0.02%) | 6795 (85%) | 391 (4.9%) | 7992 |
| SXT | 284 (3.5%) | 465 (5.8%) | 10 (0.1%) | 39 (0.5%) | 2907 (36.3%) | 4311 (53.8%) | 8016 |
| Vancomycin | 645 (92.1%) | - | 40 (5.7%) | - | 15 (2.1%) | - | 700 |
| Clindamycin | 635 (91.1%) | 4 (0.6%) | 42 (6%) | 1 (0.1%) | 14 (2%) | 1 (0.1%) | 697 |
| Tetracycline | 643 (78.8%) | 98 (12%) | 31 (3.8%) | 21 (2.6%) | 17 (2.1%) | 6 (0.7%) | 816 |
| Rifampicin | 498 (88%) | 2 (0.3%) | 47 (8.3%) | - | 19 (3.3%) | - | 566 |
| Levofloxacin | 557 (88.1%) | 7 (1.1%) | 48 (7.6%) | - | 20 (3.2%) | - | 632 |
| Ofloxacin | 92 (100%) | - | - | - | - | - | 92 |
| Cefotaxime | 111 (83.5%) | 4 (3%) | 8 (6%) | 3 (2.3%) | 6 (4.5%) | 1 (0.7%) | 133 |
| Cefuroxime | 123 (79.4%) | 12 (7.7%) | 3 (1.9%) | 8 (5.2%) | 6 (3.9%) | 3 (1.9%) | 155 |
| Meropenem | 87 (72.5%) | 13 (10.8%) | 3 (2.5%) | 8 (6.7%) | 6 (5%) | 3 (2.5%) | 120 |
| Linezolid | 169 (89.4%) | - | 11 (5.8%) | - | 9 (4.8%) | - | 189 |
| Telithromycin | 180 (93.3%) | - | 8 (4.1%) | - | 5 (2.6%) | - | 193 |
| Trovafloxacin | 2 (40%) | - | 2 (40%) | - | 1 (20%) | - | 5 |
| Quinupristin- dalfopristin | 69 (77.5%) | - | 11 (12.4%) | - | 9 (10.1%) | - | 89 |
| Amoxicillin | 111 (81.6%) | 6 (4.4%) | 8 (5.9%) | 3 (2.2%) | 6 (4.4%) | 2 (1.5%) | 136 |
| Antimicrobial Agents | Colonization Isolates | Non-Invasive Isolates | Invasive Isolates | N | |||
|---|---|---|---|---|---|---|---|
| S (%) | NS (%) | S (%) | NS (%) | S (%) | NS (%) | ||
| Chloramphenicol | 516 (6.3%) | 9 (0.1%) | 22 (0.3%) | - | 7621 (92.5%) | 73 (0.9%) | 8241 |
| Erythromycin | 596 (6.9%) | 131 (1.5%) | 20 (0.2%) | 3 (0.03%) | 6397 (74.1%) | 1487 (17.2%) | 8634 |
| SXT | 362 (4.2%) | 365 (4.2%) | 15 (0.2%) | 7 (0.08%) | 4882 (56.8%) | 2957 (34.4%) | 8588 |
| Vancomycin | 525 (96%) | - | 22 (4%) | - | - | - | 547 |
| Clindamycin | 618 (87.9%) | 63 (9%) | 21 (3%) | 1 (0.1%) | - | - | 703 |
| Tetracycline | 436 (75.3%) | 120 (20.7%) | 16 (2.8%) | 7 (1.2%) | - | - | 579 |
| Rifampicin | 524 (95.8%) | 1 (0.2%) | 22 (4%) | - | - | - | 547 |
| Levofloxacin | 565 (96.3%) | - | 22 (3.7%) | - | - | - | 587 |
| Ofloxacin | - | - | - | - | - | - | - |
| Cefotaxime | 73 (91.3%) | 7 (8.7%) | - | - | - | 80 | |
| Cefuroxime | 18 (100%) | - | - | - | - | - | 18 |
| Meropenem | 19 (51.4%) | 5 (13.5%) | 13 (35.1%) | - | - | - | 37 |
| Linezolid | 99 (100%) | - | - | - | - | - | 99 |
| Telithromycin | 82 (100%) | - | - | - | - | - | 82 |
| Trovafloxacin | - | - | - | - | - | - | - |
| Quinupristin- dalfopristin | 24 (100%) | - | - | - | - | - | 24 |
| Amoxicillin | 186 (97.4%) | 5 (2.6%) | - | - | - | - | 191 |
| Antimicrobial Agent | Origin | Meningitis | Non Meningitis | General Parameter | ||||||
| S (%) | NS (%) | N | S (%) | NS (%) | N | S (%) | NS (%) | N | ||
| (a) | ||||||||||
| Penicillin | Colonization | - | - | - | - | - | - | 1108 (74.1%) | 387 (25.9%) | 1495 |
| Non-invasive | - | - | - | - | - | - | 51 (66.2%) | 26 (33.8) | 77 | |
| Invasive | 1527 (68.1%) | 715 (31.9%) | 2242 | 767 (82.3%) | 165 (17.7%) | 932 | 3082 (73.9%) | 1087 (26.1%) | 4169 | |
| (b) | ||||||||||
| Penicillin | Colonization | - | - | - | - | - | - | 539 (53.9%) | 461 (46.1%) | 1000 |
| Non-invasive | - | - | - | - | - | - | 20 (83.3%) | 4 (16.7%) | 24 | |
| Invasive | 2545 (71.3%) | 1025 (28.7%) | 3570 | 4307 (93.7%) | 291 (6.3%) | 4598 | - | - | - | |
| Antimicrobial | Origin | Meningitis | Non Meningitis | General Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) | I (%) | R (%) | N | S (%) | I (%) | R (%) | N | S (%) | I (%) | R (%) | N | ||
| (a) | |||||||||||||
| Ceftriaxone | Colonization | - | - | - | - | - | 5 (83.3%) | 1 (16.7) | 6 | 32 (100%) | - | - | 32 |
| Non-invasive | - | - | - | - | 5 (55.5%) | 4 (44.4%) | - | 9 | - | - | - | - | |
| Invasive | 1879 (87.7%) | 174 (8.1%) | 89 (4.1%) | 2142 | 828 (92.8%) | 62 (7%) | 2 (0.2%) | 892 | 3082 (96.9%) | 87 (2.7%) | 11 (0.3%) | 3180 | |
| (b) | |||||||||||||
| Ceftriaxone | Colonization | - | - | - | - | 98 (96.1%) | 4 (3.9%) | - | 102 | 517 (95.4%) | - | 25 (4.6%) | 542 |
| Non-invasive | - | - | - | - | - | - | - | - | - | - | - | - | |
| Invasive | 3121 (89.7%) | 239 (6.9%) | 118 (3.4%) | 3478 | 4244 (96.3%) | 164 (3.7%) | - | 4408 | - | - | - | - | |
| Number | Exclusion Criteria | Inclusion Criteria |
|---|---|---|
| 1 | Review/Commentary Articles | Articles with raw data |
| 2 | Veterinary or plant isolates | Human isolates |
| 3 | Other bacterial species | Streptococcus pneumoniae |
| 4 | Out of date (before the year 2000) | Articles with data from 2000 to 2023 |
| 5 | SIREVA II data | Data not presented by SIREVA II |
| 6 | Data from other countries | Brazilian data |
| 7 | Other unrelated topics | Articles within the proposed theme |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knupp-Pereira, P.A.; Cabral, A.S.; Dolores, Í.M.; da Silva, A.B.; Póvoa, H.C.C.; Neves, F.P.G. Antimicrobial Resistance in Streptococcus pneumoniae before and after the Introduction of Pneumococcal Conjugate Vaccines in Brazil: A Systematic Review. Antibiotics 2024, 13, 66. https://doi.org/10.3390/antibiotics13010066
Knupp-Pereira PA, Cabral AS, Dolores ÍM, da Silva AB, Póvoa HCC, Neves FPG. Antimicrobial Resistance in Streptococcus pneumoniae before and after the Introduction of Pneumococcal Conjugate Vaccines in Brazil: A Systematic Review. Antibiotics. 2024; 13(1):66. https://doi.org/10.3390/antibiotics13010066
Chicago/Turabian StyleKnupp-Pereira, Patricia Alice, Amanda Seabra Cabral, Ítalo Moraes Dolores, Amanda Beiral da Silva, Helvécio Cardoso Correa Póvoa, and Felipe Piedade Gonçalves Neves. 2024. "Antimicrobial Resistance in Streptococcus pneumoniae before and after the Introduction of Pneumococcal Conjugate Vaccines in Brazil: A Systematic Review" Antibiotics 13, no. 1: 66. https://doi.org/10.3390/antibiotics13010066
APA StyleKnupp-Pereira, P. A., Cabral, A. S., Dolores, Í. M., da Silva, A. B., Póvoa, H. C. C., & Neves, F. P. G. (2024). Antimicrobial Resistance in Streptococcus pneumoniae before and after the Introduction of Pneumococcal Conjugate Vaccines in Brazil: A Systematic Review. Antibiotics, 13(1), 66. https://doi.org/10.3390/antibiotics13010066






