Abstract
Low-affinity PBP4, historically linked to penicillin resistance in Enterococcus faecalis, may still have affinity for novel cephalosporins. Ceftobiprole (BPR) is a common therapeutic choice, even with PBP4-related overexpression and amino acid substitution due to mutations. Our study aims to explore the interaction between BPR and High-Molecular-Mass (HMM) low-reactive PBPs in Penicillin-Resistant-Ampicillin-Susceptible/Ceftobiprole Non-Susceptible (PRAS/BPR-NS) E. faecalis clinical isolates. We conducted competition assays examining class A and B HMM PBPs from four PRAS/BPR-NS E. faecalis strains using purified membrane proteins and fluorescent penicillin (Bocillin FL), in treated and untreated conditions. Interaction strength was assessed calculating the 50% inhibitory concentration (IC50) values for ceftobiprole, by analyzing fluorescence intensity trends. Due to its low affinity, PBP4 did not display significant acylation among all strains. Moreover, both PBP1a and PBP1b showed a similar insensitivity trend. Conversely, other PBPs showed IC50 values ranging from 1/2-fold to 4-fold MICs. Upon higher BPR concentrations, increased percentages of PBP4 inhibition were observed in all strains. Our results support the hypothesis that PBP4 is necessary but not sufficient for BPR resistance, changing the paradigm for enterococcal cephalosporin resistance. We hypothesize that cooperation between class B PBP4 and at least one bifunctional class A PBP could be required to synthesize peptidoglycan and promote growth.
1. Introduction
As leading causes of multidrug-resistant hospital-acquired infections worldwide, enterococci represent the third most common cause of native valve endocarditis [1]. Among the Enterococcus genus, Enterococcus faecalis, and Enterococcus faecium are responsible for approximately 75% of all typed enterococcal infections [2]. Indeed, they are a common underlying cause of infections involving the urinary tract, abdomen, biliary tract, surgical wounds, as well as bacteremia, endocarditis, and burns. Their ability to thrive in healthcare settings is attributed to their intrinsic resistance to almost all cephalosporins, all semi-synthetic penicillins, aminoglycosides, clindamycin, and trimethoprim–sulfamethoxazole. In turn, this leads to several cases of multidrug resistance, extending hospitalization time, increasing the risk of treatment failure and death. Additionally, due to the remarkable genetic adaptability of enterococci, they can readily acquire new resistance traits, such as high-level aminoglycoside resistance, high-level ampicillin resistance, and vancomycin resistance, either through mutation or by horizontal gene transfer [3].
The production of β-lactamases, rare and unusual in enterococci, along with the expression of low-affinity Penicillin-Binding Proteins (PBPs), represent the two major mechanisms leading to their intrinsic resistance to most β-lactams [4]. PBPs are classified according to their masses as High-Molecular-Mass (HMM, >45 kDa) PBPs, comprising classes A and B, and Low-Molecular-Mass (LMM, <45 kDa) PBPs, consisting of class C PBPs only.
HMM PBPs are responsible for peptidoglycan polymerization and insertion into pre-existing cell walls [5]. Class A PBPs are bifunctional enzymes displaying glycosyltransferase and transpeptidase activity, whereas class B PBPs exert only transpeptidase activity [6]. The C-terminal binding domain (PB domain) of both classes catalyze peptide cross-linking between two adjacent glycan chains exerting transpeptidase activity. While the N-terminal domain of Class A PBPs displays glycosyltransferase activity only, catalyzing the elongations of uncross-linked glycan chains, in class B PBPs it is implicated in cell morphogenesis by interacting with other proteins involved in the cell cycle [7]. Class C PBPs possess peptidyl-transferases with D,D-carboxypeptidase and D,D-endopeptidase activity.
Enterococci harbor diverse PBPs, among which three belong to class A PBPs, and three to class B PBPs, while only two to class C PBPs [8]. Within the class B PBPs, low-affinity PBP4 E. faecalis and PBP5 E. faecium are known to play key roles in β-lactam resistance [9,10]. PBP4 has four structural domains: two N-terminal domains, known as the N1 domain, which anchors the enzyme to the cytoplasmic membrane, and an N2 domain, which may be involved in protein interactions; a C-terminal domain, with transpeptidase activity (TPase); and a non-penicillin-binding domain (nPB) [11]. Additionally, altered expression levels and mutations of genes encoding PBPs may also play a role in β-lactam resistance [12].
Ceftobiprole (BPR), a fifth-generation cephalosporin, has demonstrated a potent broad spectrum of activity against some Gram-positive bacteria, including E. faecalis. The high affinity of BPR to PBPs enables the drug to form a stable inhibitory complex, which determines its potent antibacterial activity.
The aim of this work was to analyze interactions occurring between BPR and High-Molecular-Mass (HMM) PBPs, with a particular focus on PBP4, to look for a possible role of PBP4 alterations influencing the binding to BPR using Penicillin-Resistant-Ampicillin-Susceptible/BPR Non-Susceptible (PRAS/BPR-NS) E. faecalis clinical isolates.
2. Results
2.1. Binding Profiles of HMM PBPs
Based on the PBP ATCC 47077 in silico analysis and the mobility on the gel, we detected six High-Molecular-Mass (HMM) and two Low-Molecular-Mass (LMM) PBPs (DD, carboxypeptidase). The exact molecular weight, the putative function of each protein, and the amino acid and nucleotide sequences listed in GenBank (https://www.ncbi.nlm.nih.gov/nuccore/CP025020.1 accessed on 13 February 2023) are shown in Table 1.

Table 1.
PBP genes of E. faecalis OG1RF (ATCC 47077) chromosome, corresponding proteins, and putative molecular weights (kDa) (this study).
We further assessed the BPR 50% inhibitory concentration (IC50) values. Figure 1 and Table 2 report detailed BPR inhibition rates and PBP binding profiles of all E. faecalis strains in this study, along with corresponding Minimum Inhibitory Concentrations (MICs) values. Supplementary Materials are also provided (Figures S1–S5).
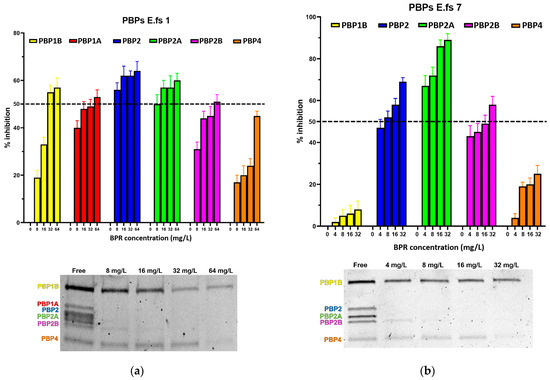
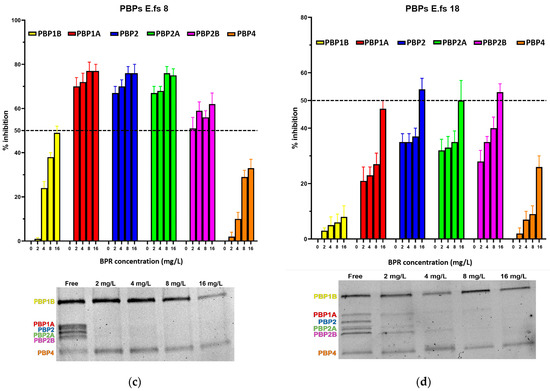
Figure 1.
Relative affinities of BPR (above) and PBP/Bocillin-labeled profiles on SDS–PAGE gel (below) for BPR-NS E. faecalis clinical isolates (a) E.fs1, (b) E.fs7, (c) E.fs8, and (d) E.fs18. Error bars indicate the average of three biological replicates in three assays ± SD.

Table 2.
Inhibition of PBPs from the E. faecalis strains in this study, and determination of IC50 value.
All strains showed similar behavior, whereby BPR consistently demonstrated low affinity for PBP4 in all isolates. Similar unresponsiveness was detected for PBP1b in almost all strains. Among the BPR non-susceptible strains (BPR-NS), E.fs7 (BPR MIC value 8 mg/L; range tested 4–32 mg/L), PBP1b and PBP4 were not inhibited by BPR, as demonstrated by their strong fluorescence to Bocillin FL, while BPR retained powerful affinity for PBP2, PBP2a, and PBP2b at different concentrations. Unexpectedly, E.fs7 lacked PBP1a. A similar inhibition profile was also recorded for E.fs8 (BPR MIC value 4 mg/L; range tested: 2–16 mg/L), but unlike E.fs7, an almost 50% inhibition was observed for PBP1b. PBP1a in E.fs18 (BPR MIC value 4 mg/L; range tested: 2–16 mg/L) did not undergo sufficient acylation even when their BPR inhibition rates were close to the IC50 value at 4× MIC; instead, PBP2, PBP2a, and PBP2b were readily inhibited at concentrations above 2× MIC. Despite the strong fluorescence shown in the gel, PBP1b and PBP4 were not significantly acylated at any concentration tested. In E.fs1 (BPR MIC value 16 mg/L; range tested 8–64 mg/L), PBP4 was inhibited by 4× MIC near to the IC50 value (45%). Conversely, other PBPs were adequately acylated, as reported by their IC50 values. (Table S1).
Focusing on PBP4 binding to BPR, we observed that its inhibition percentages increased in all strains using a higher amount of the drug although never reaching IC50, reflecting that this inhibition is concentration-related and not MIC-concentration-related (related to the respective MIC value for each strain) (Figure 2).
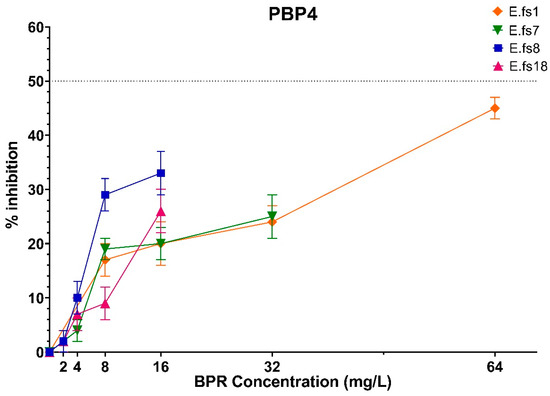
Figure 2.
Comparison of PBP4 percentages of inhibition concentrations of BPR of all strains. Orange line: E.fs1, Green line: E.fs7, Blue line: E.fs8, Fuchsia line: Efs18. GraphPad Prism 8 software (version 8.4.0).
2.2. Analysis of β-Lactamase Production
The hydrolytic activity of Nitrocefin vs. S. aureus ATCC 29213, used as Bla-positive control, was detected as an intense red color, demonstrating abundant enzyme release. On the other hand, E. faecalis ATCC 29212, as Bla-negative control, remains yellow, because of the absence of hydrolytic activity.
Efs1, Efs7, and Efs18 isolates developed a moderate red color 24 h after nitrocefin exposure, proving the enzyme production of β-lactamase and its activity. End-point PCR confirmed the presence of blaZ gene in these strains and were considered β-lactamase producers.
3. Discussion
Penicillin-resistant E. faecalis strains occur rarely but resistance can emerge following treatment with β-lactams [13]. Through the production of low-affinity PBPs, enterococci may acquire resistance to these antibiotics. Indeed, PBP4 in E. faecalis is considered a key player in intrinsic resistance against cephalosporins [8]. PBP4 operates peptidoglycan cross-linking, often in association with other PBPs such as PBP1a. Other PBPs such as PBP2a and PBP1b are thought to collaborate with PBP4, but direct interactions still require verification [14]. PBP2 is essential for cell viability. However, Dijoric et al. [15] demonstrated that PBP2, as well as PBP2b, display poor reactivity towards cephalosporins. Despite the low-affinity PBPs, BPR targets and binds these enzymes, with particular affinity for PBP4.
We related the in vitro antibacterial activity exerted by BPR to mutations of the pbp4 gene, and the affinity of BPR against PBPs. A complex mechanism of resistance to β-lactams emerged, whereby susceptibility varies for distinct β-lactams according to the PBP class with which PBP4 collaborates.
A link between resistance to benzyl-penicillins and non-susceptibility to fifth-generation cephalosporins such as BPR emerged, related to deletion of an adenine in the regulatory region upstream of the pbp4 promoter, causing altered expression levels of the pbp4 gene, along with missense mutations, destabilizing the PBP/β-lactam complex. Previous reports have described mutations in the pbp4 gene [16,17,18]. Indeed, Rice et al. [13] and Lazzaro LM et al. [12] observed that adenine deletion (delA) −35 bp upstream of the promoter region led to an over-expression of the pbp4 gene due to an alteration of the binding of regulatory proteins. As a result, increased levels of transpeptidation in peptidoglycan leading to higher levels of cross-linking in the bacterial cell wall are reached, resulting in higher MIC values. Consistently with these findings, the delA mutation carried by the four BPR-NS strains could explain the in vitro non-susceptibility, albeit still retaining bactericidal activity (Table 3) [19]. Missense mutations near the serine active site and the catalytic motifs of the enzyme may also play a role in increasing the MIC value. Such substitutions could destabilize the BPR/PBP complex, leading to a less efficient one. This was particularly true in PRAS/BPR-NS E.fs1, whereby the T418A mutation falls six amino acids upstream of the catalytic serine of motif I, leading to a BPR MIC value of 16 mg/L, and bactericidal activity only at 4× MIC. These results also led to the hypothesis that, due to the low affinity of PBP4, initial binding to BPR does not occur (explaining the in vitro antibacterial activity) but can be seen upon prolonged exposure and at higher concentrations of BPR.

Table 3.
Beta-lactam and comparator drug MIC values (mg/L) and PBP4 sequence alterations of the four E. faecalis strains included in this study.
PBP4 expression induction by BPR was not assessed in this study. Further work should also elucidate whether BPR impacts PBP4 and other PBP expression profile in different phenotypic backgrounds carrying advantageous gene mutations.
Regarding the PBP binding profiles and BPR inhibition rates analyzed by IC50 values, the major role of PBP4 as necessary and sufficient PBP conferring high-level resistance to β-lactams was not confirmed. Indeed, PBP4 is the only PBP lacking acylation, but this trend is similar to that observed for PBP1b (only acylated in E.fs1, at BPR concentration above the MIC value). Thus, it is tempting to speculate that PBP4 requires the cooperation of a bifunctional PBP to determine cephalosporin resistance.
The labeling of PBPs with Bocillin FL in competition assays is dependent on the availability of PBPs for covalent interaction with the fluorescent penicillin. The overexpression of low-affinity PBP4 could contribute to its inability to bind effectively with β-lactams and, even after treatment with increasing ceftobiprole concentrations, there may always be enough PBP4 amount available for covalent interaction with Bocillin. Moreover, the labeling efficiency of PBP4 with Bocillin can vary among the strains, according to their different levels of expression.
Taken together, no direct correlation between BPR MIC values, expression levels of pbp4, aminoacidic substitutions in the catalytic site [12], and IC50 values were found in this subset of E. faecalis strains. BPR binding affinity to PBP4 is concentration-dependent and does not influence MIC values. Moreover, BPR-NS strains showed a higher amount of PBPs other than PBP4 and PBP1b inhibited by BPR. Over-expressed PBP4 and PBP1b may be sufficient to cause reduced susceptibility to BPR. The impact of other PBP mutations should also be further analyzed to clarify their relationship in BPR resistance in E faecalis.
Moreover, although rare [20,21], the three strains were found to be weak β-lactamase producers. While the acyl–enzyme complex is initially stable, the production of β-lactamases in E.fs1, E.fs7, and E.fs18 could catalyze the deacylation reaction, leading to the inactivation and degradation of the antibiotic molecule, thus enhancing resistance against β-lactams and potentially also against fifth-generation cephalosporins. Ceftobiprole should be more prone to degradation by β-lactamase activity in E.fs1, E.fs7, and E.fs18, reducing their inhibitory potency. In contrast, Bocillin FL, being a modified penicillin, may have enhanced stability against such degradation mechanisms, leading to lower MIC values.
4. Materials and Methods
4.1. Selected Strains
Four E. faecalis strains were selected for their antibiotic-resistance behaviors, belonging to the major multi-drug resistance phenotypes: Penicillin-Resistant Ampicillin-Susceptible (PRAS), Ceftobiprole Non-Susceptible (BPR-NS), Vancomycin-Resistant (VRE), and High-level Aminoglycoside Resistant of (HLAR), from a previously characterized collection of seven E. faecalis clinical strains, isolated from bloodstream infections (BSI) in Italian hospitals, showing diverse mutations in coding and non-coding regions of pbp4 [12].
The MIC values were determined by the reference broth microdilution method and interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints (http://www.eucast.org/clinical_breakpoints/ accessed on 1 January 2021) (EUCAST, 2021). In the absence of EUCAST clinical breakpoints, those of the Clinical and Laboratory Standards Institute were applied (Clinical and Laboratory Standards Institute, 2021) (Table 3).
Two β-lactam-susceptible E. faecalis strains were also included as control: E. faecalis ATCC 29212, used as control for antimicrobial susceptibility testing [22], and E. faecalis OG1-RF, deposited in the American Type Culture Collection (ATCC) under ATCC 47077, deriving from E. faecalis OG1 by selection for resistance to rifampin and fusidic acid [23], used as control in the competition assay study.
4.2. Study of PBP4/BPR Affinity and Competition Assay
To evaluate the affinity of BPR for PBPs and determine if PBP4 significant aminoacidic substitutions could affect and alter the binding and stability of the BPR/PBP4 complex, competition assays were carried out. We used Bocillin FL (Invitrogen-life Technologies, Turin, Italy), a fluorescent β-lactam composed of penicillin V derivative linked to BODIPY fluorochrome. Due to its composition, Bocillin FL reacts with empty binding sites that remain after the reaction of PBPs with unlabeled BPR, detecting the formation of the β-lactam/PBP complex.
Therefore, through the evaluation of the Bocillin fluorescence level at different concentrations of BPR tested, we assessed the antibiotic concentration that displaces 50% of the labeled molecule from the binding site (IC50). Thus, we obtained the potential rate of inhibition of PBP acylation in PRAS/BPR-NS strains.
The relative binding of BPR was evaluated at four different concentrations (1/2-fold to 4-fold the MIC value, specifically 1/2×, 1×, 2×, and 4 × MIC).
Briefly, a bacterial colony from fresh culture plate was inoculated into 5 mL of BHI broth and incubated o/n at 37 °C. The strain culture was measured to an OD450 nm to obtain a starting inoculum of 105–106 CFU/mL. The cells were therefore incubated for two hours at 37 °C, until reaching an OD620 = 0.20, corresponding to the exponential phase. Bacteria were harvested by centrifugation for 2 min at room temperature (RT) and washed in PBS (10 mM, pH 7.4). Different BPR concentrations were added. The samples were incubated at 37 °C for 30 min and resuspended in 50 μL of PBS containing 5 μg/mL of Bocillin FL (7.6 μM).
Following incubation with lysozyme (10 mg/mL) for 30 min at 37 °C and 5 cycles of sonication in a Banderlin Sonifier (30 s, 30% duty cycle for 6 intervals), the protein concentration was determined by using the Qubit® protein Assay Kit, a fluorometric test executed using Qubit 2.0 (Invitrogen-life Technologies, Turin, Italy), and normalized at the same protein concentration; a 51 μL measure of each sample was added with 17 μL of 4× Laemmli buffer plus β-mercaptoethanol, as a reducing reagent, boiled for 5 min at 90 °C for protein denaturation, and cooled at RT. Electrophoresis was carried out in an SDS–PAGE on a Criterion™ TGX™ (Tris-Glycine extended) 8–16% polyacrylamide gel, for 2 h at a constant voltage of 100 V. To predict the molecular weight of each PBP, two different commercial pre-stained standards, SeeBlue® Plus2 Prestained Standard (Invitrogen by Thermo Fisher Scientific, Basingstoke, UK) and Thermo Scientific Spectra Multicolor High Range Protein Ladder, were used. The fluorescent Bocillin FL covalently bound to the PBPs was detected with excitation at 488 nm and emission at 520 nm (Typhoon FLA 9500; filter Alexa 488; PMT 1000; pixel 50 μm, Washington, DC, USA). To detect the PBP profile and identify their molecular weight, the SDS–PAGE Criterion™ TGX™ (Hercules, CA, USA) (Tris-Glycine extended) 8–16% polyacrylamide gel was stained for 1 h in a Coomassie brilliant blue R-250 buffer (40% methanol; 10% glacial acetic acid; 0.1% Coomassie Brilliant Blue R-250, St. Louis, MO, USA), and de-stained for 1 h (40% methanol; 10% glacial acetic acid).
The fluorescence was analyzed using ImageJ software (https://imagej.net/ij/features.html, accessed on 25 November 2023). The brightness and contrast on the image should be adjusted to optimize the signal-to-noise ratio. The background signal should be subtracted. Normalization was obtained correlating labeled PBPs with Coomassie-stained PBPs.
Our study was completed by the determination of the relative binding (IC50), i.e., the 50% inhibitory concentration values (concentration of ceftobiprole (μg/mL) needed to reduce by 50% the binding of Bocillin FL to individual PBPs), determined by plotting the PBP band volumes versus compound concentrations. The 100% binding of Bocillin FL was represented by the PBPs labeled with Bocillin FL but with no drug. IC50 values were calculated and statistically analyzed using the GraphPad Prism 8 software. We performed an in silico analysis for ATCC 47077 PBPs. We calculated the exact molecular weight of each PBP by the online bioinformatic database “Quest Calculate™ Peptide and Protein Molecular Weight Calculator” (AAT Bioquest, Inc.) (https://www.aatbio.com/tools/calculate-peptide-and-protein-molecular-weight-mw, accessed on 25 November 2023) and the amino acid and nucleotide sequences listed in GenBank (https://www.ncbi.nlm.nih.gov/nuccore/CP025020.1, accessed on 25 November 2023). The putative function of each protein was also inferred by the NCTC database.
4.3. β-Lactamase Assay
β-lactamase activity was evaluated by nitrocefin assay (OxoidTM, Thermo ScientificTM, Basingstoke, UK). Even though nitrocefin is a cephalosporin, and thus possesses a β-lactam ring susceptible to β-lactamase mediate hydrolysis, it does not have antimicrobial properties. Furthermore, if degraded, nitrocefin rapidly changes color from yellow to red.
Starting from a high inoculum (109 CFU/mL), simulating in vivo infections, cells were incubated for 24 h with and without oxacillin at a final concentration of 8 mg/mL. The detection of the β-lactamase activity on the supernatant and pellets (cellular debris) was performed using already published protocols [21], repeated in duplicate, and reconfirmed. S. aureus ATCC 29213 was used as positive control; E. faecalis ATCC 29212 was used as Bla-negative control. End-point PCR for blaZ gene was executed designing primers on the sequence of S. aureus ATCC 29213, the Bla+ control strain.
5. Conclusions
In conclusion, in PRAS/BPR-NS strains, mutations of the pbp4 gene (delA and amino acid substitutions) may influence the susceptibility profile to β-lactams, and particularly to BPR, increasing the MIC value and influencing the binding to the antimicrobial, without altering the in vivo bactericidal activity. Furthermore, the low affinity of PBP4 is a common feature of all strains, regardless of catalytic site alterations. In addition, other PBPs may be involved in intrinsic cephalosporin resistance in E. faecalis. PBP4 was necessary but not sufficient to determine BPR resistance, and β-lactam exposure requires cooperation of PBP4 and, at least, another class A PBP (preferably PBP1b). Moreover, the production of β-lactamases may contribute to enhance resistance to β-lactams and potentially also to fifth-generation cephalosporins.
Further analyses are needed to evaluate how PBPs other than PBP4 are involved in non-susceptibility to BPR, and what mutations and/or altered expression levels are required. Regarding PBP4, the role of delA and aminoacidic substitutions via site-direct mutagenesis should be analyzed, along with in vivo expression levels of pbp4 and BPR/PBP affinity at different exposure times and concentrations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13010065/s1, Figures S1–S5: Detailed PBPs inhibition rates of ATCC47077 (S1), E.fs1 (S2), e.fs7 (S), E.fs8 (S4), E.fs18 (S5).
Author Contributions
F.C. designed the research and directed the project. P.C., L.M.L., F.L. (Fabio Longo), F.L. (Federica Lenzo), A.G., S.A.F. and F.C. conducted the research. L.M.L., P.C. and F.C. analyzed the data. L.M.L., P.C., S.S. and F.C. wrote the manuscript. F.C. and S.S. edited and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by MUR PNRR-PE 13 One Health Basic and Translational Research Actions addressing Unmet Needs on Emerging Infectious Diseases, project no. PE00000007, INF-ACT (CUP E63C22002090006), and by the National Research Project of relevant interest PRIN2022 Soteria, project grant number 2022WZK874 (CUP E53D23009580006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
PBP4 sequences of the strains in this study were submitted to GenBank with ID accession no. OM032878 (Efs7); OM032880 (Efs1); OM032881 (Efs8); and OM032883 (Efs18).
Acknowledgments
We would like to thank the Bio-Nanotech Research and Innovation Tower (BRIT) service center belonging to the University of Catania (Italy) for the use of its facility, instruments, and technical assistance. We are grateful to the colleagues of “EURESTOP” COST Action CA21145 for their valuable advice.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in Enterococcus. Virulence 2012, 3, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.; Edwards, J.R.; Sievert, D.M. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti Infect Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Born, P.; Breukink, E.; Vollmer, W. In vitro synthesis of cross-linked murein and its attachment to sacculi by PBP1A from Escherichia coli. J. Biol. Chem. 2006, 281, 26985–26993. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.M.; D’Andréa, É.D.; Lee, C.W.; Soares, A.; Jakoncic, J.; Desbonnet, C.; Garcia-Solache, M.; Rice, L.B.; Page, R.; Peti, W. The structures of penicillin-binding protein 4 (PBP4) and PBP5 from Enterococci provide structural insights into β-lactam resistance. J. Biol. Chem. 2018, 293, 18574–18584. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Vernet, T.; Pinho, M. The different shapes of cocci. FEMS Microbiol. Rev. 2008, 32, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Arbeloa, A.; Segal, H.; Hugonnet, J.E.; Josseaume, N.; Dubost, L.; Brouard, J.P.; Gutmann, L.; Mengin-Lecreulx, D.; Arthur, M. Role of class A penicillin-binding proteins in PBP5-mediated β-lactam resistance in Enterococcus faecalis. J. Bacteriol. 2004, 186, 1221–1228. [Google Scholar] [CrossRef]
- Duez, C.; Zorzi, W.; Sapunaric, F.; Amoroso, A.; Thamm, I.; Coyette, J. The penicillin resistance of Enterococcus faecalis JH2-2R results from an overproduction of the low-affinity penicillin-binding protein PBP4 and does not involve a psr-like gene. Microbiology 2001, 147, 2561–2569. [Google Scholar] [CrossRef]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin binding proteins and beta-lactam resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef]
- Ghuysen, J.M. Serine β -lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 1991, 45, 37–67. [Google Scholar] [CrossRef]
- Lazzaro, L.M.; Cassisi, M.; Stefani, S.; Campanile, F. Impact of PBP4 Alterations on β-Lactam Resistance and Ceftobiprole Non-Susceptibility Among Enterococcus faecalis Clinical Isolates. Front Cell Infect. Microbiol. 2022, 11, 816657. [Google Scholar] [CrossRef]
- Rice, L.B.; Desbonnet, C.; Tait-Kamradt, A.; Garcia-Solache, M.; Lonks, J.; Moon, T.M.; D’Andréa, É.D.; Page, R.; Peti, W. Structural and regulatory changes in PBP4 trigger decreased β-lactam susceptibility in Enterococcus faecalis. mBio 2018, 9, e00361-18. [Google Scholar] [CrossRef]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef]
- Djorić, D.; Little, J.L.; Kristich, C.J. Multiple Low-Reactivity Class B Penicillin-Binding Proteins Are Required for Cephalosporin Resistance in Enterococci. Antimicrob. Agents Chemother. 2020, 64, e02273-19. [Google Scholar] [CrossRef]
- Conceição, N.; da Silva, L.E.; Darini, A.L.; Pitondo-Silva, A.; de Oliveira, A.G. Penicillin Resistant, Ampicillin-Susceptible Enterococcus faecalis of Hospital Origin: Pbp4 Gene Polymorphism and Genetic Diversity. Infect. Genet. Evol. 2014, 28, 289–295. [Google Scholar] [CrossRef]
- Gawryszewska, I.; Zabicka, D.; Hryniewicz, W.; Sadowy, E. Penicillin-Resistant, Ampicillin-Susceptible Enterococcus faecalis in Polish Hospitals. Microb. Drug Resist. 2021, 27, 291–300. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Conceic, N.; Goncalves de Oliveira, A.; Costa Darini, A.L. Evaluation of polymorphisms in pbp4 gene and genetic diversity in penicillin-resistant, ampicillin-susceptible Enterococcus faecalis from hospitals in different states in Brazil. FEMS Microbiol. Lett. 2016, 363, fnw044. [Google Scholar] [CrossRef]
- Werth, B.J.; Abbott, A.N. The combination of ampicillin plus ceftaroline is synergistic against Enterococcus faecalis. J. Antimicrob. Chemother. 2015, 70, 2414–2417. [Google Scholar] [CrossRef]
- Arias, C.A.; Singh, K.V.; Panesso, D.; Murray, B.E. Time-kill and synergism studies of ceftobiprole against Enterococcus faecalis, including beta-lactamase-producing and vancomycin-resistant isolates. Antimicrob. Agents Chemother. 2007, 51, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- Sarti, M.; Campanile, F.; Sabia, C.; Santagati, M.; Gargiulo, R.; Stefani, S. Polyclonal diffusion of beta-lactamase-producing Enterococcus faecium. J. Clin. Microbiol. 2012, 50, 169–172. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. EUCAST. 2021, Version 11.0. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 1 January 2021).
- Bourgogne, A.; Garsin, D.A.; Qin, X.; Singh, K.V.; Sillanpaa, J.; Yerrapragada, S.; Ding, Y.; Dugan-Rocha, S.; Buhay, C.; Shen, H.; et al. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 2008, 9, 1–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).